Abstract
There is accumulating evidence that peripheral motor axons deteriorate following spinal cord injury (SCI). Secondary axonal dysfunction can exacerbate muscle atrophy, contribute to peripheral neuropathies and neuropathic pain, and lead to further functional impairment. In an attempt to ameliorate the adverse downstream effects that developed following SCI, we investigated the effects of a short-term peripheral nerve stimulation (PNS) program on motor axonal excitability in 22 SCI patients. Axonal excitability studies were undertaken in the median and common peroneal nerves (CPN) bilaterally before and after a 6-wk unilateral PNS program. PNS was delivered percutaneously over the median nerve at the wrist and CPN around the fibular head, and the compound muscle action potential (CMAP) from the abductor pollicis brevis and tibialis anterior was recorded. Stimulus intensity was above motor threshold, and pulses (450 μs) were delivered at 100 Hz with a 2-s on/off cycle for 30 min 5 days/wk. SCI patients had consistently high thresholds with a reduced CMAP consistent with axonal loss; in some patients the peripheral nerves were completely inexcitable. Nerve excitability studies revealed profound changes in membrane potential, with a “fanned-in” appearance in threshold electrotonus, consistent with membrane depolarization, and significantly reduced superexcitability during the recovery cycle. These membrane dysfunctions were ameliorated after 6 wk of PNS, which produced a significant hyperpolarizing effect. The contralateral, nonstimulated nerves remained depolarized. Short-term PNS reversed axonal dysfunction following SCI, may provide an opportunity to prevent chronic changes in axonal and muscular function, and may improve rehabilitation outcomes.
Keywords: nerve excitability, rehabilitation, peripheral nerve stimulation, spinal cord injury
there is increasing evidence to suggest that, following spinal cord injury (SCI), function of peripheral motor axons caudal to the lesion is compromised. This is reflected in reduced compound muscle action potential (CMAP) amplitudes (Kirshblum et al. 2001; Lin et al. 2007; Nogajski et al. 2006; Rutz et al. 2000; Van De Meent et al. 2010), slowing of conduction velocity (Nogajski et al. 2006), increased excitability thresholds (Lin et al. 2007), and altered H-reflexes (Hiersemenzel et al. 2000; Leis et al. 1996; Nakazawa et al. 2006; Schindler-Ivens and Shields 2000) after SCI. More recently, studies using novel threshold tracking nerve excitability techniques have further identified complex changes in biophysical properties of peripheral motor axons in patients with acute (Boland et al. 2009; Boland et al. 2011), subacute, and chronic (Lin et al. 2007) SCI. Nerve excitability is a noninvasive electrophysiological technique that provides information regarding the activity of various ion channels, energy-dependent pumps, and ion exchange processes activated during impulse conduction in peripheral axons (Burke et al. 2001; Kiernan et al. 2000; Krishnan et al. 2009). This technique has been used extensively to study the biophysical properties of human peripheral nerves in vivo and have provided important mechanistic insight into axonal ion channel dysfunction in a wide range of neurological disorders, including toxic, metabolic, acquired, and inherited demyelinating neuropathies (Burke et al. 2001; Krishnan et al. 2008), amyotrophic lateral sclerosis (Vucic and Kiernan 2006), and SCI (Boland et al. 2011; Lin et al. 2007). More specifically, axonal depolarization was consistently observed in both upper and lower limb nerves following SCI (Boland et al. 2011; Lin et al. 2007). Furthermore, abnormal axonal excitability could be detected as early as 6 days postspinal injury (Boland et al. 2009), and the period of acute excitability changes coincided with the development of hyperreflexia during the later stage of spinal shock (Boland et al. 2011). The results from the aforementioned studies suggest that SCI has a profound downstream effect on the somatic motor nervous system below the neurological level of injury. The pathophysiology underlying SCI-induced axonal dysfunction is likely to be multifactorial and must involve complex interactions between decentralization, ischemia (acute and chronic), subsequent inactivity, and disuse atrophy. Irrespective of the precise mechanism, secondary peripheral nerve dysfunction will affect muscle strength and contribute to the development of peripheral neuropathies (Burke et al. 2001; Krishnan et al. 2008), thus leading to further loss of function and independence. Furthermore, peripheral nerve dysfunction may limit spontaneous recovery, particularly in patients with incomplete SCI (Van De Meent et al. 2010), and prevent motor axons from responding appropriately to rehabilitation and future regenerative therapies (Van De Meent et al. 2010). As such, a directed clinical investigation of potential therapies that could reverse secondary peripheral nerve dysfunction following SCI has important clinical implications. Maintenance of peripheral motor axonal function during the acute and subacute phases of SCI may lead to better functional and rehabilitation outcome later on. In an attempt to ameliorate the adverse downstream effects following SCI, the present study investigated whether altered axonal excitability could be reversed by an intensive, short-term therapeutic peripheral nerve stimulation program.
MATERIALS AND METHODS
Patient eligibility criteria.
Common peroneal nerve (CPN) and median nerve (MN) excitability studies were undertaken in 22 patients with first-time traumatic SCI (17 males; 5 females; age range 19–83 yr; mean age 45 ± 20.2 yr), all within 6 mo since injury, and the results were compared with 32 healthy subjects (12 peroneal nerves and 20 MN). The level of functional impairment in SCI patients was categorized using the American Spinal Injury Association (ASIA) scale (Marino et al. 2003). None of the patients suffered from pressure sores, hypotension, or vascular disorders at the time of experiment nor had history of peripheral neuropathy or other coexisting disease processes (such as diabetes and renal disorders) that would affect peripheral nerve function. All patients were recruited from the Spinal Medicine Department at The Prince of Wales Hospital in Randwick Sydney and gave informed consent to the experimental procedures, which were approved by the Human Research Ethics Committees of the South Eastern Sydney Local Health District (Northern Sector) and the University of New South Wales. The research procedures conformed to the Declaration of Helsinki. A subset of SCI patients were recruited to participate in a 6-wk unilateral peripheral nerve stimulation program targeting the MN in the upper limb and/or the CPN in the lower limb.
Axonal excitability studies.
Peripheral nerve excitability was assessed using QTRAC (Hugh Bostock, Institute of Neurology, London, UK) and follows a previously established protocol designed to measure a number of different excitability indexes (Burke et al. 2001; Kiernan et al. 2000). Recordings were made from the MN and the CPN bilaterally before and after a 6-wk unilateral peripheral nerve stimulation program (see below). For the upper limb nerve excitability studies, the MN was stimulated at the wrist and CMAPs were recorded from the abductor pollicis brevis (APB) muscle using standard surface electromyography (EMG) techniques, with active electrode on the motor point and the reference electrode over the interphalangeal joint. In the lower limb, the CPN was stimulated with the active electrode over the nerve around the fibular head and the reference electrode over the patella. CMAPs were recorded from the tibialis anterior (TA) muscle using surface EMG with the active electrode over the motor point and the reference electrode over the distal tibia. The current required to produce a CMAP that was of 40% of the maximal motor response was tracked (threshold tracking). Responses were amplified (ICP511 AC amplifier; Grass Technologies), and electronic noise was removed using Hum Bug (50/60 Hz noise eliminator; Quest, Scientific Instruments, North Vancouver, Canada). Skin temperature at the stimulation site was monitored with a thermistor thermometer (5831-A; Omega Engineering, Manchester, UK) and kept above 33°C throughout the experiment. The following excitability indexes were assessed, and the sequence of recording followed that previously described (Kiernan et al. 2000; Krishnan et al. 2004): 1) stimulus-response relationship using a stimulus duration of 1.0 ms, with the ratio between the stimulus response curve and four stimulus durations (0.2, 0.4, 0.8, and 1.0 ms) being used to calculate rheobase and strength duration time constant (SDTC), a measure of nodal persistent Na+ conductances; 2) threshold electrotonus using prolonged (100-ms) polarizing currents, a marker of intermodal axonal membrane function; 3) current-threshold relationship was assessed using polarizing currents of 200-ms duration, in incremental steps from +50 to 100% of threshold, providing information regarding the rectifying properties of nodal and internodal axolemma; and 4) recovery cycle was assessed using a paired-pulse paradigm, with threshold changes in response to a 1-ms (supramaximal) test stimulus as the conditioning test interval was increased from 2 to 200 ms was tracked. The latter data were then extrapolated to determine refractoriness [because of inactivation of nodal transient Na+ channels, and measured as the threshold change at an interstimulus interval (ISI) of 2.5 ms], superexcitability (measured as the minimum mean threshold change of three adjacent points), and late subexcitability (measured as the minimum mean threshold change after ISI of 10 ms). Superexcitability and late subexcitability are dependent on juxtaparanodal fast K+ channels and nodal slow K+ channels, respectively.
Short-term peripheral nerve stimulation therapy.
A portable neuromuscular electrical stimulation unit (NeuroTrac) was used to deliver peripheral nerve stimulation therapy five times per week for 6 wk. All therapy sessions were supervised. One limb was randomly assigned to receive peripheral nerve stimulation therapy, whereas the opposite limb acted as an internal control. Stimulus intensity was set at above motor threshold. Pulses (450 μs) were delivered at 100 Hz with a 2-s on/off cycle for 30 min. This parameter was chosen because it produces smooth tetanic contractions of the target muscles without the need of using large electrical current (between 12 and 30 mA). This protocol was well tolerated by all patients and had a 100% adherence rate over the 6-wk period.
During electrical stimulation therapy, the patients were encouraged to actively contract the target muscles during the “on” phase and relax completely during the “off” phase. For those who were not able to actively contract the target muscles, they were instructed to imagine moving the target muscles during the on phase and rest during the off phase. In total, each session generated ∼450 electrically assisted contractions. All patients continued with their usual supervised physiotherapeutic rehabilitation.
Data analysis.
A total of 36 different excitability parameters was analyzed from each nerve excitability study. Initial CPN and MN excitability data from SCI patients were compared with a group of healthy age-matched controls using t-tests for independent samples to assess for effects of SCI. Repeated-measures ANOVA with post hoc comparisons were used to assess effects of a 6-wk unilateral peripheral nerve stimulation program between limbs in SCI patients. All data are expressed as means ± SE. Statistical significance was defined as P < 0.05 for all analyses.
RESULTS
SCI patient characteristics.
The demographic and clinical data for the 22 SCI patients initially enrolled in the study are presented in Table 1. As expected, the majority (n = 17; 77%) of the patients were males, with over half of all SCI attributed to falls (55%), followed by motor vehicle accidents (23%) and recreational or sports injuries (18%; horse riding, surfing, rugby injury, etc.). All were within 6 mo since injury (mean days since injury 82 ± 57 days). Thirteen patients had sustained cervical injuries (C3-C7) while eight had thoracic lesions (T1-T11); one patient had suffered a lumbar lesion at L1.
Table 1.
Clinical data of all spinal cord injury patients in whom peripheral nerve excitability testing was attempted
| Nerve Excitability Testing |
||||||||
|---|---|---|---|---|---|---|---|---|
| MN |
CPN |
|||||||
| SCI Patient No. | Age, yr/Sex | Motor Level | ASIA Classification | Time from Injury to Test, days | Left | Right | Left | Right |
| 1 | 70/F | C3 | D | 84 | Y | Inexcitable | *X | *X |
| 2 | 20/M | C4 | A | 172 | Y | Inexcitable | Inexcitable | Inexcitable |
| 3 | 30/M | C4 | A | 47 | Y | Y | Inexcitable | Inexcitable |
| 4 | 84/M | C4 | C | 61 | Y | Y | *X | *X |
| 5 | 59/M | C4 | C | 21 | Y | Y | Y | Y |
| 6 | 46/M | C4 | D | 7 | Y | Y | *X | *X |
| 7 | 57/M | C4 | D | 75 | *X | *X | Y | Y |
| 8 | 19/M | C5 | C | 28 | Y | Y | Y | Y |
| 9 | 56/M | C5 | C | 138 | Y | *X | *X | *X |
| 10 | 65/M | C5 | D | 65 | Y | Y | *X | *X |
| 11 | 32/M | C6 | D | 28 | Y | Y | *X | *X |
| 12 | 26/M | C7 | B | 61 | Y | Y | Y | Y |
| 13 | 62/M | C7 | D | 13 | Y | Inexcitable | Y | Y |
| 14 | 32/M | T1 | A | 3 | Inexcitable | Inexcitable | Y | Y |
| 15 | 57/F | T1 | C | 165 | Y | Y | Y | Y |
| 16 | 25/M | T4 | A | 123 | Y | Y | Inexcitable | Inexcitable |
| 17 | 24/M | T6 | A | 102 | Y | Y | Inexcitable | Inexcitable |
| 18 | 24/M | T9 | A | 121 | Y | Y | Y | Y |
| 19 | 70/M | T11 | A | 180 | Y | Y | Inexcitable | Inexcitable |
| 20 | 37/F | T11 | B | 92 | Y | Y | Inexcitable | Inexcitable |
| 21 | 72/F | T11 | C | 140 | Y | Y | Y | Inexcitable |
| 22 | 26/F | L1 | C | 94 | Y | Y | Inexcitable | Y |
SCI, spinal cord injury; ASIA, American Spinal Injury Association; MN, median nerve; CPN, common peroneal nerve; F, female; M, male;
X, unable to test because of wrist/knee flexion contractures/spasticity or fracture.
Peripheral nerve excitability studies were undertaken in the median and CPN bilaterally to elucidate the downstream effects of SCI on axonal excitability. In some SCI patients, the peripheral nerves were completely inexcitable (32% of CPN tested and 11.4% of MN tested). Six patients had severe lower limb spasticity and were unable to complete the full nerve excitability protocol because of positioning difficulty and intermittent muscle spasms (see Table 1). One patient (no. 10) had a nondisplaced spiral tibial fracture and declined testing because of pain locally. Overall, full peroneal nerve excitability data were successfully obtained from 10 SCI patients (18 legs), and the results were compared with 12 healthy age-matched controls.
Seven SCI patients with injury level at or below T4 demonstrated normal intrinsic hand muscle power, hand function, and median motor axonal excitability (data not shown). As such, their MN excitability data were not included in the final analysis. Of the remaining 15 SCI patients with injury level between C3 and T1, four had inexcitable MN (1 bilaterally and 3 unilaterally) and two had severe wrist and elbow flexion contracture (1 bilaterally and 1 unilaterally), which made it impossible to conduct nerve excitability study (see Table 1). Overall, full median axonal excitability data were collected from 13 SCI patients (22 upper limbs), and the results were compared with a group of age-matched healthy controls (n = 20).
Axonal excitability changes after SCI.
The Wilcoxin Signed Rank Test showed that there was no between-limb difference for any peripheral nerve excitability indexes in SCI patients (range for P = 0.29–0.9), and, as such, the left and right CPN excitability data from all SCI patients were pooled for comparison with age-matched able-bodied controls. Compared with neurologically intact subjects, the common peroneal motor axons in SCI patients exhibited consistently high thresholds, with a significant rightward shift in the stimulus-response curve, illustrated in Fig. 1A. The mean TA peak-to-peak CMAP amplitude was smaller in SCI patients (4.35 ± 1.21 mV) compared with controls (7.72 ± 1.08 mV; P = 0.024), but there was no significant difference for the slope of the stimulus-response curves (P = 0.26) between the two groups. The SDTC (a measure of the rate at which the threshold current declines as the stimulus duration is increased), which reflects the behavior of persistent Na+ conductances, was also similar between groups (SCI 0.46 ± 0.03 ms; controls 0.44 ± 0.02 ms; P = 0.68).
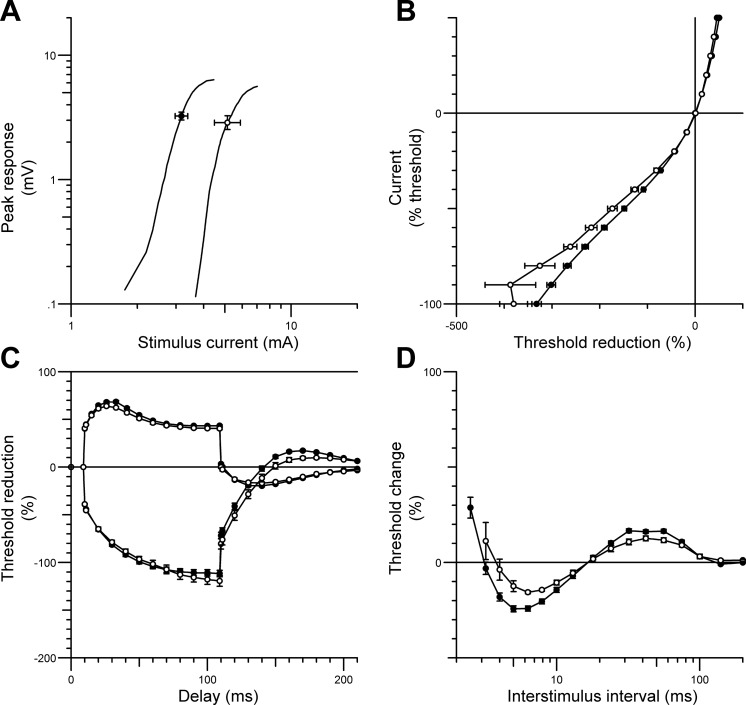
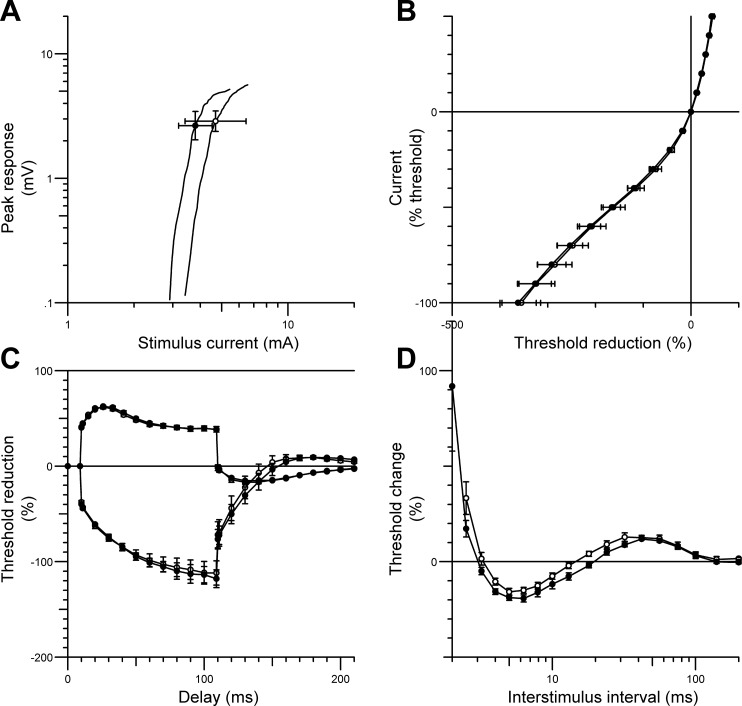
Fig. 1.
Nerve excitability data (mean ± SE) recorded from the tibialis anterior muscle during common peroneal nerve (CPN) stimulation in patients with recent traumatic spinal cord injury (SCI) (○) compared with responses from healthy controls (n = 12; ●). The figure shows the stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), and the recovery cycle (D). SCI patient data were pooled from both lower limbs.
The current-threshold relationship (Fig. 1B) reflects the rectifying properties of the nodal and internodal axolemma, and the slope of the curve provides an estimate of the threshold analog of input conductance. A decrease in threshold is demonstrated by a rightward shift of the curve, and an increase in threshold is indicated by a shift to the left. For SCI patients, the current-threshold curve was shifted to the right (Fig. 1B) in the depolarizing direction (SCI 0.33 ± 0.03; control 0.25 ± 0.01; P = 0.024). Similarly, abnormal responses were also observed throughout threshold electrotonus, during prolonged hyperpolarizing currents at durations of 10–20 ms (P = 0.002), 20–40 ms (P = 0.003), and 90–100 ms (P = 0.002), and during prolonged depolarizing currents at durations of 10–20 ms (P = 0.001), 40–60 ms (P = 0.0003), and 90–100 ms (P = 0.0016); this resulted in a “fanned in” appearance (Fig. 1C) (Kaji 2003). The abnormalities observed in SCI patients during threshold electrotonus were consistent with those observed during the recovery cycle (Fig. 1D). The recovery of excitability following a supramaximal conditioning stimulus was flatter in SCI patients compared with able-bodied controls, with a significant reduction in superexcitability (SCI −7.2 ± 1.86%; controls −19.51 ± 1.53%; P < 0.0001) and late subexcitability (SCI 8.9 ± 1.3%; controls 13 ± 1.62%; P < 0.05). Furthermore, refractoriness was increased in SCI patients (106.3 ± 16.9%; controls 63.04 ± 5.97%; P = 0.03).
Compared with able-bodied controls, the median motor axons in SCI patients with neurological levels between C3 and T1 were of high threshold, evident from a shift of the stimulus-response curve to the right (Fig. 2A). The peak-to-peak CMAP amplitude for the APB muscle was similar between groups (controls 6.48 ± 1.1 mV; SCI 5.8 ± 1.1 mV; P = 0.4), but the threshold required to produce a CMAP of 50% of maximum was increased in SCI patients (5.6 ± 1.2 mA; controls 3.2 ± 1.1 mA; P = 0.0013). There was no significant difference in the slope of the stimulus-response curves (P = 0.64) or the SDTC (P = 0.4) between groups, as also observed for the CPN. APB CMAPs in patients with SCI at or below T4 were preserved, and the excitability parameters of median axons were comparable to able-bodied controls (data not shown). This is not unexpected, given that the spinal roots that contribute to the MN exit above T4.
Fig. 2.
Nerve excitability data (mean ± SE) recorded from the abductor pollicis brevis (APB) muscle during median nerve stimulation in SCI patients with neurological level between C3 and T1 (○) compared with responses from normal controls (n = 20; ●). The figure shows the stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), and the recovery cycle (D). SCI patient data were pooled from both upper limbs.
In contrast to the motor axons studied in the CPN, the nerve current-threshold relationship and the threshold electrotonus for motor axons in the MN were relatively unaffected following SCI (range for P = 0.07–0.89; Fig. 2, B and C). However, as for the common peroneal motor axons, the recovery cycle of MN motor axons was flatter in SCI patients, with a significant reduction in both superexcitability (SCI −17.14 ± 2.3%; controls −23.51 ± 1.42%; P = 0.036; Fig. 2D) and subexcitability (SCI 12.17 ± 1.77%; controls 16.5 ± 0.9%; P = 0.034; Fig. 2D). Taken together, the results from the present study demonstrate that, in patients with recent SCI, axonal excitability changes were generally more abnormal in the lower limbs (CPN) than in the upper limbs (MN) and suggest that the adverse downstream effects of SCI may be distance or length dependent.
Effects of 6-wk unilateral peripheral nerve stimulation therapy on axonal excitability.
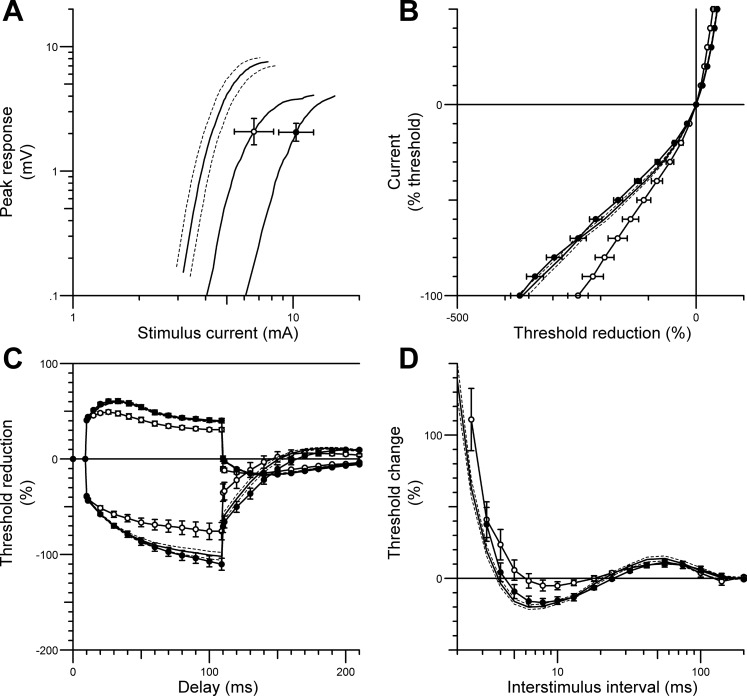
Ten patients with recent SCI (mean days since injury 65 ± 55 days) participated in the 6-wk unilateral peripheral nerve stimulation program targeting the CPN. One leg was randomly assigned to receive CPN stimulation therapy, whereas the opposite leg acted as an internal control, except in two patients in whom electrical stimulation therapy was applied to the CPN that remained excitable (patient nos. 21 and 22). Nerve excitability data pre- and post-6-wk unilateral CPN stimulation were compared between limbs and between the two time points to assess the effects of short-term electrical stimulation therapy. Repeated-measures ANOVA with post hoc analyses showed no significant difference in any excitability parameters between limbs in SCI patients at baseline (range for P = 0.29–0.9). However, 6 wk of unilateral CPN stimulation ameliorated a number of abnormal excitability parameters identified previously following SCI, including the increased superexcitability of the recovery cycle (pre −8.9 ± 2.6%; post −16.85 ± 2.9%; P = 0.04; Fig. 3D) and threshold electrotonus during both prolonged hyperpolarizing and depolarizing currents (P < 0.007), resulting in a more “fanned out” appearance (Fig. 3C) toward the normal limits. Furthermore, the current-threshold curve was shifted to the left after 6-wk CPN stimulation, in the hyperpolarizing direction, a trend toward normality (Fig. 3B). In addition, mean peak CMAP amplitude for the TA was maintained over the 6-wk period (pre 4.15 ± 1.7 mV; post 4.1 ± 1.2 mV; P = 0.92).
Fig. 3.
CPN excitability data (mean ± SE) recorded from the tibialis anterior muscle in patients with SCI before (○) and after (●) 6 wk of unilateral CPN stimulation therapy. Data from age-matched healthy controls (n = 12) are shown in solid black line with SE indicated by dotted lines. The figure shows changes in stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), and the recovery cycle (D). Six weeks of CPN stimulation therapy ameliorated abnormalities in the current-threshold relationship (B), the threshold electrotonus (C), and the recovery cycle (D) toward the normal range.
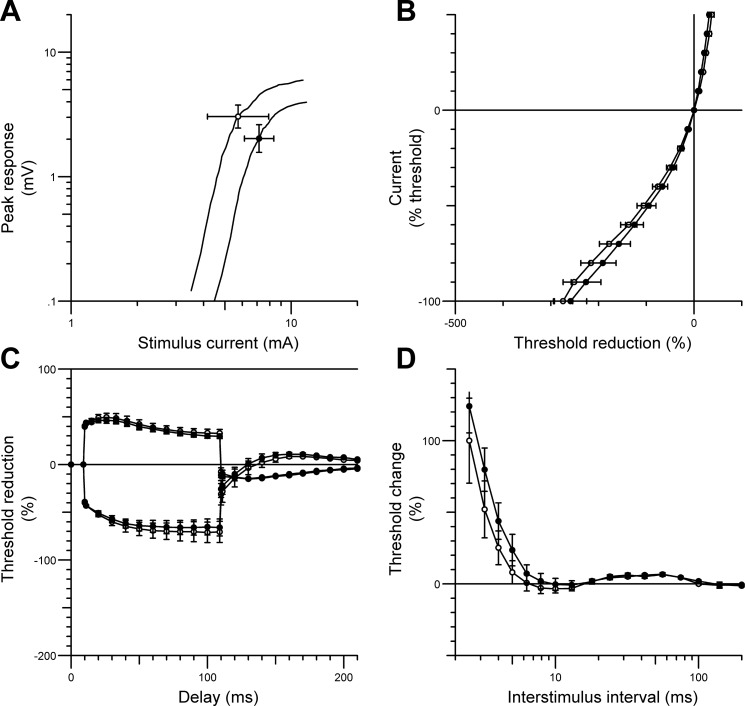
In contrast, there were no significant changes in any CPN excitability parameters recorded from the opposite, nonstimulated (control) leg over the 6-wk period (Fig. 4). The averaged peak CMAP of the nonstimulated TA muscle was smaller at 6 wk (baseline; 6.1 ± 1.2 mV; at 6 wk 4.05 ± 1.3 mV); however, this decrement in amplitude was not statistically significant (P = 0.25).
Fig. 4.
CPN excitability data (mean ± SE) recorded from the nonstimulated (control) tibialis anterior muscle in SCI patients at baseline (○) and after 6 wk of contralateral CPN stimulation (●). The figure shows the stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), and the recovery cycle (D). There were no significant changes in any excitability parameters over the 6-wk period.
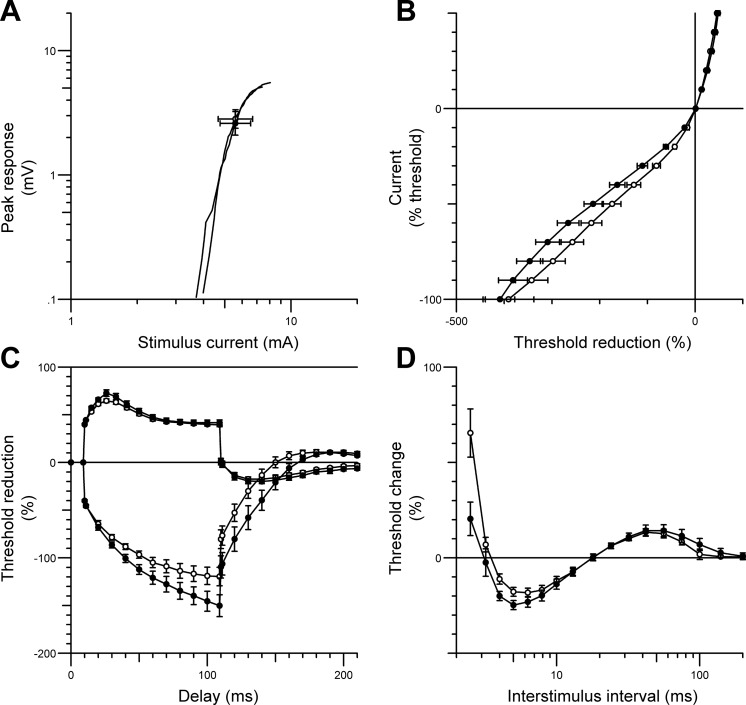
A subset of 11 SCI patients with injury levels between C3 and T1 (mean days since injury 55 ± 54 days) participated in the 6-wk MN stimulation program (3 of whom also took part in the unilateral peroneal nerve stimulation program concurrently). One arm was randomly assigned to receive MN stimulation, whereas the opposite arm acted as an internal control. Three out of 11 patients were subsequently excluded from the study because of unforseen medical complications (unrelated to the electrical stimulation therapy) that are known to affect peripheral nerve function. One patient developed acute inflammatory demyelinating polyneuropathy, one reported increased hand numbness and was diagnosed with carpal tunnel syndrome, and another developed brachial plexus palsy. Figures 5 and 6 show the mean MN excitability data (± SE) pre- and post-6-wk unilateral MN stimulation from the remaining eight SCI patients. Similar to the nerve excitability recordings obtained from the lower limb, short-term MN stimulation increased superexcitability of the recovery cycle (pre −17.14 ± 2.3%; post −23.16 ± 2.4%; P = 0.005; Fig. 5D) and threshold electrotonus during both prolonged hyperpolarizing currents at durations of 10–20 ms (P = 0.03), 20–40 ms (P = 0.009), and 90–100 ms (P = 0.004) and during depolarizing currents at durations of 10–20 ms (P = 0.006), thus producing a fanned out appearance (Fig. 5C). There were no significant differences in the stimulus-response curve and current-threshold relationship (Fig. 5, A and B, respectively). In contrast to the stimulated limb, there were no significant differences in any of the excitability parameters recorded from the opposite, nonstimulated MN (Fig. 6).
Fig. 5.
Median nerve excitability data (mean ± SE) recorded from the APB muscle in SCI patients before (○) and after (●) 6 wk of unilateral median nerve stimulation therapy. The figure shows changes in stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), and the recovery cycle (D). Six weeks of median nerve stimulation therapy produced a “fanned out” appearance in threshold electrotonus (C) and increased superexcitability of the recovery cycle (D).
Fig. 6.
Median nerve excitability data (mean ± SE) recorded from the nonstimulated (control) APB muscle in SCI patients at baseline (○) and at the 6th wk (●). There were no significant changes the stimulus-response curve (for 1-ms stimulus) (A), the current-threshold relationship (B), threshold electrotonus (C), or the recovery of excitability following a supramaximal stimulus (D) over the 6-wk period.
Effects of long-term unilateral peripheral nerve stimulation therapy on axonal excitability.
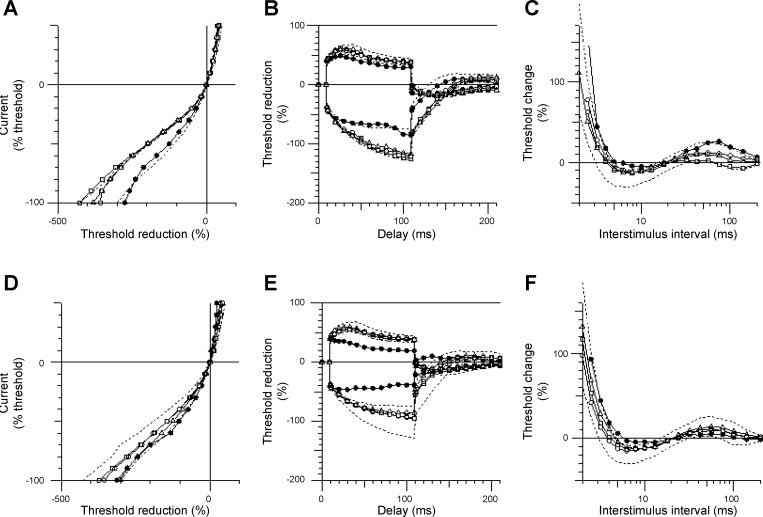
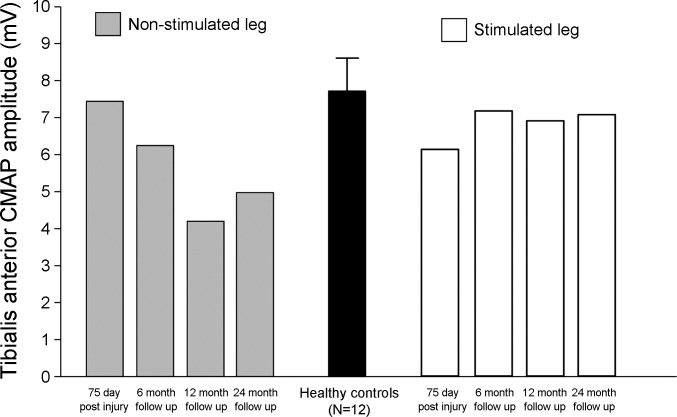
One patient with an incomplete cervical SCI (patient no. 7; C4 ASIA D) who commenced the 6-wk unilateral CPN stimulation program 75 days postinjury voluntarily continued with the same stimulation regimen (30 min/day 4–5 times/wk) for another 24 mo after completing the initial 6-wk stimulation program. Follow-up CPN excitability studies were conducted bilaterally at 6, 12, and 24 mo, and CMAP of TA was recorded. The results are presented in Figs. 7 and 8. Long-term CPN stimulation reinforced the effects produced by the 6-wk stimulation therapy, that is, a leftward shift of the current-threshold curve in the hyperpolarizing direction (Fig. 7A), increased superexcitability of the recovery cycle (Fig. 7C), and normalization of threshold electrotonus during both prolonged hyperpolarizing and depolarizing currents toward normal limits (Fig. 7B). There was some spontaneous improvement in threshold electrotonus and the recovery cycle in the opposite nonstimulated leg at 6 mo follow-up, but there was no further improvement thereafter (Fig. 7, E and F). Long-term CPN stimulation improved TA CMAP amplitude after 6 mo and maintained it over the next 24 mo (Fig. 8). In contrast, TA CMAP of the nonstimulated leg deteriorated gradually over time (by ∼1/3).
Fig. 7.
CPN excitability data from a patient with incomplete cervical SCI (C4 American Spinal Injury Association D) on day 75 postinjury (●) and after 6 mo (□), 12 mo (○), and 24 mo (△) of unilateral CPN stimulation. The figure shows the current-threshold relationship (A and D), threshold electrotonus (B and E), and the recovery cycle (C and F), with 95% confidence intervals obtained from healthy controls (n = 12) indicated by dotted lines. The panel on top (A–C) depicts data from the leg that received CPN stimulation therapy, and the panel on the bottom (D–F) shows data from the opposite, nonstimulated (control) leg.
Fig. 8.
Tibialis anterior (TA) compound muscle action potential (CMAP, mV) recorded from a patient with incomplete cervical SCI who continued with unilateral CPN stimulation for a further 24 mo after completing the 6-wk stimulation program. Data from the stimulated leg are depicted by empty bars, and data from the nonstimulated (control) leg are represented by gray-filled bars. Mean TA CMAP (± SE) from 12 healthy controls is shown in black for comparison. TA CMAP amplitude is maintained in the stimulated leg, and, in contrast, CMAP of the nonstimulated TA deteriorated over time.
DISCUSSION
There is accumulating evidence that peripheral nerve function is compromised following SCI (Boland et al. 2009; Boland et al. 2011; Lin et al. 2007; Nogajski et al. 2006; Van De Meent et al. 2010), and the results from the present study are in full agreement with this view. Using novel threshold tracking nerve excitability techniques, we have demonstrated that peripheral motor axons in both the upper and lower limbs underwent significant functional modifications within a few months of SCI. The distal axons were more severely affected, accompanied by significant reduction in CMAP amplitude. The magnitude of CMAP loss (∼44%) was significant enough to affect muscle strength and function (Van De Meent et al. 2010). Because motor axonal dysfunction is apparent in the early phases of SCI, the aim of the present study was to investigate whether the dysfunction could be reversed by a targeted peripheral nerve stimulation program. We have demonstrated that the addition of an intensive, 6-wk peripheral nerve stimulation program in the early phase of SCI ameliorated abnormalities in motor axonal excitability. Furthermore, peripheral nerve function could be maintained with long-term stimulation. It is important to note at this juncture that the abnormalities in axonal excitability did not improve with “standard” rehabilitation, but only with the addition of peripheral nerve stimulation. It is presumptuous to assume that peripheral nerve stimulation alone ameliorated axonal dysfunction. A more likely scenario is that peripheral nerve stimulation improved the biophysical properties of axonal membrane by normalizing various energy-dependent processes, and this in turn enhanced the responsiveness of motor axons to other rehabilitation therapies.
The results from our study suggest that peripheral nerve stimulation provides an opportunity to prevent chronic changes in axonal and muscular function following SCI. Maintenance of peripheral nerve function may help to reduce the risk of developing secondary peripheral neuropathy and peripheral nerve diseases that are extremely prevalent among SCI patients, especially within the first 12 mo of injury (Nogajski et al. 2006). Maintenance of peripheral nerve function in the early phases of SCI may improve long-term rehabilitation outcomes and the responsiveness of motor axons to future regenerative therapies. A longitudinal investigation with a larger cohort will be necessary to test this hypothesis.
Mechanisms underlying excitability abnormalities following SCI.
A number of mechanisms are likely to contribute to the complex downstream excitability changes after SCI. These included compression and ischemia related to the primary mechanical injury and delayed secondary processes such as cord inflammation, disuse atrophy, and increased sedentariness of motor neurones that have been disconnected from the central nervous system (Balentine 1978; Boland et al. 2011; Dietz 2010; Noble and Wrathall 1989). The “fanning-in” responses during threshold electrotonus, and a reduction in superexcitability during the recovery cycle, as well as shifting of the current-threshold curve to the right and increased refractoriness, all suggest some degree of axonal depolarization in SCI nerves. However, no change in the SDTC was observed, and a decrease in late subexcitability would argue against pure membrane depolarization as the primary pathogenic process. Ischemia is known to cause axonal depolarization through its inhibitory actions on the Na+/K+ pump (Kiernan and Bostock 2000) and is likely to contribute in part to the “depolarization-like” changes observed in SCI nerves. This may be compounded by delayed secondary metabolic processes such as upregulation of proinflammatory cytokines following SCI (Hayes et al. 2002), which is known to affect Na+ and K+ conductances similar to that observed in various channelopathies (Waxman 1998), which further disrupt the homoestasis of energy-dependent processes and together with decentralization contributed to the abnormalities in axonal excitability (Boland et al. 2011; Lin et al. 2007).
Our results argue against a generalized polyneuropathy as the cause of peripheral nerve dysfunction, since excitability of motor axons above the lesion was completely normal. Moreover, the cause of peripheral nerve dysfunction could not be attributed to direct trauma to the peripheral nerves because the excitability changes were observed in both upper and lower limb nerves and not just confined to one single nerve. This is further supported by a previous study that showed different patterns of excitability changes in patients with focal CPN palsy (Boland et al. 2011).
In accordance with previous studies, we also confirmed that the abnormalities in motor axonal excitability were more prominent in the lower limbs than in the upper limbs (Boland et al. 2011; Lin et al. 2007). The paradox that a SCI has a more detrimental effect on excitability properties of distal peripheral axons is perplexing and has been ascribed to trans-synaptic degeneration after SCI (Boland et al. 2011; Van De Meent et al. 2010). In our SCI cohort (all within 6 mo of injury), there was already evidence of significant atrophy and axonal loss in the TA (reduced TA CMAP by ∼44% and increased electrical threshold), whereas CMAP of APB was only mildly affected. A reduction in CMAP amplitudes after SCI has been reported previously (Boland et al. 2011; Dietz 2010; Kirshblum et al. 2001; Lin et al. 2007; Van De Meent et al. 2010). A recent, multicenter study with a large sample size (345 SCI patients) has also reported greater CMAP reduction in the lower limb (abductor hallucis; 36–57%) than the upper limb (abductor digiti minimi; 15–24%) in the first year after SCI, with the lowest CMAP amplitudes found between 5 and 9 mo postinjury (Van De Meent et al. 2010). The results of our study are in line with these previous studies.
Clinical implications.
The data from the current study support the view that a SCI has a profound downstream effect on the peripheral nervous system below the level of injury. As such, rehabilitation efforts must take this into account. Irrespective of the precise pathophysiological mechanisms responsible for the abnormalities in axonal excitability that developed following SCI, the present study has clearly demonstrated that an intensive 6-wk peripheral nerve stimulation program was beneficial in improving nerve excitability parameters toward the normal range. Importantly, the improvement could be maintained with long-term stimulation. The results of our study have several significant clinical implications for the management and rehabilitation of patients with SCI, particularly in the acute phase and in the context of future neuroregenerative projects. First, assessment of peripheral nerve function must commence in the acute phase of SCI and needs to be investigated routinely, especially in patients with complaints of new motor weakness, sensory loss, or pain. Second, therapies that help to maintain peripheral nerve function (such as the peripheral nerve stimulation paradigm used in the current study) need to be incorporated into the mainstream neurorehabilitation program in the early phases of SCI. Last, peripheral nerve stimulation may be used as a preventative strategy to maintain neural function in peripheral nerves that are more prone to chronic compression, such as the median and ulnar nerves at the wrist and elbows, as well as the CPN near the fibula head and the posterior tibial nerve in the popliteal fossa. In summary, maintenance of peripheral nerve function in the early phases of SCI may improve long-term rehabilitation outcomes.
GRANTS
The funding for this project was supported by the New South Wales Office for Medical Research, the Spinal Cord Injury and Related Neurological Conditions Program Grant, and the Brain Foundation. M. Lee is an Applied Spinal Cord Injury Research Fellow from the New South Wales Office for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.L., M.C.K., V.G.M., and C.S.-Y.L. conception and design of research; M.L. performed experiments; M.L. analyzed data; M.L. and V.G.M. interpreted results of experiments; M.L. prepared figures; M.L. drafted manuscript; M.L., M.C.K., V.G.M., and B.B.L. edited and revised manuscript; M.L., M.C.K., V.G.M., B.B.L., and C.S.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants, staff, interns, registrars, and nurses at the Spinal Medicine Department at the Prince of Wales Hospital in Randwick Sydney for assistance and patience throughout the study period.
REFERENCES
- Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest 39: 236–253, 1978. [PubMed] [Google Scholar]
- Boland RA, Bostock H, Kiernan MC. Plasticity of lower limb motor axons after cervical cord injury. Clin Neurophysiol 120: 204–209, 2009. [DOI] [PubMed] [Google Scholar]
- Boland RA, Lin CS, Engel S, Kiernan MC. Adaptation of motor function after spinal cord injury: novel insights into spinal shock. Brain 134: 495–505, 2011. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol 112: 1575–1585, 2001. [DOI] [PubMed] [Google Scholar]
- Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol 6: 167–174, 2010. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, Popovich PG. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma 19: 753–761, 2002. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology 54: 1574–1582, 2000. [DOI] [PubMed] [Google Scholar]
- Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle Nerve 27: 285–296, 2003. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain 123: 2542–2551, 2000. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve 23: 399–409, 2000. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Lim S, Garstang S, Millis S. Electrodiagnostic changes of the lower limbs in subjects with chronic complete cervical spinal cord injury. Arch Phys Med Rehabil 82: 604–607, 2001. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Kiernan MC. Nerve excitability properties in lower-limb motor axons: evidence for a length-dependent gradient. Muscle Nerve 29: 645–655, 2004. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Park SB, Kiernan MC. Assessment of nerve excitability in toxic and metabolic neuropathies. J Peripheral Nerv Sys 13: 7–26, 2008. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Park SB, Kiernan MC. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog Neurobiol 89: 288–313, 2009. [DOI] [PubMed] [Google Scholar]
- Leis AA, Kronenberg MF, Stetkarova I, Paske WC, Stokic DS. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology 47: 231–237, 1996. [DOI] [PubMed] [Google Scholar]
- Lin CS, Macefield VG, Elam M, Wallin BG, Engel S, Kiernan MC. Axonal changes in spinal cord injured patients distal to the site of injury. Brain 130: 985–994, 2007. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM, Committee ANS. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 26, Suppl 1: S50–S56, 2003. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Kawashima N, Akai M. Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch Phys Med Rehabil 87: 71–75, 2006. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res 482: 57–66, 1989. [DOI] [PubMed] [Google Scholar]
- Nogajski JH, Engel S, Kiernan MC. Focal and generalized peripheral nerve dysfunction in spinal cord-injured patients. J Clin Neurophysiol 23: 273–279, 2006. [DOI] [PubMed] [Google Scholar]
- Rutz S, Dietz V, Curt A. Diagnostic and prognostic value of compound motor action potential of lower limbs in acute paraplegic patients. Spinal Cord 38: 203–210, 2000. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res 133: 233–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Meent H, Hosman AJ, Hendriks J, Zwarts M, Group ESS, Schubert M. Severe degeneration of peripheral motor axons after spinal cord injury: a European multicenter study in 345 patients. Neurorehabil neural Repair 24: 657–665, 2010. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Axonal excitability properties in amyotrophic lateral sclerosis. Clin Neurophysiol 117: 1458–1466, 2006. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Demyelinating diseases–new pathological insights, new therapeutic targets. N Engl J Med 338: 323–325, 1998. [DOI] [PubMed] [Google Scholar]