Abstract
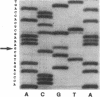
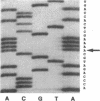
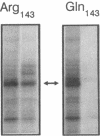
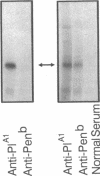
The human Pena/Penb alloantigen system represents a naturally occurring polymorphism of human platelet membrane glycoprotein (GP) IIIa, and has previously been implicated in the onset of two important clinical syndromes, neonatal alloimmune thrombocytopenic purpura and posttransfusion purpura. To investigate the molecular basis of the polymorphism underlying the Pen alloantigen system, we used the polymerase chain reaction to amplify platelet-derived GPIIIa mRNA transcripts. DNA sequence analysis of amplified GPIIIa cDNAs from nucleotides 161 to 1341 (encompassing amino acid residues 22-414) revealed a G526<==>A526 polymorphism that segregated precisely with Pen phenotype in twelve other individuals examined. This nucleotide substitution results in an Arg (CGA) to Gln (CAA) polymorphism at amino acid 143 of GPIIIa. Interestingly, this polymorphic residue is located within the putative RGD binding site (residues 109-171) of GPIIIa. Platelet aggregation patterns of a Penb/b individual, however, were nearly normal in response to all physiological agonists tested, indicating that this polymorphism does not grossly affect integrin function. Short synthetic peptides encompassing residue 143 were unable to mimic either the Pena or Penb antigenic determinants, suggesting that the Pen epitopes are dependent upon proper folding of the polypeptide chain. Finally, we constructed allele-specific recombinant forms of GPIIIa that differed only at amino acid residues 143. Whereas anti-Pena alloantibodies were able to recognize the Arg143 recombinant form of GPIIIa, anti-Penb antibodies were not. Conversely, anti-Penb alloantibodies were reactive only with the Gln143 isoform of the GPIIIa molecule. It thus appears that amino acid 143 of GPIIIa is not only associated with Pen phenotype, but specifically controls the formation and expression of the Pen alloantigenic determinants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer J., Coller B. S. Evidence that platelet glycoprotein IIIa has a large disulfide-bonded loop that is susceptible to proteolytic cleavage. J Biol Chem. 1989 Oct 15;264(29):17564–17573. [PubMed] [Google Scholar]
- Calvete J. J., Henschen A., González-Rodríguez J. Assignment of disulphide bonds in human platelet GPIIIa. A disulphide pattern for the beta-subunits of the integrin family. Biochem J. 1991 Feb 15;274(Pt 1):63–71. doi: 10.1042/bj2740063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Lam S. C., Plow E. F. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988 Oct 7;242(4875):91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- Flug F., Espinola R., Liu L. X., SinQuee C., DaRosso R., Nardi M., Karpatkin S. A 13-mer peptide straddling the leucine33/proline33 polymorphism in glycoprotein IIIa does not define the PLA1 epitope. Blood. 1991 May 1;77(9):1964–1969. [PubMed] [Google Scholar]
- Friedman J. M., Aster R. H. Neonatal alloimmune thrombocytopenic purpura and congenital porencephaly in two siblings associated with a "new" maternal antiplatelet antibody. Blood. 1985 Jun;65(6):1412–1415. [PubMed] [Google Scholar]
- Furihata K., Nugent D. J., Bissonette A., Aster R. H., Kunicki T. J. On the association of the platelet-specific alloantigen, Pena, with glycoprotein IIIa. Evidence for heterogeneity of glycoprotein IIIa. J Clin Invest. 1987 Dec;80(6):1624–1630. doi: 10.1172/JCI113250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger A., Kolodziej M., Poncz M., Bennett J. S., Newman P. J. Effect of single amino acid substitutions on the formation of the PlA and Bak alloantigenic epitopes. Blood. 1991 Aug 1;78(3):681–687. [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L., 3rd, Ginsberg M. H. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990 Aug 24;249(4971):915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- Lyman S., Aster R. H., Visentin G. P., Newman P. J. Polymorphism of human platelet membrane glycoprotein IIb associated with the Baka/Bakb alloantigen system. Blood. 1990 Jun 15;75(12):2343–2348. [PubMed] [Google Scholar]
- McFarland J. G., Aster R. H., Bussel J. B., Gianopoulos J. G., Derbes R. S., Newman P. J. Prenatal diagnosis of neonatal alloimmune thrombocytopenia using allele-specific oligonucleotide probes. Blood. 1991 Nov 1;78(9):2276–2282. [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J. Platelet GPIIb-IIIa: molecular variations and alloantigens. Thromb Haemost. 1991 Jul 12;66(1):111–118. [PubMed] [Google Scholar]
- Niewiarowski S., Norton K. J., Eckardt A., Lukasiewicz H., Holt J. C., Kornecki E. Structural and functional characterization of major platelet membrane components derived by limited proteolysis of glycoprotein IIIa. Biochim Biophys Acta. 1989 Jul 24;983(1):91–99. doi: 10.1016/0005-2736(89)90384-2. [DOI] [PubMed] [Google Scholar]
- Santoso S., Shibata Y., Kiefel V., Mueller-Eckhardt C. Identification of the Yukb allo-antigen on platelet glycoprotein IIIa. Vox Sang. 1987;53(1):48–51. doi: 10.1111/j.1423-0410.1987.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Shibata Y., Miyaji T., Ichikawa Y., Matsuda I. A new platelet antigen system, Yuka/Yukb. Vox Sang. 1986;51(4):334–336. doi: 10.1111/j.1423-0410.1986.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Simon T. L., Collins J., Kunicki T. J., Furihata K., Smith K. J., Aster R. H. Posttransfusion purpura associated with alloantibody specific for the platelet antigen, Pen(a). Am J Hematol. 1988 Sep;29(1):38–40. doi: 10.1002/ajh.2830290109. [DOI] [PubMed] [Google Scholar]