Abstract
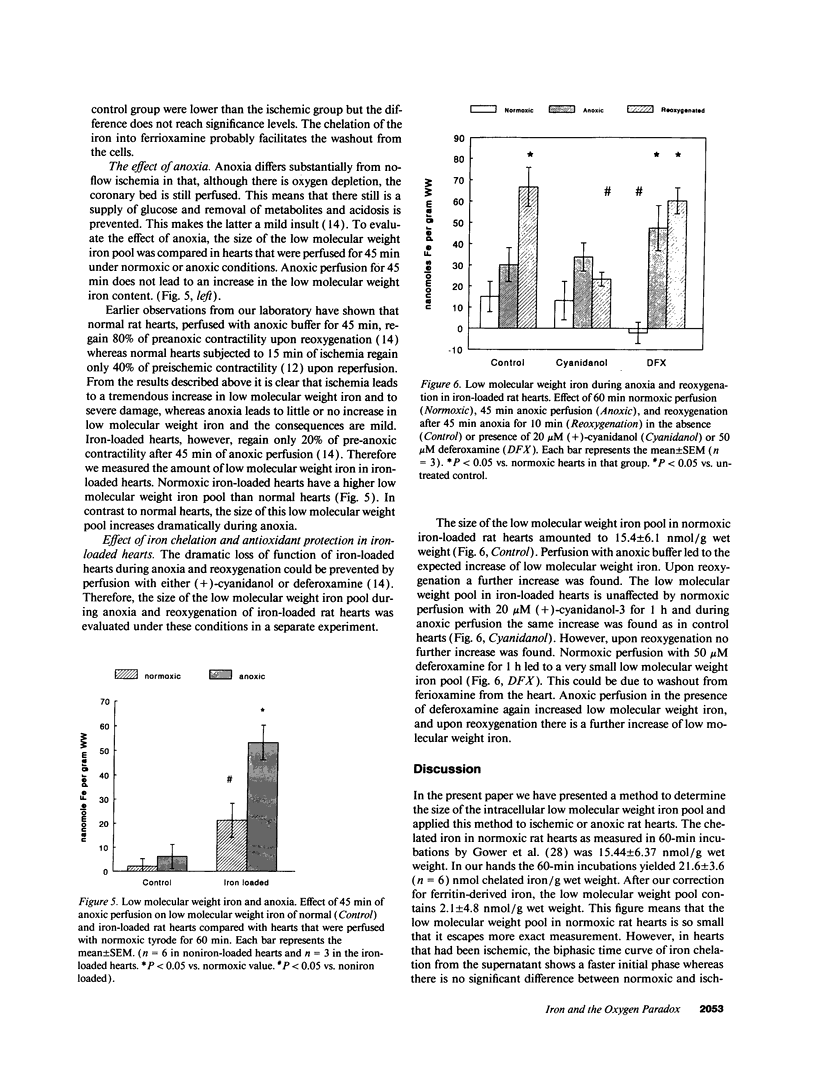
Little is known about changes in the amount of iron in the intracellular low molecular weight pool, which catalyzes the Fenton reactions during reperfusion after ischemia. In this study a new approach is presented to measure low molecular weight iron and it is applied to normal hearts during ischemia and to iron-loaded hearts during anoxia and reoxygenation. The results of this study show that (a) during ischemia in normal hearts a progressive 30-fold increase occurs in low molecular weight iron after 45 min of ischemia, whereas (b) during 45 min of anoxic perfusion the low molecular weight iron does not increase. This means that the reductive release from the storage protein ferritin is greatly enhanced by the acidification that occurs during ischemia. (c) Anoxic perfusion of iron-loaded hearts does increase low molecular weight iron and there is a further increase upon reoxygenation, which is prevented by (+)-cyanidanol-3. Based on these findings it is concluded that oxygen deprivation enhances the susceptibility of rat hearts to oxygen radicals by increasing the amount of catalytic, ferrous iron in the low molecular weight pool.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. On the limited ability of superoxide to release iron from ferritin. Eur J Biochem. 1990 Nov 13;193(3):899–904. doi: 10.1111/j.1432-1033.1990.tb19415.x. [DOI] [PubMed] [Google Scholar]
- Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991 Mar;5 (Suppl 2):249–268. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- Farber N. E., Vercellotti G. M., Jacob H. S., Pieper G. M., Gross G. J. Evidence for a role of iron-catalyzed oxidants in functional and metabolic stunning in the canine heart. Circ Res. 1988 Aug;63(2):351–360. doi: 10.1161/01.res.63.2.351. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Lewis C. A. Hydroxyl free radical formation from hydrogen peroxide by ferrous iron-nucleotide complexes. Biochemistry. 1983 May 24;22(11):2645–2649. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- Funk F., Lenders J. P., Crichton R. R., Schneider W. Reductive mobilisation of ferritin iron. Eur J Biochem. 1985 Oct 1;152(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Gower J. D., Healing G., Green C. J. Determination of desferrioxamine-available iron in biological tissues by high-pressure liquid chromatography. Anal Biochem. 1989 Jul;180(1):126–130. doi: 10.1016/0003-2697(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Healing G., Gower J., Fuller B., Green C. Intracellular iron redistribution. An important determinant of reperfusion damage to rabbit kidneys. Biochem Pharmacol. 1990 Apr 1;39(7):1239–1245. doi: 10.1016/0006-2952(90)90269-q. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Tosaki A. Free radicals and reperfusion-induced arrhythmias: protection by spin trap agent PBN in the rat heart. Circ Res. 1987 Mar;60(3):375–383. doi: 10.1161/01.res.60.3.375. [DOI] [PubMed] [Google Scholar]
- Holt S., Gunderson M., Joyce K., Nayini N. R., Eyster G. F., Garitano A. M., Zonia C., Krause G. S., Aust S. D., White B. C. Myocardial tissue iron delocalization and evidence for lipid peroxidation after two hours of ischemia. Ann Emerg Med. 1986 Oct;15(10):1155–1159. doi: 10.1016/s0196-0644(86)80857-5. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Komara J. S., Nayini N. R., Bialick H. A., Indrieri R. J., Evans A. T., Garritano A. M., Hoehner T. J., Jacobs W. A., Huang R. R., Krause G. S. Brain iron delocalization and lipid peroxidation following cardiac arrest. Ann Emerg Med. 1986 Apr;15(4):384–389. doi: 10.1016/s0196-0644(86)80171-8. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Chambers S., Hoffbrand A. V. Comparative study of iron mobilization from haemosiderin, ferritin and iron(III) precipitates by chelators. Biochem J. 1987 Jan 1;241(1):87–92. doi: 10.1042/bj2410087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G. Ferritin, a physiological iron donor for microsomal lipid peroxidation. FEBS Lett. 1986 Apr 7;199(1):85–88. doi: 10.1016/0014-5793(86)81228-5. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mulligan M., Althaus B., Linder M. C. Non-ferritin, non-heme iron pools in rat tissues. Int J Biochem. 1986;18(9):791–798. doi: 10.1016/0020-711x(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Reperfusion injury and its pharmacologic modification. Circulation. 1989 Oct;80(4):1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- Reddy B. R., Kloner R. A., Przyklenk K. Early treatment with deferoxamine limits myocardial ischemic/reperfusion injury. Free Radic Biol Med. 1989;7(1):45–52. doi: 10.1016/0891-5849(89)90099-3. [DOI] [PubMed] [Google Scholar]
- Sirivech S., Frieden E., Osaki S. The release of iron from horse spleen ferritin by reduced flavins. Biochem J. 1974 Nov;143(2):311–315. doi: 10.1042/bj1430311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosaki A., Blasig I. E., Pali T., Ebert B. Heart protection and radical trapping by DMPO during reperfusion in isolated working rat hearts. Free Radic Biol Med. 1990;8(4):363–372. doi: 10.1016/0891-5849(90)90102-o. [DOI] [PubMed] [Google Scholar]
- Vander Elst L., Goudemant J. F., Mouton J., Chatelain P., Van Haverbeke Y., Muller R. N. Amiodarone pretreatment effects on ischemic isovolumic rat hearts: a P-31 nuclear magnetic resonance study of intracellular pH and high-energy phosphates contents evolutions. J Cardiovasc Pharmacol. 1990 Mar;15(3):377–385. [PubMed] [Google Scholar]
- Vander Heide R. S., Sobotka P. A., Ganote C. E. Effects of the free radical scavenger DMTU and mannitol on the oxygen paradox in perfused rat hearts. J Mol Cell Cardiol. 1987 Jun;19(6):615–625. doi: 10.1016/s0022-2828(87)80367-x. [DOI] [PubMed] [Google Scholar]
- Weaver J., Zhan H., Pollack S. Mitochondria have Fe(III) receptors. Biochem J. 1990 Jan 15;265(2):415–419. doi: 10.1042/bj2650415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]
- van der Kraaij A. M., Mostert L. J., van Eijk H. G., Koster J. F. Iron-load increases the susceptibility of rat hearts to oxygen reperfusion damage. Protection by the antioxidant (+)-cyanidanol-3 and deferoxamine. Circulation. 1988 Aug;78(2):442–449. doi: 10.1161/01.cir.78.2.442. [DOI] [PubMed] [Google Scholar]
- van der Kraaij A. M., van Eijk H. G., Koster J. F. Prevention of postischemic cardiac injury by the orally active iron chelator 1,2-dimethyl-3-hydroxy-4-pyridone (L1) and the antioxidant (+)-cyanidanol-3. Circulation. 1989 Jul;80(1):158–164. doi: 10.1161/01.cir.80.1.158. [DOI] [PubMed] [Google Scholar]