Abstract
Although species-specific differences in ion channel properties are well-documented, little has been known about the properties of the human Nav1.8 channel, an important contributor to pain signaling. Here we show, using techniques that include voltage clamp, current clamp, and dynamic clamp in dorsal root ganglion (DRG) neurons, that human Nav1.8 channels display slower inactivation kinetics and produce larger persistent current and ramp current than previously reported in other species. DRG neurons expressing human Nav1.8 channels unexpectedly produce significantly longer-lasting action potentials, including action potentials with half-widths in some cells >10 ms, and increased firing frequency compared with the narrower and usually single action potentials generated by DRG neurons expressing rat Nav1.8 channels. We also show that native human DRG neurons recapitulate these properties of Nav1.8 current and the long-lasting action potentials. Together, our results demonstrate strikingly distinct properties of human Nav1.8, which contribute to the firing properties of human DRG neurons.
Keywords: sodium channel, Nav1.8, persistent current, ramp current, dorsal root ganglion
voltage-gated sodium channels play a critical role in the generation and propagation of action potentials in excitable cells, including cardiomyocytes, muscle cells, and neurons. Nine voltage-gated sodium channels (Nav1.1–Nav1.9) have been identified in mammals (Catterall et al. 2005). Different sodium channel subtypes have specific distributions and functions. Sodium channel Nav1.8 is preferentially expressed in peripheral neurons, in particular within small and medium-sized dorsal root ganglion (DRG) neurons (Akopian et al. 1996; Djouhri et al. 2003; Sangameswaran et al. 1996; Shields et al. 2012). Nav1.8 is known to produce a slow-inactivating, tetrodotoxin (TTX)-resistant current and is characterized by significantly depolarized activation and inactivation compared with other sodium channels (Akopian et al. 1996; Sangameswaran et al. 1996). This enables Nav1.8 to act as a major contributor to the action potential upstroke during repetitive firing of DRG neurons evoked by sustained depolarization (Blair and Bean 2002; Renganathan et al. 2001).
Studies in knockout animals have clearly established a role of Nav1.8 in pain (Akopian et al. 1999; Zimmermann et al. 2007). Furthermore, gain-of-function mutations of Nav1.8 have been found in human subjects with painful neuropathies; relatively subtle gain-of-function changes in channel biophysics due to these mutations markedly alter the excitability of DRG neurons (Faber et al. 2012; Han et al. 2014; Huang et al. 2013), underscoring the potential importance of even small differences in human Nav1.8 channel properties, compared with those in rodents, for pain signaling. Nav1.8 mutations linked to small-fiber neuropathy have been functionally profiled after expression in rodent DRG neurons (Faber et al. 2012; Han et al. 2014; Huang et al. 2013). Although rodent DRG neurons provide a tractable heterologous expression system for the functional assays that have been used to assess these mutations (Dib-Hajj et al. 2009), notable differences between rodent and human DRG sodium channels have been reported, including, e.g., an ∼10-mV difference in voltage dependence of human Nav1.9 currents compared with rodent Nav1.9 currents (Dib-Hajj et al. 1999). Given the important role of Nav1.8 in pain, there is a need for information about the functional properties of human Nav1.8 channels and their roles in human DRG neuron firing.
Persistent sodium current and ramp current are produced by some sodium channels, and it has been known that persistent and ramp sodium currents play critical functional roles in modulating the excitability of neurons (Cummins et al. 1998; Fleidervish and Gutnick 1996; Kiss 2008; Stafstrom 2007). Persistent sodium currents have been associated with several hyperexcitability disorders, including epilepsy and LQT3 disease (Christe et al. 2008; Saint 2008; Stafstrom 2007; Veeramah et al. 2012), and pain (Fertleman et al. 2006). In the present study, using dynamic clamp as well as voltage clamp and current clamp to study human Nav1.8 both after transfection into rodent DRG neurons and in native human DRG neurons, we investigated the biophysical properties of human Nav1.8. We demonstrate larger persistent current and ramp current as well as slower inactivation of human Nav1.8 channels and demonstrate longer-lasting action potentials in DRG neurons carrying human Nav1.8.
MATERIALS AND METHODS
Plasmid Constructs
The pcDNA5-SCN10A (Nav1.8) plasmid construct that encodes human Nav1.8 protein was purchased from Genionics, and the plasmid pRK-Nav1.8 carrying rat Nav1.8 insert was a gift from Dr. John Wood (University College London, London, UK). Both human and rat Nav1.8 constructs were driven by the CMV promoter.
Primary Mouse DRG Neuron Isolation and Transfection
Animal studies were approved by Yale University and Department of Veterans Affairs West Haven Hospital Animal Use Committees. DRG neurons were isolated, as previously reported (Dib-Hajj et al. 2009), from homozygous Nav1.8-cre mice (4–8 wk of age, both male and female) that lack endogenous Nav1.8. Briefly, 24 DRGs (from T8 to L6, both sides) were harvested, incubated at 37°C for 20 min in complete saline solution [CSS; in mM: 137 NaCl, 5.3 KCl, 1 MgCl2, 25 sorbitol, 3 CaCl2, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), adjusted to pH 7.2 with NaOH] containing 0.5 U/ml Liberase TM (Roche Diagnostics) and 0.6 mM EDTA before 15-min incubation at 37°C in CSS containing 0.5 U/ml Liberase TL (Roche Diagnostics), 0.6 mM EDTA, and 30 U/ml papain (Worthington). Tissue was then centrifuged and triturated in 0.5 ml of DRG medium: Dulbecco's modified Eagle's medium-F12 (1:1) with 100 U/ml penicillin, 0.1 mg/ml streptomycin (Invitrogen), and 10% fetal bovine serum (Hyclone), containing 1.5 mg/ml bovine serum albumin (BSA) (low endotoxin; Sigma) and 1.5 mg/ml trypsin inhibitor (Sigma). After trituration, Nav1.8 constructs (2 μg of Nav1.8 wild type plus 0.2 μg of EGFP) were transfected into DRG neuron suspension by electroporation using Nucleofector IIS (Lonza) and Amaxa SCN Nucleofector (VSPI-1003). After electroporation, 100 μl of calcium-free Dulbecco's modified Eagle's medium (Invitrogen) was added and cells were incubated at 37°C for 5 min in a 95% air-5% CO2 (vol/vol) incubator to allow neurons to recover. The cell mixture was then diluted with DRG medium containing 1.5 mg/ml BSA (low endotoxin; Sigma) and 1.5 mg/ml trypsin inhibitor (Sigma), seeded onto poly-d-lysine-laminin-coated coverslips (BD), and incubated at 37°C to allow DRG neurons to attach to the coverslips. After 40 min, DRG medium was added into each well to a final volume of 1.0 ml [for current-clamp recording culture, medium was supplemented with 50 ng/ml mouse nerve growth factor (mNGF) (Alomone Labs) and 50 ng/ml recombinant human glial cell line-derived neurotrophic factor (hGDNF) (PeproTech)] and the DRG neurons were maintained at 37°C in a 95% air-5% CO2 (vol/vol) incubator for ∼40–48 h before recording.
Primary Human DRG Neuron Isolation
Human DRGs [lumbar (L)3, L4, or L5] were obtained from the National Disease Research Interchange (NDRI). Studies with human tissues were approved by human investigation committees at Yale University and Department of Veterans Affairs West Haven Hospital. The DRG neurons were harvested and dissociated within 24 h of clamping the aorta. DRGs were harvested from six Caucasian donors (woman, age 53 yr, L5; woman, age 51 yr, L3; man, age 46 yr, L3; woman, age 53 yr, L3; woman, age 51 yr, L3; man, age 75 yr, L3 and L4). The causes of death were anoxia, cardiac arrest, head trauma, and intracranial hemorrhage. None of these patients had been diagnosed with a pain or inflammatory syndrome or with peripheral neuropathy prior to death.
Nerve roots and as much connective tissue as possible were removed, and DRGs were sliced into small fragments in CSS and then incubated on a rotating shaker at 37°C for 40 min in CSS containing 0.5 U/ml Liberase TM (Roche) and 0.6 mM EDTA, followed by a 25-min incubation at 37°C in CSS containing 0.5 U/mL Liberase TL (Roche), 0.6 mM EDTA, and 30 U/ml papain (Worthington). DRGs were then centrifuged (1,000 rpm for 30 s) and triturated in DRG medium containing 1.5 mg/ml BSA (low endotoxin; Sigma) and 1.5 mg/ml trypsin inhibitor (Sigma). After filtering with 100-μm nylon mesh cell strainer (BD), the cell suspension was centrifuged (1,000 rpm for 10 min), the cell pellet was resuspended in 1.2 ml of DRG medium, and 80 μl of cell suspensions were plated onto each poly-d-lysine-laminin-coated coverslip (BD). After incubation at 37°C in a 95% air-5% CO2 (vol/vol) incubator for 60 min to allow neurons to adhere, 0.92 ml of DRG medium supplemented with mNGF (50 ng/ml; Alomone Labs) and hGDNF (50 ng/ml; PeproTech) was added into each well. In preliminary experiments to optimize the culture conditions, we determined that supplements of these trophic factors were necessary to obtain viable neurons for recordings. DRG neurons were maintained at 37°C in a 95% air-5% CO2 (vol/vol) incubator and recorded by whole cell patch clamp within 24 h after plating. A total of 86 DRG neurons (size range 25–60 μm) were selected for recording by voltage clamp and current clamp.
Primary Rat DRG Neuron Isolation
DRG neurons were isolated from male Sprague-Dawley rats (4–8 wk of age) as described previously (Rizzo et al. 1994). The tissue was then enzymatically digested at 37°C for 25 min with collagenase A (1 mg/ml; Roche) in CSS and for 25 min with collagenase D (1 mg/ml; Roche) and papain (30 U/ml; Worthington) in CSS at 37°C. Treated tissues were gently centrifuged (100 g for 3 min), and the pellets were triturated in DRG medium containing 1.5 mg/ml BSA (low endotoxin; Sigma) and 1.5 mg/ml trypsin inhibitor (Sigma). Cells were then plated on poly-l-ornithine-laminin-coated glass coverslips (BD), flooded with DRG medium after 1 h, and incubated at 37°C in a humidified 95% air-5% CO2 (vol/vol) incubator. Small DRG neurons were recorded by whole cell patch clamp within 24 h after plating.
Electrophysiology
Voltage-clamp recording on transfected mouse DRG neurons.
Small transfected mouse DRG neurons (<25-μm diameter) with robust green fluorescence and no apparent neurites were selected for voltage-clamp recording. Fire-polished electrodes (1–2 MΩ) were fabricated from 1.6-mm-outer diameter borosilicate glass micropipettes (World Precision Instruments, Sarasota, FL). The pipette potential was adjusted to zero before seal formation, and liquid junction potential was not corrected. Capacitive transients were canceled, and voltage errors were minimized with 80–90% series resistance (1.5–5 MΩ) compensation; cells were excluded from analysis if the predicted voltage error exceeded 3 mV. Currents were acquired with Pulse Software (HEKA Electronics) 5 min after whole cell configuration was established, sampled at a rate of 50 kHz, and filtered at 2.9 kHz. The pipette solution contained the following (in mM): 140 CsCl, 10 NaCl, 0.5 EGTA, 3 MgATP, and 10 HEPES, pH 7.3 with CsOH (adjusted to 315 mosM with dextrose). The extracellular bath solution contained the following (in mM): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 5 CsCl, and 20 tetraethylammonium chloride (TEA·Cl), pH 7.32 with NaOH (327 mosM). TTX (0.5 μM), CdCl2 (0.1 mM), and 4-aminopyridine (1 mM) were added to the bath solution to block endogenous voltage-gated sodium currents, calcium currents, and potassium currents, respectively.
We cotransfected Nav1.8 channels with EGFP and were able to record the Nav1.8 slow-inactivating TTX-R current in every GFP-positive neuron (48/48 cells transfected with rat or human Nav1.8 channels) in voltage-clamp mode, indicating that in our transfection method all neurons with green fluorescence also produced Nav1.8 sodium currents. For voltage-clamp recording, DRG neurons were held at −70 mV to inactivate Nav1.9 channels and we recorded both activation and fast inactivation of every cell. We have shown previously (Cummins et al. 1999; Dib-Hajj et al. 1999) that the fast inactivation of Nav1.9 starts at much hyperpolarized voltage (around −100 mV) but that Nav1.8 starts to inactivate at much depolarized voltage (around −70 mV). For our analysis, we discarded the cells that displayed TTX-R current with hyperpolarized fast inactivation (less than −70 mV) to exclude potential contamination of Nav1.9 current.
For current-voltage (I-V) relationships, cells were held at −70 mV and stepped to a range of potentials (−70 to +50 mV in 5-mV increments) for 100 ms. Persistent current was measured as described above. Peak inward currents (I) were plotted as a function of depolarization potential to generate I-V curves. Activation curves were obtained by converting I to conductance (G) at each V with the equation G = I/(V − Vrev), where Vrev is the reversal potential that was determined for each cell individually. Activation curves were then fit with Boltzmann functions in the form of G = Gmax/{1 + exp[(V1/2,act − V)/k]}, where Gmax is the maximal sodium conductance, V1/2,act is the potential at which activation is half-maximal, V is the test potential, and k is the slope factor.
Steady-state fast inactivation was achieved with a series of 500-ms prepulses (−90 to +10 mV in 5-mV increments), and the remaining noninactivated channels were activated by a 40-ms step depolarization to 0 mV. Peak inward currents obtained from steady-state fast inactivation protocols were normalized to the maximal peak current (Imax) and fit with Boltzmann functions: I/Imax = A + (1 − A)/{1 + exp[(V − V1/2,inact)/k]}, where V represents the inactivating prepulse potential and V1/2,inact represents the midpoint of the inactivation.
Persistent currents were measured as mean amplitudes of currents recorded between 90 and 95 ms after the onset of depolarization and are presented as a percentage of the maximal transient peak current. Ramp currents were elicited with slow ramp depolarization over a 600-ms period at 0.2 mV/ms. The amplitude of ramp current was presented as a percentage of the maximal peak current.
Voltage-clamp recording on native human DRG neurons.
Voltage-clamp recordings were obtained from native human DRG neurons within 24 h after plating, with rat DRG neurons (<30-μm diameter) as a comparator. The pipette solution was the same as for voltage-clamp recording on transfected mouse neurons. For the extracellular bath solution, 140 mM NaCl was substituted with 70 mM NaCl and 70 mM choline-Cl, to minimize voltage errors caused by large peak currents. TTX (0.5 μM), CdCl2 (0.1 mM), and 4-aminopyridine (1 mM) were also added to the bath solution to block endogenous voltage-gated sodium currents, calcium currents, and potassium currents, respectively. DRG neurons were held at −60 mV to inactivate Nav1.9 channels. Persistent current was measured as described above.
Current-clamp recording on transfected mouse DRG neurons.
We used the same method of DRG neuron transfection for current-clamp recording as for voltage-clamp recording; thus we expected the 100% coincidence of green fluorescence and Nav1.8 TTX-R current as observed in the voltage-clamp studies. Current-clamp recordings were obtained from small (<25-μm diameter) GFP-labeled DRG neurons 40–48 h after transfection with EPC-10 (HEKA). Electrodes had a resistance of 1–3 MΩ when filled with the pipette solution, which contained the following (in mM): 140 KCl, 0.5 EGTA, 5 HEPES, and 3 Mg-ATP, pH 7.3 with KOH (adjusted to 315 mosM with dextrose). The extracellular solution contained the following (in mM): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, and 10 HEPES, pH 7.3 with NaOH (adjusted to 320 mosM with dextrose). Whole cell configuration was obtained in voltage-clamp mode before proceeding to the current-clamp recording mode. Cells with stable (<10% variation) resting membrane potential (RMP) more negative than −40 mV were used for data collection.
Current-clamp recording on native DRG neurons.
Current-clamp recordings were performed with native human or rat (<30-μm diameter) DRG neurons within 24 h after plating. Pipette solution and extracellular solution were the same as for the current-clamp recordings on transfected mouse DRG neurons.
Dynamic clamp recording.
DRG neurons obtained from homozygous Nav1.8-cre mice (4–8 wk of age, both male and female) were enzymatically isolated as described above and were used for dynamic clamp recording after 40–48 h. Small DRG neurons (<25-μm diameter) were dynamically clamped in whole cell configuration (Kemenes et al. 2011; Samu et al. 2012; Sharp et al. 1993; Vasylyev et al. 2014). Electrode resistance was 1–3 MΩ when filled with the intracellular solution, which had the same composition as the intracellular solution used for current-clamp recording described above. The extracellular solution was the same as the extracellular solution used for current-clamp recording described above. Membrane voltages and currents were recorded in dynamic clamp with a MultiClamp 700B amplifier (Molecular Devices) interfaced with CED Power 1401 mk II DAI and Signal software (CED), digitized by Digidata 1440A DAC, and stored on hard disk with pCLAMP 10 software (Molecular Devices).
Kinetic model of Nav1.8 channel.
The Nav1.8 channel model used for dynamic clamp was based on Hodgkin-Huxley equations dm/dt = αm(1 − m) − βmm and dh/dt = αh(1 − h) − βhh, where m and h are channel activation and inactivation variables and α and β are forward and backward rate constants, respectively. The following rate constants were used for the human Nav1.8 channel model:
Rat Nav1.8 channel was described by the following rate constant:
Sodium current was described by INa = gmax·m3·h·(Vm − ENa), where gmax is maximal conductance and was set to 125 nS to match our voltage-clamp recordings, which show ∼6 nA of current, Vm is membrane voltage, and ENa = 65 mV is sodium reversal potential. Currents evoked by voltage clamp were calculated in 10-μs precision with a custom program written in OriginPro 8.5 LabTalk. Human and rat Nav1.8 channel steady-state parameters and kinetics were obtained from our voltage-clamp recording on transfected mouse DRG neurons.
Data Analysis
Voltage-clamp and current-clamp data were analyzed with FitMaster (HEKA) and OriginPro 8.5 (OriginLab). Dynamic clamp data were analyzed with pCLAMP 10 (Molecular Devices) and OriginPro 8.5. All data are presented as means ± SE. Statistical significance was examined with two-sample Student's t-test, Mann-Whitney test, or two-portion z-test, except for dynamic clamp data, which was examined with two-sample paired Student's t-test.
RESULTS
Human Nav1.8 Displays Distinct Gating Properties in Transfected Mouse DRG Neurons
There are marked differences in properties of ion channels in human versus rodent neurons (Serrano et al. 2012; Valeyev et al. 1996); for example, there are large differences in the voltage-gated sodium channel Nav1.9, which shows an ∼10-mV hyperpolarizing shift of activation in human DRG neurons compared with rodent neurons (Dib-Hajj et al. 1999). Whether data from rodent Nav1.8 can be extrapolated to human Nav1.8 is not clear. To assess the gating properties of human Nav1.8 in DRG neurons, we transfected human Nav1.8 into Nav1.8-null mouse DRG neurons and performed voltage-clamp analysis. As a comparator, rat Nav1.8 was also transfected into Nav1.8-null mouse DRG neurons.
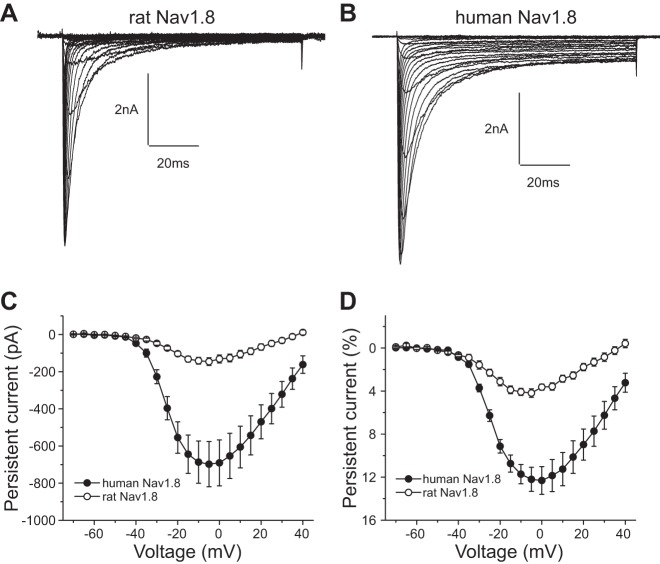
Figure 1, A and B, show representative sodium channel current traces recorded from DRG neurons transfected with rat (Fig. 1A) and human (Fig. 1B) Nav1.8 channels. DRG neurons transfected with both rat and human Nav1.8 channels generated voltage-dependent, slowly inactivating inward currents. There was no significant difference in the densities of peak sodium channel current between the two groups of cells [rat Nav1.8: 356 ± 74 pA/pF (n = 25), human Nav1.8: 447 ± 90 pA/pF (n = 23); P > 0.05]. Notably, DRG neurons transfected with human Nav1.8 display a threefold larger persistent current compared with DRG neurons transfected with rat Nav1.8. Figure 1, C and D, compare the absolute and normalized amplitudes of persistent current in DRG neurons expressing rat and human Nav1.8 channels, respectively. The persistent currents display voltage dependence with a peak within the voltage range −5 to 0 mV for both channels. However, the normalized amplitude of persistent current of DRG neurons expressing human Nav1.8 channels (12.3% ± 1.3%, n = 17) is 2.9-fold larger than that of DRG neurons expressing rat DRG neurons (4.2% ± 0.4%, n = 17; P < 0.001) (Table 1).
Fig. 1.
Human Nav1.8 channels produce a larger persistent current than rat Nav1.8 channels in transfected Nav1.8-knockout dorsal root ganglion (DRG) neurons. A and B: representative Nav1.8 current family traces recorded from mouse Nav1.8-knockout DRG neurons transfected with rat (A) or human (B) Nav1.8 channels. Cells were held at −70 mV and stepped to a range of potentials (−70 to +40 mV in 5-mV increments) for 100 ms. C and D: comparison of the absolute value (C) and normalized value (D) of persistent currents between rat and human Nav1.8 channels.
Table 1.
Comparison of biophysical properties between human Nav1.8 and rat Nav1.8
| Activation |
Fast Inactivation |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| V1/2,act, mV | n | V1/2,fast, mV | Offset | n | Ramp Current | n | Persistent Current | n | |
| Rat Nav1.8 | −6.21 ± 1.62 | 17 | −35.53 ± 1.6 | 2.25 ± 0.42% | 16 | 12.1 ± 2.1% | 9 | 4.2 ± 0.4% | 17 |
| Human Nav1.8 | −11.12 ± 1.76* | 17 | −31.86 ± 0.58* | 9.2 ± 1.61%*** | 15 | 21.9 ± 1.1%** | 12 | 12.3 ± 1.3%*** | 17 |
Values are means ± SE. V1/2,act, V1/2,fast, potential at which activation or fast inactivation is half-maximal.
P < 0.05,
P < 0.01,
P < 0.001.
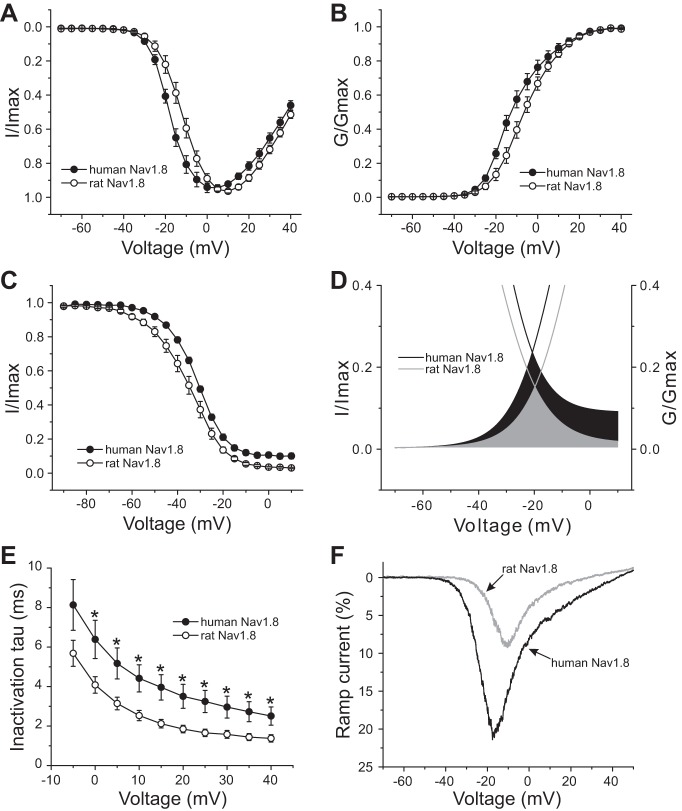
We also compared the voltage dependence of activation and steady-state fast inactivation between human and rat Nav1.8 channels. As Fig. 2A shows, human Nav1.8 channels display hyperpolarized activation compared with rat Nav1.8 channels. The V1/2 of activation for human Nav1.8 is −11.12 ± 1.76 mV (n = 17), which represents an ∼5-mV hyperpolarizing shift compared with the V1/2 of activation for rat Nav1.8 (−6.21 ± 1.62 mV, n = 17; P < 0.05) (Fig. 2B, Table 1). Human Nav1.8 channels display depolarized steady-state fast inactivation compared with rat Nav1.8 channels. The V1/2 of fast inactivation for human Nav1.8 channels (−31.86 ± 0.58 mV, n = 15) is significantly different from that for rat Nav1.8 channels (−35.53 ± 1.6 mV, n = 16; P < 0.05) (Fig. 2C, Table 1). In addition, the offset of fast inactivation for human Nav1.8 channels (9.2 ± 1.61%, n = 15) is fourfold bigger compared with rat Nav1.8 channels (2.25 ± 0.42%, n = 16; P < 0.001) (Table 1). The hyperpolarized activation combined with depolarized fast inactivation of human Nav1.8 produce a large overlap that predicts a large window current (Fig. 2D). The kinetics for open-state inactivation, which reflect the transition from the open to the inactivated state, were significantly slowed for human Nav1.8 channel compared with rat Nav1.8 channel from 0 to +40 mV (Fig. 2E).
Fig. 2.
Voltage-clamp analysis of human and rat Nav1.8 channels in transfected Nav1.8-knockout mouse DRG neurons. A: normalized current-voltage (I–V) curves for human and rat Nav1.8 channels. B: comparison of voltage-dependent activation between human and rat Nav1.8 channels. Activation of human Nav1.8 channels is hyperpolarized by ∼5 mV compared with rat Nav1.8 channels. G/Gmax, normalized sodium conductance. C: fast inactivation of human Nav1.8 is depolarized by 3.7 mV compared with rat Nav1.8. D: magnified view of overlap between activation and fast inactivation for rat and human Nav1.8. The overlap of Nav1.8 channels for rat is shown in gray, whereas the black area shows the difference of the overlap between human and rat Nav1.8. E: kinetics of open-state inactivation measured as a function of voltage for rat (n = 17) and human (n = 17) Nav1.8 channel. Time constants (τ) were obtained by fitting currents elicited as described in Fig. 1, A and B, with a single-exponential function. *P < 0.05. F: representative ramp currents elicited with 600-ms ramp depolarization from −70 mV to 50 mV for human or rat Nav1.8 channel recorded from transfected DRG neurons. Human Nav1.8 produces a ramp current that is almost double that produced by rat Nav1.8 channels.
We also measured the response to a slow ramp stimulus (−70 to +50 mV over 600 ms). Human Nav1.8 channels produce almost twofold larger ramp current (21.9 ± 1.1%, n = 12) compared with rat Nav1.8 channels (12.1 ± 2.1%, n = 9; P < 0.01) (Fig. 2F, Table 1).
Threshold Is Lower in DRG Neurons Transfected with Human Nav1.8 Channels
Our previous work has shown that Nav1.8 contributes significantly to the production of sodium-dependent action potentials in small DRG neurons (Renganathan et al. 2001). The species-specific differences in gating properties of Nav1.8 suggested that there might be a marked difference in the effect of human versus rat Nav1.8 on the firing properties of DRG neurons. Here we transfected human Nav1.8 channels into Nav1.8-null mouse DRG neurons so that we could correlate action potential characteristics in these cells with our transfected Nav1.8 channels. As a comparator, rat Nav1.8 channels were also transfected into Nav1.8-null mouse DRG neurons.
The mean RMP was −51.1 ± 1.2 mV (n = 26) in DRG neurons expressing human Nav1.8 channels, which was not significantly different from the mean RMP in DRG neurons expressing rat Nav1.8 channels (−51.6 ± 0.8 mV, n = 36; P > 0.05). There was no significant difference for input resistance between DRG neurons expressing human Nav1.8 channels (566 ± 78 MΩ, n = 26) or rat Nav1.8 channels (461 ± 45 MΩ, n = 36; P > 0.05).
The current threshold, at which DRG neurons generate the first all-or-none action potential, was smaller by nearly one-half for DRG neurons expressing human Nav1.8 channels (68 ± 12 pA, n = 26) compared with rat Nav1.8 channels (137 ± 13 pA, n = 36; P < 0.001). The amplitudes of the action potential for DRG neurons transfected with human Nav1.8 channels and DRG neurons transfected with rat Nav1.8 channels were 109.8 ± 1.3 mV (n = 26) and 107.8 ± 1.4 mV (n = 36; P > 0.05), respectively.
DRG Neurons Transfected with Human Nav1.8 Channels Display Two Firing Patterns
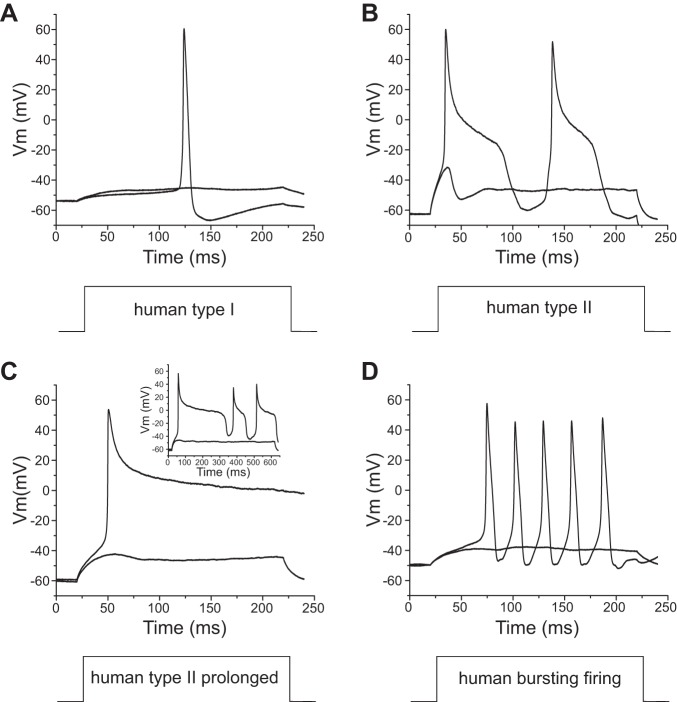
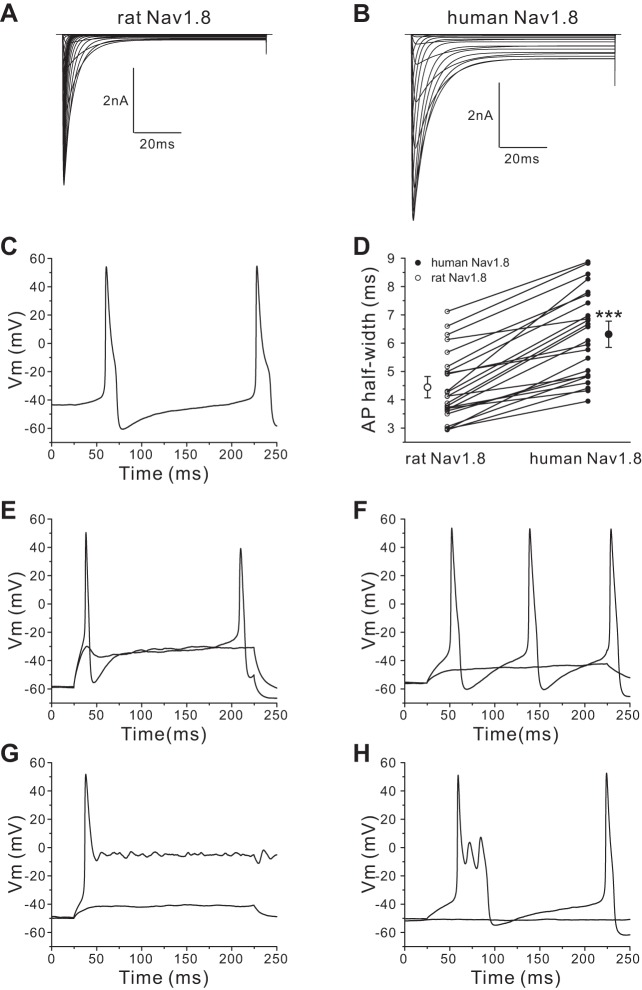
Using a 200-ms stimulus at the current threshold for individual neurons, we studied firing of Nav1.8-null DRG neurons transfected with rat Nav1.8 cDNA (42 neurons) or human Nav1.8 cDNA (45 neurons). DRG neurons expressing human Nav1.8 channels produced different firing patterns based on the width of the first action potential, which we grouped into type I, with a half-width shorter than 10 ms (Fig. 3A), and type II, with a half-width of longer than 10 ms (Fig. 3B). DRG neurons (36 of 36 cells) expressing rat Nav1.8 channels demonstrated only a type I firing pattern with a narrow action potential spike in response to 200-ms stimuli. In contrast, DRG neurons expressing human Nav1.8 channels demonstrated two firing patterns, types I (Fig. 3A) and II (Fig. 3B): 77% (20 of 26 cells) were type I, and 23% (6 of 26 cells) were type II. As Fig. 3C shows, some type II neurons transfected with human Nav1.8 display extremely prolonged action potentials with a plateau around 0 mV. In addition, we observed that 40% (8 of 20 cells) of type I DRG neurons transfected with human Nav1.8 channels demonstrated burstlike responses in which the first response to 200-ms stimuli was composed of multiple spikes (Fig. 3D). However, there were no DRG neurons transfected with rat Nav1.8 channels that demonstrated burst firing in type I neurons or a type II firing pattern. In addition, we compared the average half-width of action potentials (using the first spike for both types) between DRG neurons transfected with human Nav1.8 channels and DRG neurons transfected with rat Nav1.8 channels. The half-width of the DRG neurons transfected with human Nav1.8 channels (8.69 ± 1.51 ms, n = 26) was significantly larger than that of DRG neurons transfected with rat Nav1.8 channels (5.13 ± 0.26 ms, n = 36; P < 0.01).
Fig. 3.
DRG neurons transfected with human Nav1.8 channels generate different firing patterns. A: representative type I response: the response to a 200-ms depolarizing stimulus has only 1 single spike, and the action potential half-width is smaller than 10 ms. Vm, membrane voltage. B and C: representative type II response: half-width of the first action potential larger than 10 ms (B) and extremely long action potential (C). Inset in C shows the full length of this extremely broad action potential. D: representative bursting firing response: the first all-or-none response has a burst of multiple spikes.
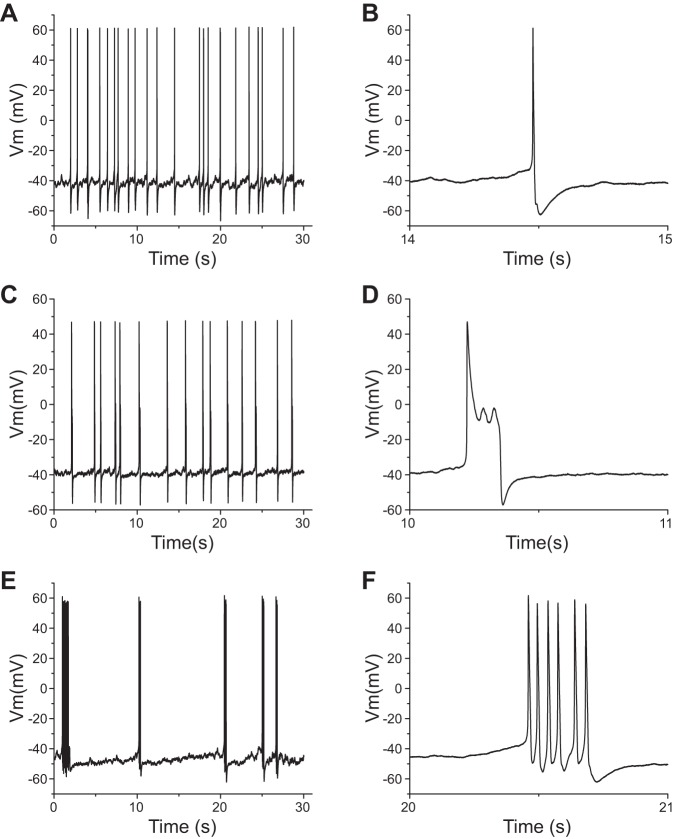
The expression of human Nav1.8 channels produced a significant increase in the proportion of spontaneously firing DRG neurons. Six of 42 (14%) recorded DRG neurons expressing rat Nav1.8 channels displayed spontaneous firing. In contrast, nearly three times more DRG neurons transfected with human Nav1.8 channels were spontaneously active (42%, 19 of 45 neurons; P < 0.01). Moreover, as for non-spontaneously active DRG neurons, spontaneously firing DRG neurons expressing human Nav1.8 channels also displayed different firing patterns (Fig. 4). The percentages for type I (Fig. 4, A and B) and II (Fig. 4, C and D) firing patterns were 84% (16 of 19 neurons) and 16% (3 of 19 neurons), respectively. Among 16 type I DRG neurons, 5 cells displayed burst firing (Fig. 4, E and F). In contrast, spontaneously firing DRG neurons expressing rat Nav1.8 channels demonstrated only a type I firing pattern, with no burstlike firing.
Fig. 4.
Spontaneously active DRG neurons transfected with human Nav1.8 channels display different firing patterns. A, C, and E: representative spontaneous type I (A), type II (C), and bursting (E) firing. B, D, and F: portions of the traces from the 30-s trace displayed on an expanded timescale for traces shown in A (B), C (D), and E (F).
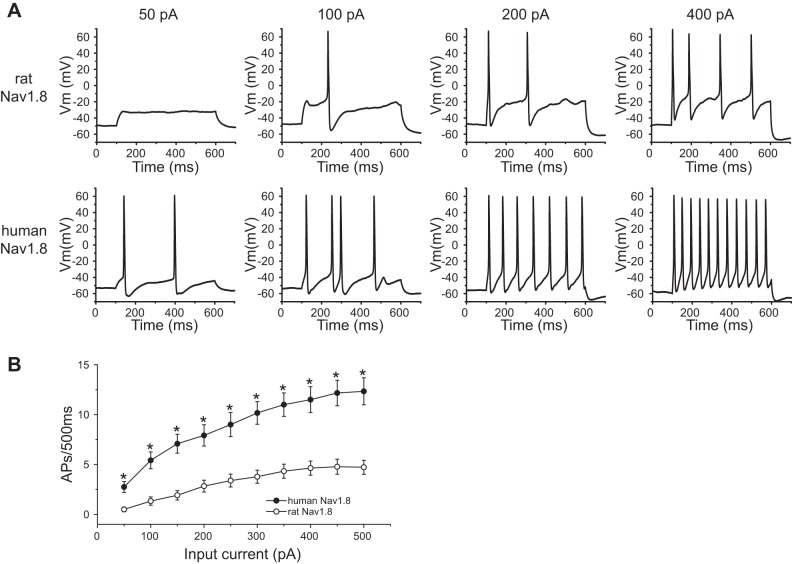
We also compared the evoked responses to a series of 500-ms depolarizing stimuli of different magnitudes (50–500 pA) between DRG neurons expressing human Nav1.8 channels and DRG neurons expressing rat Nav1.8 channels. Since DRG neurons expressing rat Nav1.8 channels generate only type I action potentials, we confined this analysis to DRG neurons expressing human Nav1.8 channels that displayed type I firing. Figure 5A shows the responses of representative DRG neurons that expressed human and rat Nav1.8 channels to 500-ms steps at 50-, 100-, 200-, and 400-pA current stimuli. DRG neurons expressing human Nav1.8 channels generated significantly more action potentials over the stimulation range from 50 to 500 pA (Fig. 5B).
Fig. 5.
For DRG neurons displaying type I evoked firing, neurons expressing human Nav1.8 channels are more excitable than neurons expressing rat Nav1.8 channels. A: responses of representative DRG neurons expressing rat (top) and human (bottom) Nav1.8 channels to 50-pA, 100-pA, 200-pA, and 400-pA (from left to right), 500-ms depolarization current injections. B: comparison of responses (number of impulses evoked by a 500-ms stimulus) in DRG neurons expressing human (n = 26) or rat (n = 36) Nav1.8 channels across a range of step current injections from 50 to 500 pA. *P < 0.05.
Dynamic Clamp Recordings Confirm that Human Nav1.8 Channel Contributes to the Broad Action Potential
Our results indicate that for the population of transfected mouse DRG neurons, human Nav1.8 produces a larger persistent current compared with neurons transfected with rat Nav1.8 channels and suggest that this results in action potentials with longer duration, a more pronounced inflection on the falling phase, and type II action potentials with a plateaulike falling phase. Since these results were obtained in transfected cells where it is not possible to control the expression level, we also examined action potential electrogenesis using dynamic clamp recording where we can precisely titrate the level of human and rat Nav1.8 expression. Dynamic clamp recording allowed us to compare, in the same cell, the effects of addition of a physiological level of rat Nav1.8 conductance versus human Nav1.8 conductance; the current amplitude (∼6 nA) injected by the dynamic clamp was adjusted to be the mean level of conductance observed after transfection of human Nav1.8 in Nav1.8-null mouse DRG neurons of <25-μm diameter, which is comparable to the average Nav1.8 current amplitude that we have previously measured in wild-type mouse DRG neurons (Shields et al. 2012).
For dynamic clamp recording, a single neuron from a Nav1.8-null mouse was endowed sequentially with 100% human Nav1.8 channel conductance or 100% rat Nav1.8 channel conductance. First we performed in silico evaluation of human and rat Nav1.8 channels. Figure 6, A and B, show computer simulation of current traces obtained from the rat (Fig. 6A) and human (Fig. 6B) Nav1.8 channel model INa = gmax·m3·h·(Vm − ENa) that we developed and used for the dynamic clamp recordings. Compared with modeled rat Nav1.8 channels, modeled human Nav1.8 channels clearly display larger persistent currents. We performed dynamic clamp recording with 200-ms-long stimulation (the same protocol as we used for our current-clamp recordings on transfected mouse DRG neurons) by injecting either human Nav1.8 conductance or rat Nav1.8 conductance. Among 39 cells we recorded, 9 cells (23%) displayed spontaneous firing after the injection of human Nav1.8 conductance (Fig. 6C); in contrast, no cells showed spontaneous firing after we injected rat Nav1.8 conductance. For the other 30 non-spontaneously active neurons, the RMP was not significantly different between the human Nav1.8 channel recordings (−55.3 ± 1.0 mV, n = 30) and rat Nav1.8 channel recordings (−55.3 ± 1.0 mV, n = 30; P > 0.05). However, the current threshold required to produce the first all-or-none action potential was significantly reduced after the injection of human Nav1.8 conductance (73 ± 7 pA, n = 30) compared with the injection of rat Nav1.8 conductance (129 ± 11 pA, n = 30; P < 0.001).
Fig. 6.
Dynamic clamp recording confirms the effect of human Nav1.8 on DRG neuron behavior. A and B: current traces obtained from rat (A) and human (B) Nav1.8 model INa = gmax·m3·h·(Vm − ENa). C: representative spontaneously firing action potential trace after input of human Nav1.8 channel conductance. D: comparison of action potential half-width between human Nav1.8 group and rat Nav1.8 group. ***P < 0.001. Action potential width in each neuron was measured after injection of human Nav1.8 conductance and subsequently in the same cell with injection of rat Nav1.8 conductance (open and filled symbols connected for each neuron). Two larger symbols indicate means ± SE. E and F: type I representative response to rat (E) or human (F) Nav1.8 conductance injection. G: type II representative response displaying extremely long action potential to human Nav1.8 conductance injection. H: type II response displaying early afterdepolarization-like action potential to human Nav1.8 conductance injection.
As described above, mouse DRG neurons transfected with rat Nav1.8 channels displayed only a type I firing pattern. Here, in the dynamic clamp recording, we also observed that DRG neurons demonstrated only type I firing behavior after the injection of rat Nav1.8 channel conductance. In contrast, after injection of human Nav1.8 channel conductance 80% (24 of 30 neurons) of DRG neurons displayed a type I firing pattern, while 20% (6 of 30 neurons) of DRG neurons produced type II broad action potentials. No burst firing behavior was observed. For each DRG neuron that displayed type I firing pattern, we compared the action potential width with input of human Nav1.8 conductance and with rat Nav1.8 conductance in the same cell. As Fig. 6D shows, the half-width of the action potential was significantly wider after inputting human Nav1.8 conductance (6.31 ± 0.31 ms, n = 24) compared with inputting rat Nav1.8 conductance (4.44 ± 0.25 ms, n = 24; P < 0.001). Figure 6, E and F, show representative type I action potential traces for the same DRG neuron after inputting rat (Fig. 6E) or human (Fig. 6F) Nav1.8 conductance. For the six DRG neurons that demonstrated type I action potentials after inputting rat Nav1.8 but type II broad action potentials after inputting human Nav1.8 conductance, five of six cells produced extremely prolonged action potentials that did not repolarize to RMP even after the 200-ms stimulation. The plateaus of responses generated by these five cells were around 0 mV. Figure 6G shows representative action potential traces recorded from one of these five cells. Figure 6H shows the action potential traces recorded from the sixth type II firing cell. The action potential configuration of this cell is similar to the action potential shape shown in Fig. 4D from a spontaneously active DRG neuron transfected with human Nav1.8, with one major spike and two minor spikes.
Human Nav1.8 Displays Larger Persistent Current and Ramp Current in Native Human DRG Neurons
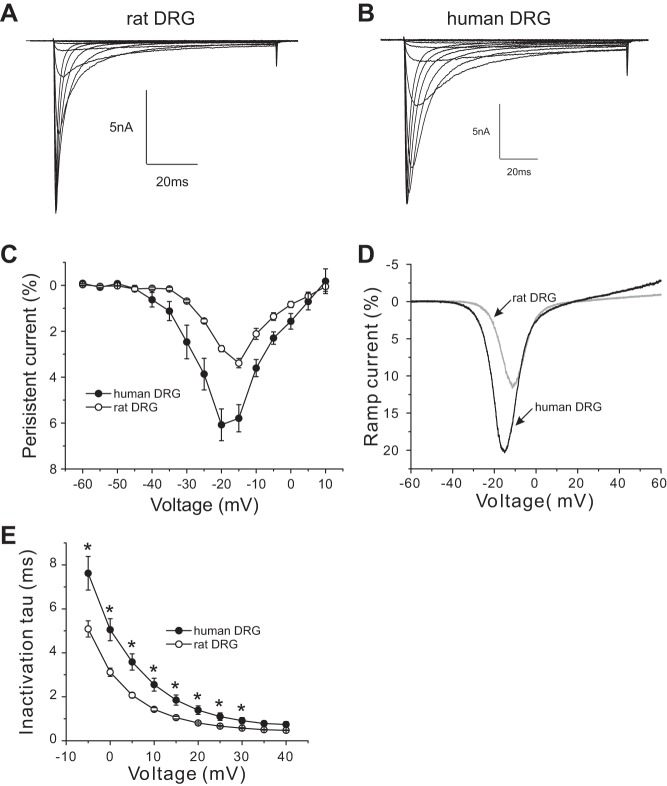
Our data above show that the human Nav1.8 channel displays larger persistent current and ramp current when assessed in transfected mouse DRG neurons. To determine whether Nav1.8 channels also produces a large persistent current or ramp current within native human DRG neurons, we performed voltage-clamp recording on acutely isolated human DRG neurons. As in the experiments described above, rat DRG neurons were used as a comparator. Figure 7, A and B, show representative Nav1.8 current traces recorded from rat and human DRG neurons, respectively. As Fig. 7C shows, compared with Nav1.8 channels of rat DRG neurons, Nav1.8 channels of human DRG neurons produce significantly larger persistent current between −40 mV and 0 mV. The normalized peak persistent current for Nav1.8 channels of human DRG neurons was 6.1 ± 0.7% (n = 9), which was ∼1.8-fold larger than that of Nav1.8 channels of rat DRG neurons (3.4 ± 0.2%, n = 19; P < 0.01) (Fig. 7C). We also measured the response to a slow ramp stimulus and show that Nav1.8 channels in human DRG neurons produce almost twofold larger ramp current (18.6 ± 1.7%, n = 8) compared with Nav1.8 channels in rat DRG neurons (9.8 ± 0.7%, n = 15; P < 0.001) (Fig. 7D). The rate of fast inactivation as a function of stimulus pulse potential is shown in Fig. 7E, which shows that the kinetics of Nav1.8 channel in human DRG neurons is significantly slower over the voltage range between −5 and +30 mV compared with Nav1.8 channel in rat DRG neurons.
Fig. 7.
Human DRG neurons demonstrate larger Nav1.8 persistent currents. A and B: representative Nav1.8 current family traces recorded from a rat DRG neuron (A) or a human DRG (B). Cells were held at −60 mV and stepped to a range of potentials (−60 to +40 mV in 5-mV increments) for 100 ms. C: comparison of the normalized amplitude of Nav1.8 persistent currents between rat DRG neurons and human Nav1.8 DRG neurons. D: representative ramp currents, elicited with 600-ms ramp depolarization from −60 mV to +60 mV, for Nav1.8 channel in a human and a rat DRG neuron. Nav1.8 channels in human DRG neurons produce a ramp current that is almost double that produced by Nav1.8 channels in rat DRG neurons. E: kinetics of open-state inactivation measured as a function of voltage for Nav1.8 channel in rat DRG neurons (n = 19) and in human DRG neurons (n = 9). Time constants were obtained by fitting currents elicited as described in A and B with a single-exponential function. *P < 0.05.
Human DRG Neurons Generate Broader Action Potentials
Our observation that human DRG neurons produce a larger Nav1.8 persistent current and ramp current, and slower kinetics of inactivation, suggested that the properties of action potentials generated by human DRG neurons might be different from those of rat DRG neurons. To test this hypothesis, we performed current-clamp recording from both rat and human DRG neurons.
Human DRG neurons manifested distinct properties compared with rat DRG neurons. The RMP for human DRG neurons was −55.0 ± 0.8 mV (n = 59), which was depolarized by 6 mV compared with the RMP of rat DRG neurons (−61.0 ± 1.9 mV, n = 30; P < 0.01). The input resistance of human DRG neurons (153 ± 20 MΩ, n = 55) was significantly smaller than that of rat DRG neurons (659 ± 51 MΩ, n = 30; P < 0.001). The action potential amplitude was different between human (111.7 ± 1.6 mV, n = 59) and rat (118.5 ± 1.8 mV, n = 30; P < 0.01) DRG neurons, consistent with the more depolarized RMP. The current threshold of human DRG neurons was 964 ± 85 pA (n = 59), which was significantly larger than that of rat DRG neurons (251 ± 29 pA, n = 30; P < 0.001), consistent with the smaller input resistance of human DRG neurons.
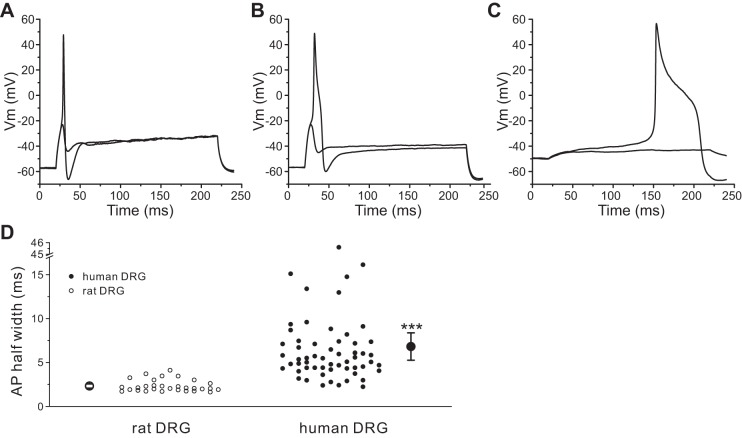
Figure 8A shows representative action potentials recorded from a rat DRG neuron. All of the rat DRG neurons that we recorded (30 of 30 cells) displayed only type I firing pattern. In contrast, 90% (53 of 59 cells) of human DRG neurons demonstrated a type I firing pattern (Fig. 8B), while 10% (6 of 59 cells) of human DRG neurons demonstrated a type II firing pattern with prolonged action potentials. Figure 8C shows the prolonged action potential recorded from one of these human DRG neurons. We also measured the duration of the action potential (Fig. 8D). For human DRG neurons, the mean half-width of the action potential was 6.83 ± 0.78 ms (n = 59), which is threefold larger than that of rat DRG neurons, where it was 2.33 ± 0.12 ms (n = 30; P < 0.001) (Fig. 8D). A higher proportion of native human DRG neurons (18 of 77 cells, 23.4%) displayed spontaneous firing compared with rat DRG neurons (2 of 32 cells, 6.3%; P < 0.05).
Fig. 8.
Human DRG neurons generate broader action potentials. A and B: representative action potential traces recorded from a rat (A) or a human (B) DRG neuron. Action potentials were elicited by 200-ms step depolarizing current injections from resting membrane potential. C: action potential traces recorded from a human DRG neuron with prolonged duration. D: comparison of the half-width of action potential between rat and human DRG neurons. Two larger symbols indicate means ± SE. ***P < 0.001.
DISCUSSION
In this study, we demonstrate differences in gating of human Nav1.8 compared with rat Nav1.8 channels, paralleled by different action potential properties of human DRG neurons compared with rat DRG neurons. We demonstrate two- to threefold larger persistent current and ramp current and slower kinetics of fast inactivation in human Nav1.8 channels, both in native human DRG and in transfected mouse DRG neurons. We demonstrate significantly broader action potentials in human DRG neurons, with an average half-width that is nearly threefold longer compared with rat DRG neurons. Nav1.8-null DRG neurons that express human Nav1.8 channels, or are endowed with the same conductance level of human Nav1.8 current by dynamic clamp, fire action potentials that are unexpectedly long-lasting, similar to those produced by native human DRG neurons.
The basic biophysical properties of native human and rodent DRG neurons show large and statistically significant differences that are predicted to alter excitability of these neurons. The mean input resistance (153 ± 20 MΩ) and action potential threshold (964 ± 85 pA) of human DRG neurons are different from the input resistance (659 ± 51 MΩ) and threshold for action potential (251 ± 29 pA) for native rat DRG neurons but are comparable to those reported by Davidson et al. (2014) from a different set of human donors (input resistance: 97.51 ± 10.09 MΩ; threshold: 1,430 ± 110 pA), confirming that these properties are intrinsic to human DRG neurons. The action potential width in human DRG neurons (6.83 ± 0.78 ms) was threefold greater than that of rat DRG neurons (2.33 ± 0.12 ms), and human DRG neurons manifested increased firing frequencies compared with rat DRG neurons. Despite higher threshold for the initial spike in human DRG neurons, which is attributable at least in part to the lower input resistance in these neurons, they fire more action potentials once they reach that threshold. These data demonstrate clear species-specific differences in firing properties of DRG neurons, consistent with species-specific differences in ion channel conductances that underlie electrogenesis in these neurons.
Although multiple ion conductances contribute to electrogenesis in human and rodent DRG neurons, Nav1.8 has been shown to be a major contributor to the action potential upstroke in these neurons and is essential for repetitive firing (Blair and Bean 2002; Renganathan et al. 2001). The slower kinetics of inactivation and the twofold increase in ramp and persistent currents of human Nav1.8 channels compared with rat Nav1.8 channels suggest that this channel is a major contributor to regulating action potential width and the increase in firing frequency in native human DRG neurons. Several factors may contribute to this species-specific difference in Nav1.8 properties, including the substantial divergence of the primary sequence of the two channels (83% identical) and a possible difference in the distribution and affinity of interaction with channel partners, including β-subunits. It is important to note that the main differences between human and rodent Nav1.8 were recapitulated when the channels were expressed in the Nav1.8-null DRG neurons, suggesting a more prominent effect of divergent sequence that may be reflected in altered channel folding or in altering the affinity of interaction with auxiliary proteins. Although we cannot exclude a contribution of β-subunits or other channel partners to the different properties of human and rat Nav1.8 channels, our data are more consistent with a major role of intrinsic Nav1.8 gating properties in conferring species-specific differences in excitability of DRG neurons.
The Nav1.8 persistent current in native human DRG neurons (6.1%) is smaller than the human Nav1.8 persistent current recorded in transfected rodent DRG neurons (12.3%), but the molecular basis for the smaller persistent current in native human DRG neurons is not clear. The persistent current in native rat DRG neurons (3.4% of peak current) is comparable to the rat Nav1.8 persistent current recorded in transfected rodent DRG neurons (4.3% of peak current), suggesting that overexpression of Nav1.8 channels per se would not cause the large persistent human Nav1.8 current in these neurons. One possibility is that the modulation of Nav1.8 channels differs in different cell backgrounds. Other possibilities include interindividual variability in the expression levels of Nav1.8 among the different donors or donor-specific differences in Nav1.8, for example, single-nucleotide polymorphisms that may impact channel function.
Persistent and ramp sodium currents have been shown to control the excitability of many different cell types (Bennett et al. 2000; Brumberg et al. 2000; Crill et al. 1996; Cummins et al. 1998; Enomoto et al. 2006; Fleidervish and Gutnick 1996; Golomb et al. 2006; Kuo et al. 2006; Lamas et al. 2009; Vervaeke et al. 2006; Wu et al. 2005). Our observation that the persistent and ramp currents are severalfold larger for human Nav1.8 compared with rat Nav1.8 suggests that these aspects of Nav1.8 play an important role in controlling the excitability of DRG neurons. First, our observations indicate that enhanced persistent and ramp currents of human Nav1.8 channels are associated with reduced current threshold and increased firing frequency in transfected DRG neurons. It is interesting in this regard that mutations of human Nav1.8 that increase the ramp current reduce threshold in DRG neurons (Faber et al. 2012; Huang et al. 2013). Second, we found that DRG neurons display different firing patterns after expression of human Nav1.8 channels compared with rat Nav1.8 channels. Previously, Theiss et al. (2007) reported that persistent sodium currents also play a major role in determining firing patterns in rat ventral horn spinal interneurons. In that study, cells with repetitive-firing patterns had significantly larger sodium persistent currents, and treatment with riluzole, which is known to reduce the persistent currents, changed repetitive or burst firing into a single-spiking pattern. Third, we found that human native human DRG neurons, Nav1.8-transfected DRG neurons, and DRG neurons after input of human Nav1.8 conductance via dynamic clamp all display prolonged action potentials. This result is in agreement with previous reports that persistent sodium currents maintain prolonged action potentials in other types of neurons (Fleidervish and Gutnick 1996; Maltsev et al. 2007; Song et al. 2006). It is also supported by the observation (Mantegazza et al. 1998) that during perfusion with ATXII, a toxin that is known to increase sodium persistent currents, neocortical pyramidal neurons display a metastable condition characterized by very long-lasting plateau action potentials. We in fact observed these “long-lasting” plateau action potentials within DRG neurons endowed with human Nav1.8 currents (Fig. 3C and Fig. 6G). The plateaus of these prolonged action potentials occur close to 0 mV, a potential that coincides with the peak of the Nav1.8 persistent sodium current, around 0 mV, as observed in voltage-clamp recordings. Fourth, our observations suggest that the enhanced human Nav1.8 persistent current may also contribute to the spontaneous activity of DRG neurons. We found that native human DRG neurons and DRG neurons transfected with human Nav1.8 channels or after input of human Nav1.8 conductance all demonstrate enhanced levels of spontaneous firing activity. In this regard, it is notable that spontaneous activity in injured DRG neurons is inhibited by riluzole (Xie et al. 2011). These observations provide evidence that intrinsic biophysical properties of Nav1.8 channels contribute to regulation of firing in human native DRG neurons, and to the marked difference in the firing of human and rodent DRG neurons.
NGF and GDNF have been known to play important roles in the regulation of sodium channel expression in DRG neurons. In rat DRG neurons, NGF has been shown to increase Nav1.8 expression and affect the electrophysiological properties of DRG neurons (Fang et al. 2005; Fjell et al. 1999). GDNF has also been shown to significantly increase Nav1.8 currents in rat DRG neurons (Cummins et al. 2000; Fjell et al. 1999). However, these effects are generally seen in DRG neurons in culture for several days. As described in materials and methods, our preliminary experiments indicated that supplements of these trophic factors were necessary to obtain viable human DRG neurons for recordings, in agreement with recently published data (Davidson et al. 2014); thus both NGF and GDNF were added into the primary human DRG culture. Therefore it is possible that differences in exposure to NGF and/or GDNF could have contributed to the different firing behavior that we observed between human and rat DRG neurons. We observed, in Nav1.8-null neurons treated with NGF and GDNF and transfected with rat versus human Nav1.8, a pattern of differences in firing properties that paralleled the differences between native human and rodent DRG neurons, suggesting a minimal effect of the acute application of these factors in our culture system. Our data, however, do not formally preclude an effect of NGF and/or GDNF on Nav1.8 expression or modulation that may have contributed to the different firing behavior that we observed between human and rat DRG neurons.
Although it is possible that production of bigger current by transfected human Nav1.8 channels compared with rat Nav1.8 channels could contribute to the different firing patterns of DRG neurons expressing these channels, our voltage-clamp data show comparable levels of human Nav1.8 and rat Nav1.8 current densities. Since persistent current levels were normalized to peak currents, the increased persistent current of human Nav1.8 reflects a difference in the gating properties of the channel, and the effect on neuronal firing cannot be attributed to higher levels of expression of human Nav1.8 channels. Our dynamic clamp recording (Kemenes et al. 2011; Samu et al. 2012; Sharp et al. 1993; Vasylyev et al. 2014) permitted us to more directly examine the effect on the same DRG neuron of precisely calibrated levels of human or rat Nav1.8 channel currents. Our dynamic clamp recordings in DRG neurons recapitulate the differential effects of human Nav1.8 channels compared with rat Nav1.8 channels on firing properties of DRG neurons, further supporting the conclusion that the unique properties of human Nav1.8 channels shape the pattern of DRG neuron firing.
A role of Nav1.8 in human pain has been demonstrated by the link of Nav1.8 mutations to human pain syndromes (Faber et al. 2012), validating this channel as a target for pain treatment. Our results show that human Nav1.8 channels produce a large persistent current that contributes to determining the firing patterns of DRG neurons. Nav1.8 is known to be present in the central portion of primary afferent axons in the dorsal horn (Amaya et al. 2000), and functional Nav1.8 channels are known to be present along the neurites of DRG neurons in vitro (Vasylyev and Waxman 2012). It has been demonstrated that in some presynaptic terminals persistent currents can modulate transmitter release (Engel and Jonas 2005; Huang and Trussell 2008). Thus it is reasonable to suggest that the presence of Nav1.8 in or close to the central terminals of DRG neurons may have an effect on transmitter release in the dorsal horn, with enhanced synaptic transmission at the terminals of C-type cells. Whether this occurs within the human dorsal horn remains to be determined. Notwithstanding this uncertainty, the distinct properties of human Nav1.8 and the distinct properties of action potentials in human DRG neurons compared with rodent DRG neurons should be taken into account when extrapolating from rodent studies of pain to humans and testing novel blockers for treatment of pain.
GRANTS
This work was supported in part by grants from the Rehabilitation Research Service and Medical Research Service, Department of Veterans Affairs (S. G. Waxman and S. D. Dib-Hajj). The Center for Neuroscience and Regeneration Research is a Collaboration of the Paralyzed Veterans of America with Yale University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H., S.D.D.-H., and S.G.W. conception and design of research; C.H., M.E., J.H., D.V.V., and P.Z. performed experiments; C.H., M.E., and J.H. analyzed data; C.H., M.E., J.H., S.D.D.-H., and S.G.W. interpreted results of experiments; C.H. prepared figures; C.H., S.D.D.-H., and S.G.W. drafted manuscript; C.H., S.D.D.-H., and S.G.W. edited and revised manuscript; C.H., M.E., J.H., D.V.V., P.Z., S.D.D.-H., and S.G.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lawrence J. Macala, Shujun Liu, Fadia Dib-Hajj, and Palak Shah for technical assistance. We thank Dr. Xiaoyang Cheng and Dr. Yang Yang for valuable comments.
REFERENCES
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379: 257–262, 1996. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2: 541–548, 1999. [DOI] [PubMed] [Google Scholar]
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci 15: 331–342, 2000. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci 20: 8493–8503, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 22: 10277–10290, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci 20: 4829–4843, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005. [DOI] [PubMed] [Google Scholar]
- Christe G, Chahine M, Chevalier P, Pasek M. Changes in action potentials and intracellular ionic homeostasis in a ventricular cell model related to a persistent sodium current in SCN5A mutations underlying LQT3. Prog Biophys Mol Biol 96: 281–293, 2008. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci 20: 8754–8761, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19: RC43, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Howe JR, Waxman SG. Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci 18: 9607–9619, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau RW 4th. Human sensory neurons: membrane properties and sensitization by inflammatory mediators. Pain 155: 1861–1870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Choi JS, Macala LJ, Tyrrell L, Black JA, Cummins TR, Waxman SG. Transfection of rat or mouse neurons by biolistics or electroporation. Nat Protoc 4: 1118–1126, 2009. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Cummins TR, Black JA, Wood PM, Waxman SG. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett 462: 117–120, 1999. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45: 405–417, 2005. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci 26: 3412–3422, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, Persson AK, Hoeijmakers JG, Gerrits MM, Pierro T, Lombardi R, Kapetis D, Dib-Hajj SD, Waxman SG. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci USA 109: 19444–19449, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci 25: 4868–4878, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52: 767–774, 2006. [DOI] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res 67: 267–282, 1999. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J Neurophysiol 76: 2125–2130, 1996. [DOI] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol 96: 1912–1926, 2006. [DOI] [PubMed] [Google Scholar]
- Han C, Vasylyev D, Macala LJ, Gerrits MM, Hoeijmakers JG, Bekelaar KJ, Dib-Hajj SD, Faber CG, Merkies IS, Waxman SG. The G1662S NaV1.8 mutation in small fibre neuropathy: impaired inactivation underlying DRG neuron hyperexcitability. J Neurol Neurosurg Psychiatry 85: 499–505, 2014. [DOI] [PubMed] [Google Scholar]
- Huang H, Trussell LO. Control of presynaptic function by a persistent Na+ current. Neuron 60: 975–979, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yang Y, Zhao P, Gerrits MM, Hoeijmakers JG, Bekelaar K, Merkies IS, Faber CG, Dib-Hajj SD, Waxman SG. Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J Neurosci 33: 14087–14097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes I, Marra V, Crossley M, Samu D, Staras K, Kemenes G, Nowotny T. Dynamic clamp with StdpC software. Nat Protoc 6: 405–417, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T. Persistent Na-channels: origin and function. A review. Acta Biol Hung 59, Suppl: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas JA, Romero M, Reboreda A, Sanchez E, Ribeiro SJ. A riluzole- and valproate-sensitive persistent sodium current contributes to the resting membrane potential and increases the excitability of sympathetic neurones. Pflügers Arch 458: 589–599, 2009. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9: 219–227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Franceschetti S, Avanzini G. Anemone toxin (ATX II)-induced increase in persistent sodium current: effects on the firing properties of rat neocortical pyramidal neurones. J Physiol 507: 105–116, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629–640, 2001. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol 72: 2796–2815, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint DA. The cardiac persistent sodium current: an appealing therapeutic target? Br J Pharmacol 153: 1133–1142, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samu D, Marra V, Kemenes I, Crossley M, Kemenes G, Staras K, Nowotny T. Single electrode dynamic clamp with StdpC. J Neurosci Methods 211: 11–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem 271: 5953–5956, 1996. [DOI] [PubMed] [Google Scholar]
- Serrano A, Mo G, Grant R, Pare M, O'Donnell D, Yu XH, Tomaszewski MJ, Perkins MN, Seguela P, Cao CQ. Differential expression and pharmacology of native P2X receptors in rat and primate sensory neurons. J Neurosci 32: 11890–11896, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AA, O'Neil MB, Abbott LF, Marder E. Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol 69: 992–995, 1993. [DOI] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153: 2017–2030, 2012. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–222, 2006. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr 7: 15–22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurones. J Physiol 580: 507–522, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeyev AY, Hackman JC, Wood PM, Davidoff RA. Pharmacologically novel GABA receptor in human dorsal root ganglion neurons. J Neurophysiol 76: 3555–3558, 1996. [DOI] [PubMed] [Google Scholar]
- Vasylyev DV, Han C, Zhao P, Dib-Hajj S, Waxman SG. Dynamic-clamp analysis of wild-type human Nav1.7 and erythromelalgia mutant channel L858H. J Neurophysiol 111: 1429–1443, 2014. [DOI] [PubMed] [Google Scholar]
- Vasylyev DV, Waxman SG. Membrane properties and electrogenesis in the distal axons of small dorsal root ganglion neurons in vitro. J Neurophysiol 108: 729–740, 2012. [DOI] [PubMed] [Google Scholar]
- Veeramah KR, O'Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, Talwar D, Girirajan S, Eichler EE, Restifo LL, Erickson RP, Hammer MF. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet 90: 502–510, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Hu H, Graham LJ, Storm JF. Contrasting effects of the persistent Na+ current on neuronal excitability and spike timing. Neuron 49: 257–270, 2006. [DOI] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic V neurons participate in burst generation and control of membrane excitability. J Neurophysiol 93: 2710–2722, 2005. [DOI] [PubMed] [Google Scholar]
- Xie RG, Zheng DW, Xing JL, Zhang XJ, Song Y, Xie YB, Kuang F, Dong H, You SW, Xu H, Hu SJ. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PloS One 6: e18681, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858, 2007. [DOI] [PubMed] [Google Scholar]