Abstract
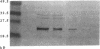
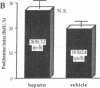
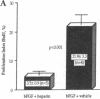
Heparin inhibits smooth muscle cell (SMC) proliferation after arterial injury by mechanisms that have yet to be defined. Since the initiation of SMC proliferation is mediated by basic fibroblast growth factor (bFGF), we have investigated the possibility that heparin inhibits SMC proliferation by displacing bFGF from the arterial wall. Using a rat carotid artery model of balloon catheter injury, we demonstrate that a bolus injection of heparin depletes the arterial wall of both systemically administered bFGF and of endogenous bFGF. Heparin, however, does not reduce the bFGF content of unmanipulated arteries. Further, a single injection of heparin given at the time of balloon injury reduces SMC proliferation by 55% but has no effect when given 6 h after injury. SMC proliferation induced in a denuded artery by injection of bFGF is inhibited almost completely by a bolus injection of heparin; however, pretreatment with a bolus of heparin does not prevent SMC from responding to a subsequent bolus of bFGF. These experiments suggest that heparin can inhibit SMC proliferation in part by removal of released bFGF from sites of injury.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Meklir B., Joyce N. C. In situ injury-induced release of basic-fibroblast growth factor from corneal epithelial cells. Am J Pathol. 1991 Nov;139(5):961–967. [PMC free article] [PubMed] [Google Scholar]
- Au Y. P., Kenagy R. D., Clowes A. W. Heparin selectively inhibits the transcription of tissue-type plasminogen activator in primate arterial smooth muscle cells during mitogenesis. J Biol Chem. 1992 Feb 15;267(5):3438–3444. [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Bârzu T., Van Rijn J. L., Petitou M., Molho P., Tobelem G., Caen J. P. Endothelial binding sites for heparin. Specificity and role in heparin neutralization. Biochem J. 1986 Sep 15;238(3):847–854. doi: 10.1042/bj2380847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wright T. C., Karnovsky M. J. Regulation of vascular smooth muscle cell growth by heparin and heparan sulfates. Semin Thromb Hemost. 1987 Oct;13(4):489–503. doi: 10.1055/s-2007-1003525. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Dryjski M., Mikat E., Bjornsson T. D. Inhibition of intimal hyperplasia after arterial injury by heparins and heparinoid. J Vasc Surg. 1988 Nov;8(5):623–633. doi: 10.1067/mva.1988.avs0080623. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Moscatelli D., Saksela O., Rifkin D. B. Role of extracellular matrix in the action of basic fibroblast growth factor: matrix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J Cell Physiol. 1989 Jul;140(1):75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hardonk M. J., Harms G. The use of 5'-bromodeoxyuridine in the study of cell proliferation. Acta Histochem Suppl. 1990;39:99–108. [PubMed] [Google Scholar]
- Hondermarck H., Courty J., Boilly B., Thomas D. Distribution of intravenously administered acidic and basic fibroblast growth factors in the mouse. Experientia. 1990 Sep 15;46(9):973–974. doi: 10.1007/BF01939392. [DOI] [PubMed] [Google Scholar]
- Kent K. M., Bentivoglio L. G., Block P. C., Bourassa M. G., Cowley M. J., Dorros G., Detre K. M., Gosselin A. J., Gruentzig A. R., Kelsey S. F. Long-term efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA Registry. Am J Cardiol. 1984 Jun 15;53(12):27C–31C. doi: 10.1016/0002-9149(84)90741-0. [DOI] [PubMed] [Google Scholar]
- Leimgruber P. P., Roubin G. S., Hollman J., Cotsonis G. A., Meier B., Douglas J. S., King S. B., Jr, Gruentzig A. R. Restenosis after successful coronary angioplasty in patients with single-vessel disease. Circulation. 1986 Apr;73(4):710–717. doi: 10.1161/01.cir.73.4.710. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Schwartz S. M., Clowes M. M., Clowes A. W. Heparin regulates smooth muscle S phase entry in the injured rat carotid artery. Circ Res. 1987 Aug;61(2):296–300. doi: 10.1161/01.res.61.2.296. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan L., Warder E., McNeil P. L. Basic fibroblast growth factor is efficiently released from a cytolsolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991 Jul;148(1):1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Olson N. E., Chao S., Lindner V., Reidy M. A. Intimal smooth muscle cell proliferation after balloon catheter injury. The role of basic fibroblast growth factor. Am J Pathol. 1992 May;140(5):1017–1023. [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Heparin inhibition of smooth muscle cell proliferation: a cellular site of action. J Cell Physiol. 1986 Oct;129(1):11–19. doi: 10.1002/jcp.1041290103. [DOI] [PubMed] [Google Scholar]
- Reilly T. M., Taylor D. S., Herblin W. F., Thoolen M. J., Chiu A. T., Watson D. W., Timmermans P. B. Monoclonal antibodies directed against basic fibroblast growth factor which inhibit its biological activity in vitro and in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):736–743. doi: 10.1016/0006-291x(89)91521-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. W., Whalen G. F., Saunders K. B., Hores T., D'Amore P. A. Heparin-mediated release of fibroblast growth factor-like activity into the circulation of rabbits. Growth Factors. 1990;3(3):221–229. doi: 10.3109/08977199009043906. [DOI] [PubMed] [Google Scholar]
- Whalen G. F., Shing Y., Folkman J. The fate of intravenously administered bFGF and the effect of heparin. Growth Factors. 1989;1(2):157–164. doi: 10.3109/08977198909029125. [DOI] [PubMed] [Google Scholar]