Abstract
To understand the mechanisms underlying visual motion analyses for perceptual and oculomotor responses and their similarities/differences, we analyzed eye movement responses to two-frame animations of dual-grating 3f5f stimuli while subjects performed direction discrimination tasks. The 3f5f stimulus was composed of two sinusoids with a spatial frequency ratio of 3:5 (3f and 5f), creating a pattern with fundamental frequency f. When this stimulus was shifted by 1/4 of the wavelength, the two components shifted 1/4 of their wavelengths and had opposite directions: the 5f forward and the 3f backward. By presenting the 3f5f stimulus with various interstimulus intervals (ISIs), two visual-motion-analysis mechanisms, low-level energy-based and high-level feature-based, could be effectively distinguished. This is because response direction depends on the relative contrast between the components when the energy-based mechanism operates, but not when the feature-based mechanism works. We found that when the 3f5f stimuli were presented with shorter ISIs (<100 ms), and 3f component had higher contrast, both perceptual and ocular responses were in the direction of the pattern shift, whereas the responses were reversed when the 5f had higher contrast, suggesting operation of the energy-based mechanism. On the other hand, the ocular responses were almost negligible with longer ISIs (>100 ms), whereas perceived directions were biased toward the direction of pattern shift. These results suggest that the energy-based mechanism is dominant in oculomotor responses throughout ISIs; however, there is a transition from energy-based to feature-tracking mechanisms when we perceive visual motion.
Keywords: visual motion analysis, eye movement, perception, interstimulus interval, illusion
presentation of two successive static images causes a sensation of motion (Anstis 1980; Braddick 1980), and such apparent motion is usually perceived in the direction of the phase shift. However, when the two frames are presented with an interstimulus interval (ISI), during which a uniform screen of mean luminance is presented, observers often experience reversal of the motion direction (Boulton and Baker 1993; Braddick 1980; Pantle and Turano 1992; Shioiri and Cavanagh 1990). Some illusory motion stimuli causing reverse motion perception are perceived in the direction of phase shift if successive motion frames are presented with ISIs of >10 ms or >40 ms (Georgeson and Harris 1990; Hammett et al. 1993; Shioiri and Cavanagh 1990). Similar ISI effects on eye movement responses to an illusory motion stimulus were reported by Sheliga et al. (2006a). Previous psychophysical studies demonstrated that there are two distinct motion-sensing mechanisms: low-level energy-based and high-level feature-tracking mechanisms (Lu and Sperling 2001; Nishida 2011). Previous ISI experiments provided insights into the mechanisms underlying visual motion analyses. Two mechanisms have been suggested for the reversals of the reversed motion percepts: a transition from energy-based to feature-tracking motion sensing caused by the ISI (Georgeson and Harris 1990; Hammett et al. 1993) and an effect of the biphasic impulse response function of temporal filters embedded in energy-based motion sensing (Sheliga et al. 2006a; Shioiri and Cavanagh 1990).
Georgeson and Harris (1990) and Sheliga et al. (2006a) examined ISI effects using the missing fundamental stimulus (Adelson 1982; Adelson and Bergen 1985). This stimulus is constructed by subtracting the fundamental sine-wave component from a square wave, which consists of the sum of odd harmonics with progressively decreasing amplitudes. When this stimulus is shifted by 1/4 of the wavelength, the principal Fourier component (the 3rd harmonic) shifts 3/4 of its wavelength, which is equivalent to a 1/4 wavelength step in the opposite direction. Thus, the pattern feature and its principal Fourier component move in opposite directions. The direction of perceived motion, and that of ocular responses, was opposite to the stimulus motion when successive 1/4-wavelength steps were applied without ISIs (Adelson 1982; Miura et al. 2006; Sheliga et al. 2005). When a 1/4-wavelength step was presented with ISIs, the direction of perceptual and oculomotor responses were in the direction of the phase shift (Georgeson and Harris 1990; Sheliga et al. 2006a). A closer look of their results revealed quantitative differences in perceptual and oculomotor responses. The reversal of reversed motion percept occurred when ISIs were >40 ms and was sustained up to an ISI of at least 320 ms (Georgeson and Harris 1990), whereas the reversal of reversed ocular responses occurred when ISIs were >10 ms, and the response almost disappeared with ISIs >100 ms (Sheliga et al. 2006a). These differences might reflect a difference in the visual motion mechanisms underlying perceptual and oculomotor responses. However, there are large differences in the experimental conditions between these two studies. Thus, the differences in the responses might be due to the stimulus configuration.
In the present study, we aimed to elucidate the mechanisms of visual motion analyses underlying perceptual and oculomotor responses and understand their similarities/differences. We used a dual grating stimulus composed of two sinusoids with a spatial frequency ratio of 3:5 (3f and 5f), creating a pattern with fundamental frequency f (Matsuura et al. 2008; Sheliga et al. 2006b). When this stimulus shifted by 1/4 of the wavelength, the two components stepped 1/4 wavelength and had opposite directions due to spatial aliasing (5f forward and 3f backward). Note that the 5f component moved 5/4 of its wavelength (equivalent to a forward 1/4 wavelength step) and the 3f component moved 3/4 of its wavelength (equivalent to a backward 1/4 wavelength step). The major Fourier component can be alternated by manipulating the relative contrast between the 3f and 5f components, in contrast to the missing fundamental stimulus in which relative contrast between 3f and 5f is fixed. If the energy-based motion sensors mediate observers' responses, then they are expected to change in association with alternation of the major Fourier component. On the other hand, if the feature-tracking mechanism is operative, then the observer's responses are independent of this alternation. Of particular interest are situations in which 5f is the major component. The response is expected to be in the direction of the phase shift if the feature-based mechanism predominates, whereas the opposite will occur, depending on ISIs, if the energy-based mechanism predominates. Thus, this stimulus configuration allows distinction between the energy-based and feature-tracking mechanisms in the presence of ISIs. Note that the missing fundamental stimulus does not distinguish between these mechanisms, even in the presence of ISIs.
In the present study, we examined perceptual and oculomotor responses to two-frame animations of the 3f5f stimuli presented with various ISIs. The subjects performed direction discrimination tasks during which oculomotor responses were also recorded. This experimental arrangement allowed direct comparison of the perceived motion and ocular responses induced by the same visual motion stimuli. To our knowledge, no study has compared perceptual and oculomotor responses using exactly the same motion stimuli. Our findings revealed similarities and differences in the mechanisms of visual motion analyses underlying motion perception and ocular responses.
METHODS
Subjects.
Eye movements were recorded in five 20 to 42 yr old subjects (S1–S5). Two of the subjects (S1 and S2) were authors, and three (S3–S5) were naïve and unaware of the experimental design. Each subject had normal or corrected-to-normal vision, normal visual fields, and clinically normal eye movements. Informed consent, based on the Helsinki Declaration, was obtained from all subjects. All experimental procedures were approved by the Kyoto University Graduate School and Faculty of Medicine, Ethics Committee.
Visual display and stimuli.
Subjects faced a 22-inch cathode ray tube monitor (Mitsubishi RDF223G) positioned 70.0 cm in front of their eyes in a dark room. Visual stimuli were presented on the monitor (resolution: 1,024 × 768 pixels, vertical refresh rate: 75 Hz). Gamma correction was performed for the monitor (8-bit grayscale resolution).
In experiment 1, we used 3f5f stimuli with luminance profiles created by the sum of two cosine waves with a spatial frequency ratio of 3:5 and zero phase difference, creating a pattern with fundamental frequency f. Each image extended 39.6 cm horizontally (31.6°, 1,024 pixels), 29.7 cm vertically (24.0°, 768 pixels) and had a mean luminance of 40.0 cd/m2. The initial phase of a given grating was randomized from trial to trial at intervals of 1/4 of the wavelength. The second frame was of the same pattern except phase shifted horizontally by 1/4 of the wavelength. An ISI was inserted between the first and second frames, during which a uniform gray image with the mean luminance was presented (0, 1, 2, 4, 8, 16, 32, or 64 frames at 75 Hz). The step direction was rightward or leftward. The total contrast of the 3f5f stimuli was fixed at 32% (Michelson contrast). The contrast ratios between the 3f and 5f components were 7/3, 5/5, or 3/7 (C3f/C5f). The fundamental frequency of the 3f5f patterns was determined as 0.066 cycles/° so that the spatial frequencies of the 3f and 5f stimuli had similar efficacy in inducing eye movement responses, which were 0.20 and 0.33 cycles/°, respectively. Note that the optimal spatial frequency for eye movement responses was ∼0.25 cycles/°, and its dependence was well characterized by Gaussian functions of log spatial frequency (see Miura et al. 2009; Sheliga et al. 2005).
Two additional ISI experiments were performed to interpret data from the main experiment. In experiment 2, four single sinusoidal gratings matching the component gratings of the 3f5f stimuli with contrast ratios of 7/3 (contrast, 3f stimulus, 22.4%; the 5f stimulus, 9.6%) and 3/7 (contrast, 3f stimulus, 9.6%; the 5f stimulus, 22.4%) were used. In experiment 3, a 3f5f stimulus with a contrast ratio of 5/5 and two single sinusoidal gratings matching the component gratings of the 3f5f stimulus were used. The ISI duration was randomly selected from 0, 1, 2, 3, 4, 5, 6, 7, 8, or 12 frames (at 75 Hz) in any given trial.
Procedures.
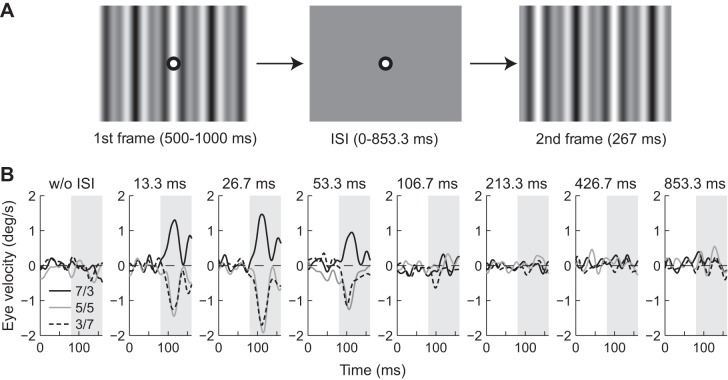
Subjects were requested to fixate on a central target (diameter, 0.4°) that appeared at the beginning of each trial along with a grating pattern (Fig. 1A). After the right eye had been positioned within 3° of the fixation target for a randomized period (500–1,000 ms), the pattern was replaced by a uniform gray image (ISI). The fixation target then disappeared, and the second frame was presented for 267 ms (20 frames), after which the screen turned a uniform gray at the same mean luminance. The subject was instructed to press a button (left or right) to report the perceived direction. After a 1,000 ms intertrial interval, a new grating pattern appeared along with a fixation point to start a new trial. Data were collected over several sessions on consecutive days until each condition had repeated at least 30 times.
Fig. 1.
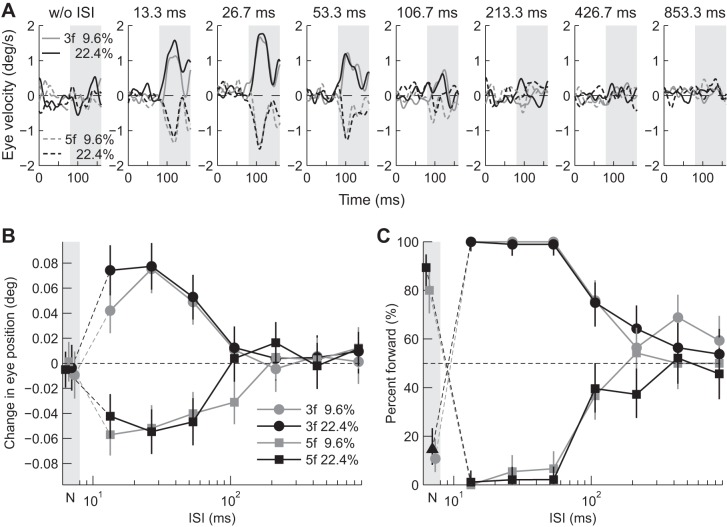
Visual stimuli and ocular responses. A: schematic diagram of visual stimuli. The grating pattern and a central fixation target appeared at the beginning of each trial. After the subject fixated on the central target, the pattern was replaced by a uniform gray image [interstimulus interval (ISI)]. The fixation target then disappeared, and the 2nd frame was presented for 267 ms. The 3f5f stimulus with a contrast ratio of 5/5 is shown. B: R-L eye velocity profiles of ocular responses to 3f5f stimuli with contrast ratios (C3f/C5f) of 7/3 (solid black), 5/5 (solid gray), and 3/7 (broken black) from subject S1. Upward deflections denote eye movements in the direction of stimulus motion. The gray-hatched area indicates the open-loop response interval during which the change in eye position was measured. The corresponding ISI is indicated on the top of each panel.
Data collection and analyses.
All aspects of the experimental paradigm were controlled by two computers, as in our previous studies (Miura et al. 2006, 2009). One computer ran the Real-time EXperimentation software package (REX) (Hays et al. 1982) for overall experimental protocol control, acquisition, display, and data storage. The other computer ran MATLAB subroutines utilizing Psychophysics Toolbox extensions (Brainard 1997) and generated the visual stimuli upon receiving a start signal from the REX machine.
Eye movements were measured by a limbus eye tracker (YKK 2930a, Takei, Osaka). Voltage signals encoding the horizontal and vertical components of eye position were passed through an analog low-pass filter (−3 dB at 100 Hz) and digitized to a resolution of 12 bits, sampling at 1 kHz. All data were stored and transferred to another computer for analysis with computer programs based on MATLAB (The MathWorks). Eye-position data were smoothed with a four-pole digital Butterworth filter (−3 dB at 25 Hz), and eye velocity traces were derived from the two-point backward difference. Eye acceleration profiles were derived from the two-point backward difference of the eye velocity traces and used to detect saccades. Trials with saccadic intrusions during the eye movement response time window were discarded.
Changes in horizontal eye position were measured over 80 ms periods beginning 80 ms after stimulus motion onset. Because the minimum latency for eye movement responses was ∼80 ms from motion onset, response measurements were restricted to the initial open-loop period. To improve the signal-to-noise ratio, the difference between the mean response to right- and leftward motion of each stimulus was calculated (R-L response). Based on convention (rightward deflection was positive), ocular responses in the direction of phase shift (referred to as forward) were positive, while those in the opposite direction (backward) were negative. The same procedure was applied to the mean eye velocity profiles (referred to as R-L velocity profiles).
To examine whether trial-by-trial variability was consistent with the perceived direction and direction of eye movements, we carried out receiver operating characteristic (ROC) analyses (Fawcett 2006). The perfcurve function in MATLAB was used to calculate the area under the ROC curve (AUC) and its 95% confidence interval.
RESULTS
Responses to 3f5f stimuli.
Ocular responses elicited by a 1/4-wavelength step of the 3f5f stimuli without an ISI were small (Fig. 1B, w/o ISI, leftmost panel). However, when shorter ISIs were introduced, eye movement responses were observed (Fig. 1B, 13.3, 26.7, and 53.3 ms). When 3f was the major Fourier component (solid black lines), eyes moved in the direction of the pattern phase shift (i.e., forward). When 5f was the major component (broken black lines), or when the two components had equal contrast (solid gray lines), eyes moved in the direction opposite the pattern (i.e., backward). Average eye movement responses were small, or almost zero, with ISIs ≥106.7 ms. The R-L response confirmed this and showed these characteristics were consistent for all subjects (Fig. 2, A–C for representative three subjects, Fig. 3A for the average of five subjects).
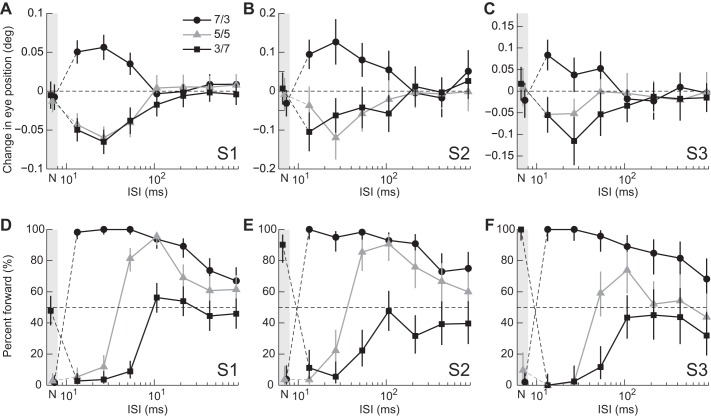
Fig. 2.
Dependence of eye movement and perceptual responses on ISI for individual subjects. Responses to 3f5f stimuli with contrast ratios (C3f/C5f) of 7/3, 5/5, and 3/7 are indicated as black circles, gray triangles, and black squares, respectively. Responses when the ISI was zero (Null) are plotted in the gray-hatched region, the leftmost part of individual panels (labeled “N”). Error bars are 95% confidence intervals. Note the logarithmic abscissa.
Fig. 3.
Dependence of eye movement and perceptual responses on ISI averaged over individuals (n = 5). Mean eye movement responses with standard deviation and mean perceptual responses are shown in A and B, respectively. Symbols are as in Fig. 2. Responses when the ISI was zero (Null) are plotted on the y-axis.
The perceived direction depended on the composition of the 3f5f stimuli (Fig. 2, D–F). When motion was applied without an ISI, subjects reported backward motion when 3f was the major component (black circles). Two (S2 and S3) of three subjects reported forward motion when 5f was the major component (black squares). Shorter ISIs (13.3, 26.7, and 53.3 ms) altered the perceived direction. When 3f was the major component, subjects reported forward motion, whereas backward motion was reported when 5f was the major component. Directional biases attenuated very gradually with longer ISIs when 3f was the major component, however, rapidly when 5f was the major component (Fig. 2, D–F for representative three subjects, Fig. 3B for the average over five subjects).
When 3f and 5f had equal contrast, the perceived direction was backward with an ISI of zero or ≤26.7 ms, and forward with an ISI > 53.3 ms (Fig. 2, D–F, and Fig. 3B, gray triangles). Direction reversal with longer ISIs was not observed in eye movement responses (Fig. 2, A–C and Fig. 3A, gray triangles, see below for more details).
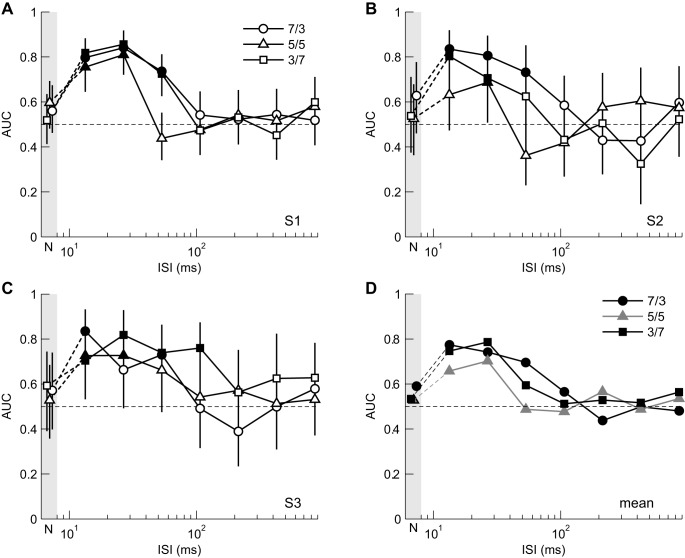
ROC analyses were carried out to examine how well ocular responses predicted the perceived direction (leftward or rightward) on a trial-by-trial basis. The AUC depended on the ISI and contrast ratio of the 3f5f stimuli (Fig. 4). For contrast ratios (C3f/C5f) of 7/3 or 3/7 (circles and squares), AUCs were generally >0.5 with ISIs of 13.3, 26.7, or 53.3 ms, indicating that eye movement was a significant predictor of perceived motion. In contrast, AUCs did not differ significantly from 0.5 with an ISI of zero or ≥106.7 ms, even when the perceived direction was significantly biased. When the contrast ratio was 5/5 (triangles), AUCs differed significantly from 0.5 only with an ISI of 13.3 or 26.7 ms.
Fig. 4.
Predictability of perceived direction from eye movement responses. Areas under the receiver operating characteristic (ROC) curves are plotted for individual subjects (A–C) and averaged over the 5 subjects (D). Data for zero (null) ISI are plotted in the gray areas (labeled “N”). Closed symbols in A–C indicate that areas under the curves (AUCs) are significantly different from 0.5.
Responses to single grating stimuli.
The direction of ocular responses and perceived motion depended strongly on the major Fourier component of the 3f5f stimuli. In experiment 2, we examined the characteristics of responses to the single sinusoidal gratings used for the 3f5f stimuli with a contrast ratio of 7/3 or 3/7. The 3f stimulus was shifted by 3/4 wavelengths (equivalent to −1/4 the wavelength), whereas the 5f stimulus was shifted by 5/4 wavelengths (equivalent to 1/4 the wavelength). Thus, the motion was equivalent to the dual-grating stimuli.
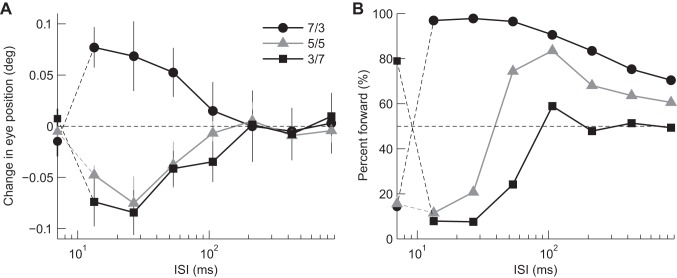
When a shorter ISI (13.3, 26.7, or 53.3 ms; Fig. 5A) was introduced, vigorous eye movement responses were observed. The 3f stimulus elicited eye movements in the forward direction, whereas 5f stimulus elicited in the backward direction. Eye movement responses were not observed with zero or long ISIs, as seen in Fig. 5A. The dependence on ISI is shown in Fig. 5B. The perceived direction showed dependence on ISI similar to eye movement responses, except for the zero ISI.
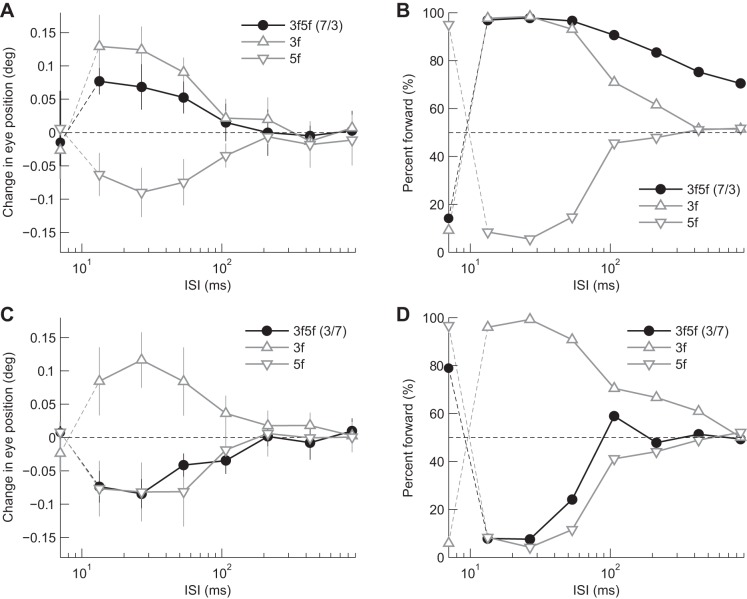
Fig. 5.
Dependence on ISI: responses to single grating stimuli in subject 1. A: R-L eye velocity profiles of ocular responses to component gratings consisting of 3f5f stimuli with contrast ratios (3f:5f) of 7/3 (3f, solid black lines; 5f, broken gray lines) and 3/7 (3f, solid gray lines; 5f, broken black lines). Upward deflections of the traces from zero denote eye movements in the direction of the stimulus motion. Note that 3f stimuli were moved 3/4 of their wavelength (equivalent to a −1/4 wavelength shift). The other conventions are as in Fig. 1. Dependence of eye movement (B) and perceptual responses (C) on ISI. See keys for the component gratings. The conventions are as in Fig. 2.
Comparing the responses to 3f5f and component alone stimuli, we found that the directions of the ocular responses to composite stimuli were consistent with those of the corresponding major Fourier components, although the response amplitudes of 3f5f stimuli were generally smaller compared with the major gratings stimuli alone (Fig. 6, A and C). The perceived directions of 3f5f stimuli were also consistent with those of the corresponding major Fourier component (Fig. 6, B and D). However, the perceived direction for a contrast ratio of 7/3 tended to be biased in the forward direction with longer ISIs (≥106.7 ms).
Fig. 6.
Dependence on ISI: comparisons between single- (△) and dual- (●) grating stimuli. A and B: responses to a 3f5f stimuli with contrast ratio of 7:3. C and D: responses to a 3f5f stimuli with contrast ratio of 3:7. Data are averaged over 5 individuals (A and C: eye movement response with standard deviation; B and D: perceptual responses). Responses when the ISI was zero (null) are plotted on the y-axis.
Responses to 3f5f stimuli in which the two components had equal contrast.
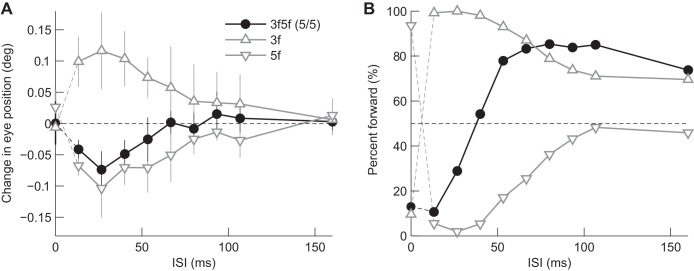
When the 3f and 5f components of the 3f5f stimuli had equal contrast, perceptual responses showed a unique dependence on ISI. To understand the mechanisms underlying this phenomenon, we compared the responses to each component alone, similar to the previous section, but with a finer temporal scale.
Consistent with experiment 1, clear backward eye movement responses (Fig. 7A, black circles) were observed with ISIs ≤40.0 ms, although their amplitudes were slightly smaller than for the 5f stimulus (lower triangles). Responses decreased with longer ISIs and almost disappeared by 66.7 ms. Thus, ocular responses might be dominated by the 5f component with short ISIs.
Fig. 7.
Dependence on ISI: comparison between single-grating (△) and 3f5f stimuli with components of equal contrast (●). Data are averaged over 5 individuals. (A: eye movement response with SD; B: perceptual responses). Note the linear abscissa.
The perceived direction showed different ISI dependence compared with simultaneously recorded eye movement responses. Subjects perceived backward motion when the 3f5f stimulus was presented without an ISI (Fig. 7B, black circle). This response coincided with responses to the 3f stimulus (upper triangles). At the minimum ISI (13.3 ms), the perceived motion to 3f5f stimulus was backward, which, in contrast to no ISI, coincided with the response to the 5f stimulus (lower triangles). The perceived direction for the 3f5f stimulus rapidly alternated as the ISI became longer, but then reached a value similar to the response to the 3f stimulus.
DISCUSSION
In this study, we simultaneously recorded ocular and perceptual responses to visual motion. This arrangement allowed direct comparison of these two responses. We will discuss visual motion analyses underlying these responses and their similarities/differences below.
Comparisons with previous studies of ocular responses.
In humans and nonhuman primates, sudden motion in a visual scene elicits ocular following responses with latencies of <80 ms and <60 ms, respectively (Gellman et al. 1990; Miles et al. 1986; Miura et al. 2006; Sheliga et al. 2005). Extensive studies are underway to understand visual motion analyses underlying eye movement responses (Aoki et al. 2012; Benson and Guo 1999; Hayashi et al. 2008; Masson and Castet 2002; Miura et al. 2006; Quaia et al. 2012; Sheliga et al. 2005, 2012, 2013). Visual motion mechanisms were the central focus of previous studies of ocular following responses. In these studies, the contributions of low-level energy-based or higher-order mechanisms involving second-order motion and feature-tracking mechanisms were examined. Note that second-order and feature-tracking mechanisms are not isolated easily, hence we considered them higher-level mechanisms. There is significant evidence that the energy-based mechanism underlies ocular following responses (Hayashi et al. 2008; Miura et al. 2006; Sheliga et al. 2005), whereas second, or higher, order mechanisms might also be related to these responses (Benson and Guo 1999).
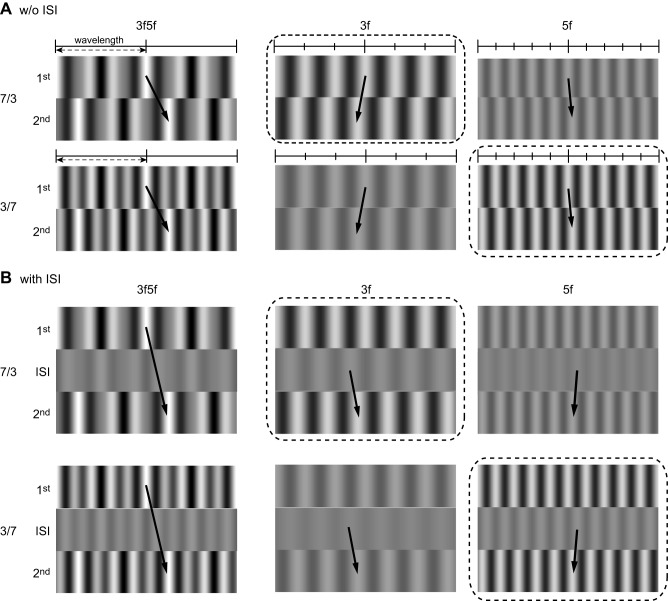
Sheliga et al. (2006a) performed ISI experiments using a missing fundamental stimulus (Adelson 1982; Adelson and Bergen 1985). When this stimulus was presented with ISIs > 10 ms, ocular responses were elicited in the direction of the pattern phase shift (Sheliga et al. 2006a). They argued that the energy-based motion detectors receive a visual input whose temporal impulse response is biphasic and that this mechanism explains the response to the missing fundamental stimulus presented with the ISI. However, responses in the direction of pattern shift could also result from the feature-tracking mechanism, as argued by Georgeson and Harris (1990). In the present study, we used 3f5f stimuli to resolve this issue. Figure 8 shows schematic diagrams describing the assumed effects of ISIs on 3f5f stimuli based on the energy-based mechanism involving temporal filters with biphasic impulse responses. When ISIs are introduced (Fig. 8B), the contrast of the original image is inverted, as argued by Shioiri and Cavanagh (1990), and this inverted pattern involves 3f and 5f components with contrast reversed. The motion between these inverted patterns and the corresponding components of the second frames (Fig. 8B) have directions opposite to those under no ISI (Fig. 8A). Given that the major Fourier component dominates the output of the energy-based mechanism, the directions of responses depend on which component dominates. When the 3f component is predominant, the response will be in the direction of the pattern phase shift (Fig. 8B, top), whereas when the 5f is predominant, the direction of the response will be opposite the phase shift (Fig. 8B, bottom). A clear difference is seen when 5f predominates: backward for the energy-based mechanism and forward for the feature-based mechanism.
Fig. 8.
Schematic diagrams of the effect of ISIs on the 3f5f stimuli processed by the energy-based mechanism. A: X-T images of the 1/4 wavelength rightward step of the 3f5f stimuli and motion of the component gratings. Images with a 2-wavelength width are shown. Top and bottom: the 3f5f stimuli with contrast ratios of 7:3 and 3:7 (3f:5f), respectively. The major Fourier component having a larger contrast is emphasized by dotted squares. Directions defined by the nearest neighbor matching are indicated by the arrows. B: X-T images of the 1/4 wavelength step of the 3f5f stimuli when an ISI is introduced between the 1st and 2nd frames. The inverted version of the 1st frame is created by temporal filters with biphasic impulse responses during an ISI. This inverted pattern is then decomposed into contrast-inverted versions of the components. The motion defined by the nearest neighbor matching between the contrast-inverted components during ISIs and the components of the second frame had opposite directions to the ones without an ISI (see short arrows in the 3f and 5f components).
Using the two-frame apparent motion of the 3f5f stimuli, we obtained three critical findings. First, when 5f was the major component, ocular responses occurred in the direction opposite to the phase shift with ISIs, which is evidence against the feature-tracking mechanism. Second, the direction of eye movement responses to 3f5f stimuli strongly depended on the major Fourier components, particularly when ISI was shorter, which is consistent with the energy-based mechanism with competitive interactions between components. Third, eye movement directions in the presence of ISI were opposite to the motion of the major component, consistent with energy-based motion detectors involving temporal filters with biphasic impulse responses. Thus, this study produced findings that reveal the mechanisms of visual motion analyses underlying ocular responses.
Comparison with previous studies of perceived motion.
The effects of ISI on perceived motion have been attributed to a biphasic impulse response function of the visual system, or to a transition from the energy-based to feature-tracking mechanisms (Boulton and Baker 1993; Georgeson and Harris 1990; Hammett et al. 1993; Shioiri and Cavanagh 1990; Strout et al. 1994; Takeuchi and De Valois 1997). The present findings add novel evidence for visual motion analysis underlying perceived motion, particularly for large-field visual stimuli.
This study provides evidence for the superiority of the energy-based mechanism over the feature-tracking mechanism when the ISI was zero or <100 ms. Without an ISI, the perceived direction of 3f5f stimuli tended to be in the direction of the major Fourier component. When 3f was predominant, the perceived direction was opposite to the actual image shift, suggesting the energy-based mechanism dominates perceptual responses when there is no ISI (Fig. 8A). The perceived direction usually reversed by introduction of ISIs. As in the argument for ocular responses, when 5f was predominant, the perceived direction was opposite to the actual pattern shift when the ISI was ≤53.3 ms, providing evidence against the dominance of the feature-tracking mechanism in this ISI range (Fig. 8B).
Takeuchi and De Valois (1997) showed backward motion perception with a shorter ISI (<100 ms) and forward motion perception with a longer ISI using single sinusoidal grating stimuli. Based on these results, it was argued that the energy-based mechanism with biphasic temporal impulse response function mediates perceived motion with shorter ISIs, whereas the feature-based mechanism mediates forward motion perception with longer ISIs. Our observations using sinusoidal gratings (experiment 2) are not completely consistent with these findings since forward motion perception was not observed with longer ISIs [note: 3f stimuli stepped 3/4 (−1/4) the wavelength]. This discrepancy might be due to a difference in the visual fields stimulated. Takeuchi and De Valois (1997) stimulated only the central part of the retina, whereas we stimulated a large visual field. In fact, the feature-based mechanism is known to operate only for the central visual field (Takeuchi and De Valois 2009). Thus, our results suggest large-field stimulation weakens the impact of the feature-tracking mechanism.
Although our data for perceptual responses to single sinusoids showed no evidence for the feature-tracking mechanism, those to dual-grating motion stimuli provided such evidence when the stimuli were presented with ISIs ≥100 ms. The perceived direction to the 3f5f stimulus with a contrast ratio of 7/3 tended to be biased more to forward compared with those predicted from the 3f stimulus. These results might be explained by differences in temporal properties of the different motion mechanisms. The low-level energy-based mechanism might have a faster temporal property compared with the feature-based mechanism.
A difference in fundamental frequencies, or different combinations of the two components (such as 5f+7f stimuli), might have an influence on the relative contribution of the energy-based and feature-tracking mechanisms. Changing the relative contrasts of the 3f and 5f components also influenced pattern appearance. These differences in the pattern appearance also might influence the contribution of the feature-tracking mechanism. These questions remain unanswered and should be addressed in future studies.
Comparison between perceived motion and ocular responses.
The principal difference between ocular responses and perceived motion in this study might be the involvement of the feature-tracking mechanism in perceived motion. Ocular responses were robustly observed when ISIs were ≤53.3 ms. Within this ISI range, eye movement is a significant predictor of the perceived direction of visual motion stimuli (Fig. 4). This suggests that similar mechanisms mediate motor and perceptual responses. With longer ISIs, ocular responses were fairly small and the trial-by-trial variance of eye movement measurements were no longer related to the perceived direction (Fig. 4), suggesting an additional mechanism underlies perceptual responses. As argued previously, the perceived direction tended to be biased toward forward movement with longer ISIs, suggesting a contribution of the feature-tracking mechanism to the perceived motion. Below we discuss two more differences that provide insight into these mechanisms.
When the two-frame animations were presented without ISI, clear biases were observed in the perceived direction, whereas ocular responses were fairly small. These findings suggest a difference in temporal impulse response functions that underlie motor and perceptual responses. A biphasic impulse response function is formed by a positive phase followed by a negative phase, and the difference between areas of the positive and negative phases determines the amplitude of the sustained state of the step response. Therefore, a likely explanation is that the temporal impulse response function mediating perceived motion may have a smaller negative phase and may be closer to monophasic compared with that mediating ocular responses. In the frequency domain, this characteristic is seen as a difference in temporal frequency tuning. In fact, Gomi et al. (2006) demonstrated that the optimal temporal frequency for visual motion perception was lower (4.4 Hz) than that of ocular responses (17.4 Hz).
A unique dependence on the ISI was found in perceived motion when the 3f and 5f components had equal contrast. When the ISI was zero or <53.3 ms, the perceived direction was backward. However, this changed to forward when the ISI became longer. Interestingly, a similar dependence on ISI was demonstrated in the perceived direction to the missing fundamental stimulus (Georgeson and Harris 1990). This dependence clearly differed from those when there was a major grating component (the 3f5f stimuli), which showed a clear reversal of perceived direction by introducing the minimum ISI (13.3 ms). Given that the energy-based mechanism operates at zero or short ISIs, a likely explanation is an alternation of the predominant motion components with or without an ISI (see Fig. 7). The 3f component might have more impact compared with 5f when there was no ISI, but the 5f component might be superior at shorter ISIs. Furthermore, the 3f component might become stronger again with longer ISIs. This ISI dependence might be due to differences in temporal properties for different spatial frequencies in motion perception. It is also possible that the feature-tracking mechanism might replace the energy-based mechanism with longer ISIs. Such dependence was not observed in ocular responses, which were backward when the ISI was ≤53.3 ms and decreased in amplitude with longer ISIs, suggesting 5f had more influence. This difference in ISI effects on motor and perceptual responses might be due to differences in competitive interactions between motion components and/or different contributions of the feature-tracking mechanism.
The present findings strongly suggest different neural systems underlie visual motion analyses for ocular and perceptual responses. There is evidence showing that short-latency ocular responses to large-field motion are primarily mediated by the middle temporal (MT) and medial superior temporal (MST) areas in monkeys (Kawano et al. 1994; Miura et al. 2014; Takemura et al. 2007). These areas are also related to perceived motion in monkeys (Newsome et al. 1990). In the human MT, separate neural populations are responsible for detecting first- and second-order motion (Ashida et al. 2007). Ocular responses might be dominated by the neural population responsible for first-order motion, whereas perceptual responses might be mediated by both neural populations. However, there are no neurophysiological findings showing differences in temporal dynamics and competitive interactions between motion components. Future studies should explore the neural mechanisms underlying these differences.
GRANTS
This work was supported by the Global COE Program “Center for Frontier Medicine,” MEXT, Japan and JSPS KAKENHI Grants 23500467 and 21240037.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.N. and K.M. performed experiments; S.N. and K.M. analyzed data; S.N., K.K., and K.M. interpreted results of experiments; S.N. and K.M. prepared figures; S.N. and K.M. drafted manuscript; S.N., K.K., and K.M. approved final version of manuscript; K.K. and K.M. conception and design of research; K.K. and K.M. edited and revised manuscript.
REFERENCES
- Adelson EH. Some new motion illusions, and some old ones, analysed in terms of their Fourier components (Abstract). Invest Ophthalmol Vis Sci 34, Suppl: 144, 1982. [Google Scholar]
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2: 284–299, 1985. [DOI] [PubMed] [Google Scholar]
- Anstis SM. The perception of apparent movement. Philos Trans R Soc Lond B Biol Sci 290: 153–168, 1980. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Kawano K, Miura K. Facilitative integration of local motion signals in the peripheral visual field observed in monkey ocular following responses. Neurosci Res 74: 48–58, 2012. [DOI] [PubMed] [Google Scholar]
- Ashida H, Lingnau A, Wall MB, Smith AT. FMRI adaptation reveals separate mechanisms for first-order and second-order motion. J Neurophysiol 97: 1319–1325, 2007. [DOI] [PubMed] [Google Scholar]
- Benson PJ, Guo K. Stages in motion processing revealed by the ocular following response. Neuroreport 10: 3803–3807, 1999. [DOI] [PubMed] [Google Scholar]
- Boulton JC, Baker CL Jr. Dependence on stimulus onset asynchrony in apparent motion: evidence for two mechanisms. Vision Res 33: 2013–2019, 1993. [DOI] [PubMed] [Google Scholar]
- Braddick OJ. Low-level and high-level processes in apparent motion. Philos Trans R Soc Lond B Biol Sci 290: 137–151, 1980. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Patt Recogn Lett 27: 861–874, 2006. [Google Scholar]
- Gellman RS, Carl JR, Miles FA. Short latency ocular-following responses in man. Vis Neurosci 5: 107–122, 1990. [DOI] [PubMed] [Google Scholar]
- Georgeson MA, Harris MG. The temporal range of motion sensing and motion perception. Vision Res 30: 615–619, 1990. [DOI] [PubMed] [Google Scholar]
- Gomi H, Abekawa N, Nishida S. Spatiotemporal tuning of rapid interactions between visual-motion analysis and reaching movement. J Neurosci 26: 5301–5308, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett ST, Ledgeway T, Smith AT. Transparent motion from feature- and luminance-based processes. Vision Res 33: 1119–1122, 1993. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Miura K, Tabata H, Kawano K. Eye movements in response to dichoptic motion: evidence for a parallel-hierarchical structure of visual motion processing in primates. J Neurophysiol 99: 2329–2346, 2008. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc 2: 1–10, 1982. [Google Scholar]
- Kawano K, Shidara M, Watanabe Y, Yamane S. Neural activity in cortical area MST of alert monkey during ocular following responses. J Neurophysiol 71: 2305–2324, 1994. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: review and update. J Opt Soc Am A Opt Image Sci Vis 18: 2331–2370, 2001. [DOI] [PubMed] [Google Scholar]
- Masson GS, Castet E. Parallel motion processing for the initiation of short-latency ocular following in humans. J Neurosci 22: 5149–5163, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Miura K, Taki M, Tabata H, Inaba N, Kawano K, Miles FA. Ocular following responses of monkeys to the competing motions of two sinusoidal gratings. Neurosci Res 61: 56–69, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol 56: 1321–1354, 1986. [DOI] [PubMed] [Google Scholar]
- Miura K, Inaba N, Aoki Y, Kawano K. Difference in visual motion representation between cortical areas MT and MST during ocular following responses. J Neurosci 34: 2160–2168, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kobayashi Y, Kawano K. Ocular responses to brief motion of textured backgrounds during smooth pursuit in humans. J Neurophysiol 102: 1736–1747, 2009. [DOI] [PubMed] [Google Scholar]
- Miura K, Matsuura K, Taki M, Tabata H, Inaba N, Kawano K, Miles FA. The visual motion detectors underlying ocular following responses in monkeys. Vision Res 46: 869–878, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Salzman CD, Movshon JA. Neuronal mechanisms of motion perception. Cold Spring Harb Symp Quant Biol 55: 697–705, 1990. [DOI] [PubMed] [Google Scholar]
- Nishida S. Advancement of motion psychophysics: review 2001–2010. J Vis 11: 11, 2011. [DOI] [PubMed] [Google Scholar]
- Pantle A, Turano K. Visual resolution of motion ambiguity with periodic luminance- and contrast-domain stimuli. Vision Res 32: 2093–2106, 1992. [DOI] [PubMed] [Google Scholar]
- Quaia C, Sheliga BM, Fitzgibbon EJ, Optican LM. Ocular following in humans: spatial properties. J Vis 12: 13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, FitzGibbon EJ, Miles FA. Initial ocular following in humans: a response to first-order motion energy. Vision Res 45: 3307–3321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, FitzGibbon EJ, Miles FA. The initial ocular following responses elicited by apparent-motion stimuli: reversal by inter-stimulus intervals. Vision Res 46: 979–992, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Kodaka Y, FitzGibbon EJ, Miles FA. Human ocular following initiated by competing image motions: evidence for a winner-take-all mechanism. Vision Res 46: 2041–2060, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Quaia C, Cumming BG, Fitzgibbon EJ. Spatial summation properties of the human ocular following response (OFR): dependence upon the spatial frequency of the stimulus. Vision Res 68: 1–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Quaia C, FitzGibbon EJ, Cumming BG. Retinal visual processing constrains human ocular following response. Vision Res 93: 29–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioiri S, Cavanagh P. ISI produces reverse apparent motion. Vision Res 30: 757–768, 1990. [DOI] [PubMed] [Google Scholar]
- Strout JJ, Pantle A, Mills SL. An energy model of interframe interval effects in single-step apparent motion. Vision Res 34: 3223–3240, 1994. [DOI] [PubMed] [Google Scholar]
- Takemura A, Murata Y, Kawano K, Miles FA. Deficits in short-latency tracking eye movements after chemical lesions in monkey cortical areas MT and MST. J Neurosci 27: 529–541, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, De Valois KK. Motion-reversal reveals two motion mechanisms functioning in scotopic vision. Vision Res 37: 745–755, 1997. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, De Valois KK. Visual motion mechanisms under low retinal illuminance revealed by motion reversal. Vision Res 49: 801–809, 2009. [DOI] [PubMed] [Google Scholar]