Abstract
In this work, we studied autoantibody repertoires and Ig isotypes in 71 mothers and their 104 healthy newborns (including twins and triplets delivered term or premature). Newborns receive maternal IgG Abs via the placenta before birth, but developing infants must produce their own IgM and IgA Abs. We used an Ag microarray analysis to detect binding to a selection of 295 self-Ags, compared with 27 standard foreign Ags. The magnitude of binding to specific self-Ags was found to be not less than that to the foreign Ags. As expected, each newborn shared with its mother a similar IgG repertoire—manifest as early as the 24th week of gestation. IgM and IgA autoantibody repertoires in cord sera were highly correlated among the newborns and differed from their mothers’ repertoires; the latter differed in sera and milk. The autoantibodies bound to self-Ags known to be associated with tumors and to autoimmune diseases. Thus, autoantibody repertoires in healthy humans—the immunological homunculus—arise congenitally, differ in maternal milk and sera, and mark the potential of the immune system to attack tumors, beneficially, or healthy tissues, harmfully; regulation of the tissue site, the dynamics, and the response phenotype of homuncular autoimmunity very likely affects health.
Introduction
The immune system is a key player in body maintenance and defense, and its proper functioning is vital to the survival and well-being of the individual. The immune system is composed of complex networks of molecules and cells that act together to orchestrate the beneficial inflammation needed to maintain and repair the body as well as to protect it from neoplastic cells and invading pathogens (1–6). Abs binding to body molecules—autoantibodies—would appear to mark the self-reactivity needed for tissue healing (7) and for tumor surveillance (8); autoantibodies also mark autoimmune diseases (9). Thus, it would be important to investigate the characteristics of autoantibodies at birth in healthy humans as a starting point for subsequent evolution of the autoreactive repertoire.
The healthy newborn enters the environment armed with maternal IgG Abs actively transported across with placenta; after delivery the newborn receives mother’s secretory IgG, IgM, and IgA Abs from her colostrum. In addition, the healthy newborn produces IgM and IgA Abs in utero, detectable in cord blood. Previously, we studied sera obtained from 10 pairs of mothers and their newborns reactive to 295 defined self-Ags. In this work, we constructed a new Ag microarray chip that included a modified selection of 295 self-Ags along with 27 nonself Ags as a benchmark for the magnitude of Ab binding. We included a larger dataset of newborns (104), and their mothers (71), and we included premature births to learn whether gestational age might influence the newborn’s Ab repertoires. We also included twins and triplets to analyze the effects of close genetic similarity. Finally, we compared maternal milk samples with their corresponding serum samples. Inspired by the ideas of Jerne (10–12), we adopted a correlation-based system-level informatics approach to extract information about functional relations between Ag reactivities; we computed the matrices of subject correlations in addition to the reactivity matrices, as is usually done.
Materials and Methods
Serum samples
Blood samples were obtained by random availability from 71 healthy women at the onset or immediately after labor and from 104 serum samples of the cord blood of their newborns, in the neonatal department, Sheba Medical Center. All samples were collected with informed consent and approval of the Helsinki committee of the Sheba Medical Center. The newborns’ gestational age ranged from week 24 to 41. The newborns at the term of pregnancy (weeks 38–41; n = 31) were all normal in development and weight for their gestational age. The cord samples included 26 twins and 1 triplet. The blood samples were allowed to clot at room temperature. After centrifugation, sera were collected and stored at −20°C (13, 14).
Milk samples
Colostrum samples, along with matched serum samples, were obtained by random availability from 22 healthy women at the onset or up to 24 h after delivery, in the neonatal department, Sheba Medical Center. All samples were collected with informed consent and approval of the Helsinki committee of the Sheba Medical Center. The women were all healthy, and their newborns’ gestational age ranged from week 27 to 41. The soluble fraction of the milk was separated from the fat by centrifugation, collected, and stored at −20°C (13, 14).
Ags
A total of 322 Ags was spotted on each microarray, as described previously (9, 14). We used some of the same Ags as in the previous studies of healthy autoimmune repertoires (13, 14); these included proteins, synthetic peptides from the sequences of key proteins, nucleotides, phospholipids, and other self and nonself molecules. See Supplemental Table I for the full list.
Ag microarray
Ag microarrays were prepared, as described previously (9). Briefly, Ags were spotted in replicates of 4, and the microarrays were blocked for 1 h at 37°C with 1% BSA and incubated under a coverslip overnight at 4°C with a 1:10 dilution of the test serum in blocking buffer. The quantitative range of signal intensity of binding to each Ag spot was 0.01–65,000, and this range of detection made it possible to record reliable data with this low dilution of test samples. The arrays were then washed and incubated for 1 h at 37°C with a 1:500 dilution of detection Abs. Three detection Abs were used, as follows: a goat anti-human IgG Cy3-conjugated Ab, a goat anti-human IgM Cy5-conjugated Ab, and a goat anti-human IgA Cy5-conjugated Ab (all purchased from Jackson ImmunoResearch Laboratories, West Grove, PA). Each sample was analyzed using two microarray slides: one with the IgG and IgM fluorescence-labeled anti-isotypes, and one with the IgG and IgA fluorescence-labeled anti-isotypes. Thus, the IgG repertoires—which showed no significant difference between maternal and cord samples because maternal IgG Abs cross the placenta to the fetus (15)—served as controls for the maternal and cord IgM and IgA determinations measured simultaneously along with the IgG on each slide. These detection Abs could not distinguish between the particular isotype found in serum and the secretory isotype found in milk. Image acquisition by laser and quantification were performed, as previously described (9, 14).
Ethics statement
All procedures were performed in compliance with Tel Aviv University, Sackler School of Medicine guidelines, and all samples were collected with informed consent and approval of the Helsinki committee of the Sheba Medical Center.
Data preprocessing, background filtering, and analysis
Problematic spots due to smudges or grainy texture were removed manually upon inspection. We then subtracted the background from the foreground for each of the test spots. Ag reactivity was defined by the mean intensity of three replicates binding to that Ag on the microarray (the fourth replicate had to be removed due to a technical problem with the robot); Ag intensities with a mean value lower than zero were removed from further calculations. Each chip was then normalized by its mean reactivity divided by the SD. This was done to account for differences in total protein concentrations that affect the background intensity level. Analysis of the microarray data were done using GenePix Pro 7 Microarray Acquisition & Analysis Software. Statistical tests of significance were done using Statistics Toolbox functions. We have previously compared our microarray reactivities with a standard ELISA to heat shock protein 60 molecules and to other salient self-Ags and found that the microarray was at least one to two orders of magnitude more sensitive (16) (see Supplemental Table II); similar results have been reported by others (17) (see Supplemental Fig. 1 for additional details). For dimensional reduction we used the Principal Component Analysis (PCA) algorithm (18) on the normalized Pearson correlation matrices, as previously described (13). Note that subjects manifesting relatively high normalized correlations are closely located in the three-dimensional space. To retrieve information embedded in higher dimensions that might have been lost in the dimension reduction process, we linked each pair of nodes by lines colored according to their normalized correlations.
Results
Maternal serum and milk Ag reactivities
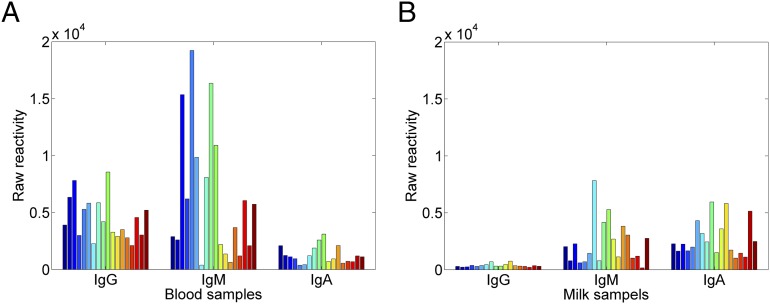
To characterize at a coarse level the isotypes of Abs in human milk and sera, we examined the Ab binding of 18 mothers’ milk samples and their matching serum samples to the 322 self and foreign Ags in the microarray (see Supplemental Table I for the full list). Fig. 1 shows the averaged reactivity (foreground–background) of each sample for each of the three isotypes; this provides an integrated overview of total reactivity of each isotype to all of the Ags on the microarray. It can be seen that the most reactive Ig isotypes in serum are IgG and IgM; the most reactive isotypes in milk are IgA and IgM (19). What are the Ag-binding specificities of the Abs present in mother’s serum and milk and in baby’s cord blood serum?
FIGURE 1.
Averaged isotype reactivity of individual maternal serum and milk integrated to all of the Ags on the microarrays. The averaged reactivity (foreground–background) (A) for the 18 serum and (B) corresponding milk samples observed for the three isotypes: IgG, IgM, and IgA. The different colors correspond to the different samples of milk and serum.
The most highly reactive Ags in milk and sera
To obtain a high-level view of the healthy repertoire, we focused on the most highly reactive and prevalent Abs. Using a relative binding threshold, we sorted the Abs from the most reactive to the least reactive determined by their amount of labeled second Ab binding; we then marked the top 5% as the most highly reactive Ags. To provide some measure of the prevalence of a given reactivity in the tested groups, we required that a highly reactive Ag had to be shared by at least 50% for the serum samples and 15% for the milk samples to enter the list; this percentage determination was chosen to avoid outliers, and as an indication of group robustness. Thus, the results reported in this work are restricted to the most reactive and abundant Abs in the repertoires of reactivity to our Ag set. Table I summarizes the highly reactive Ags of the three isotypes (IgG, IgM, and IgA) for both mothers and newborns (see Supplemental Tables 2 and 3 for additional comparisons). Note that both self and nonself Ags appear in the list.
Table I. Ags bound by highly reactive Abs in maternal milk and serum and in newborn cord serum.
| IgG |
IgM |
IgA |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Associations |
Serum |
Milk |
Serum |
Milk |
Serum |
Milk | |||||||
| Ags | Description | Cancer | Autoimmune Disease | M/C | M | C | M | C | |||||
| Viral/Bacterial | Diphtheria toxoid | 100% | 78% | 88% | 72% | 71% | 78% | ||||||
| HBcAg | Hepatitis B protein | 100% | 83% | 50% | 50% | 100% | 89% | ||||||
| HBsAg ayw | Hepatitis B surface Ag | 79% | 21% | 15% | |||||||||
| Varicella zoster virus | Herpes, Chickenpox | 90% | |||||||||||
| EBV EBNA1 | EBV | 94% | 78% | 22% | |||||||||
| West Nile | 66% | 15% | 15% | ||||||||||
| HSV-1 gG | Herpes virus | 86% | 39% | ||||||||||
| Vaccines | Influenza | 98% | 15% | 21% | |||||||||
| Pneumococcal saccharide | 79% | 21% | 82% | 37% | |||||||||
| Priorix tetra | 89% | 31% | |||||||||||
| Hormones | Atosiban | Inhibits oxytocin, vasopressin | Inhibits cell growth (24) | 22% | |||||||||
| G-CSF | G-CSF | Stimulates WBCs and stem cells | Immune regulator (25) | 50% | |||||||||
| GHRH | Growth hormone–releasing hormone | Potentiates chemotherapy (26, 27) | Deficient in Hashimoto's thyroiditis (28) | 83% | 92% | ||||||||
| Glucagon | Stimulates glucose production | Inhibits pancreatic cancer cells via PI3K/Akt pathway (29) | Blocking glucagon affects mouse diabetes (30) | 84% | 22% | 78% | |||||||
| HGH | Growth hormone | Enhances cancer growth (31) | Autoantibodies in idiopathic hypopituitary disease (32) | 87% | 68% | 66% | |||||||
| Leptin tA | Regulates energy use | Regulates hepatocellular carcinoma (33) | Promotes lupus; affects T cell autoimmunity: T regulatory cells (34) and Th17 cells (35) | 97% | 91% | 28% | 50% | ||||||
| Plasma proteins | C protein | Blood coagulation factor | 50% | ||||||||||
| F3 coagulation factor III | Reduced survival in NSCLC (36) | 56% | |||||||||||
| HDL | High-density lipoprotein | Associated with survival in NSCLC (37) | Anti-inflammatory (38) | 80% | 28% | 57% | 39% | ||||||
| LDL | Low-density lipoprotein | Level is predictor of breast tumor progression (39) | Autoantibodies in myasthenia gravis (40) | 55% | 59% | 33% | 50% | 33% | |||||
| Tissue | BIRC2 | Baculoviral IAP repeat-containing protein 2 | Cervical squamous cell carcinomas (41) | Elevated in MS (42) | 33% | ||||||||
| CA125 | Mucin family | Levels related to ovarian cancer (43) | Elevated in SLE (44) | 79% | 100% | 99% | 61% | 89% | 100% | ||||
| EEF1A1 | Eukaryotic translation elongation factor 1A | Oncogenic effects (45) | 73% | 51% | |||||||||
| EIF4G1 | Eukaryotic translation initiation factor 4 γ 1 | Nasopharyngeal carcinoma (46) | Citrullinated autoantigen in RA (47) | 22% | |||||||||
| Fibronectin | Correlates with invasion and metastasis (48); (49) | Impairs remyelination in MS (50) | 100% | ||||||||||
| Gelsolin | Actin-binding protein | Affects cancer cell adhesion (51) | Expressed in RA (52) | 50% | 94% | ||||||||
| MOG | MS (53) | 22% | 63% | 55% | 69% | ||||||||
| MUC1 | Mucin 1 | Oncoprotein active in progression (54) | Affects EAE (55) | 65% | |||||||||
| Myosin | Breast cancer (56) | Carditis (57) | 52% | 44% | |||||||||
| Neurotrophin-3 | In breast cancer metastasis (58) | Protects against CNS inflammation (59) | 73% | ||||||||||
| SCF | Stem cell factor | Cancer cells (60) | 22% | ||||||||||
| S100A9 | Migration inhibitory protein 14 | In muscle invasive bladder cancer (61) | In RA synovial fluid (62) | 58% | |||||||||
| Enzymes | BCMO1 | β-carotene metabolism to vitamin A | Affects cancer cell invasion (63) | 50% | |||||||||
| Catalase | Downregulated in tumors (64) | Autoantibodies in RA and SLE (65) | 90% | ||||||||||
| Citrate synthase | Enhanced in pancreatic cancer (66) | Autoantibodies allograft nephropathy (67) | 58% | 89% | 83% | ||||||||
| GST | Prognostic in breast cancer (68) | Autoantibodies in various autoimmune diseases (69) | 55% | 72% | 44% | 50% | |||||||
| PTGDS | PG-H2 d-isomerase | In testis cancer (70) | Suppresses CNS inflammation (71) | 89% | |||||||||
| RIPK2 | Receptor-interacting serine/threonine-protein kinase 2 | Affects hepatoma cells (72), (73) | In asthma, arthritis, and MS (74) | 60% | |||||||||
| Transglutaminase 2-tgm2 | Gliomas (75), (76) | In celiac disease and diabetes (77) | 33% | 22% | |||||||||
| Immune modulator | BETA2 microglobulin | Autoantibodies are tumoricidal (78) | Increased in SLE (79) | 58% | |||||||||
| HSP60 | Heat shock protein 60 | Autoantibodies in ovarian cancer (80) | In various diseases (81) | 83% | |||||||||
| HSP60-23 | Heat shock protein 60-23 | 50% | |||||||||||
| HSP70B | Heat shock protein 70B | Expression affects colon cancer cells (82) | 91% | 39% | 28% | ||||||||
| Laminin | Tumor invasion (83) | SLE (84) | 78% | 72% | |||||||||
| MIF | Macrophage migration inhibitory factor | Squamous cell carcinomas (85) | Affects EAE (86) | 71% | 91% | 61% | 79% | 50% | |||||
| RELA | NF | Inhibits the NF-κB pathway (87) | Inhibits psoriatic skin (88) | 65% | 56% | ||||||||
| IMP-2 | IGF-II mRNA-binding protein | Muscle cell motility (89) | 90% | 33% | 74% | 62% | 72% | 69% | |||||
Shown are Ags that were highly reactive (95th percentile) to IgG, IgM, and IgA Abs in >50% of the individual mother (M) or newborn cord (C) sera or 15% for the milk. Missing values indicate that less than this percentage of the subjects were highly reactive to the Ag. In terms of raw reactivities: the 95th percentile in the maternal dataset corresponds to 10,000–55,000 ruf (see signal intensity in Materials and Methods) for IgG, 17,000–31,000 for IgM, and 3,800–18,000 for IgA. The 95th percentile in the newborns dataset corresponds to 10,000–55,000 for IgG, 20,000–40,000 for IgM, and 5,000–25,000 for IgA. The 95th percentile in the milk corresponds to 1,000–9,000 for IgG, 7,000–19,000 for IgM, and 9,000–24,000 for IgA.
EAE, experimental autoimmune encephalomyelitis; IAP, inhibitor of apoptosis protein; IGF, insulin-like growth factor; MS, multiple sclerosis; NSCLC, non–small cell lung cancer; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Antiforeign pathogen reactivities
The category of Abs binding to foreign viral or bacterial Ags can be divided into two groups. The first is composed of reactivities probably resulting from previous maternal vaccination—diphtheria, hepatitis B, and varicella/zoster virus. The second group includes HSV, EBV, and West Nile virus, which can be attributed to natural exposure of the mothers to these viruses. Compatible with maternal transfer, the only isotype of these Abs detected in cord sera was IgG—IgM and IgA Abs to these Ags were found only in maternal sera. Note that some of the viral and bacterial Ags highly reactive with serum Abs were less reactive with milk Abs; thus, systemic and secretory immunization appear to differ (19–23).
Healthy repertoires include autoantibodies binding to disease-associated and tumor-associated self-Ags
Similar to our previous results (14), many self-Ags were bound by newborn and maternal serum Abs; the present results show that autoantibodies are also present in mother’s milk. Table I shows that the degree of reactivity to some of the self-Ags was comparable to the reactivity detected to some nonself, foreign Ags. The lists of self-Ags bound by prevalent, highly reactive autoantibodies can be categorized as hormones, plasma proteins, tissue Ags, enzymes, and immune modulators; Table I also marks whether the self-Ag molecule has been associated with an autoimmune disease or with tumors. It can be seen that many of the bound self-Ags manifest such associations; we shall touch upon specific associations in Discussion. In the remaining sections, we shall examine the global Ig isotype associations of the various repertoires without limitation to highly reactive and prevalent reactivities.
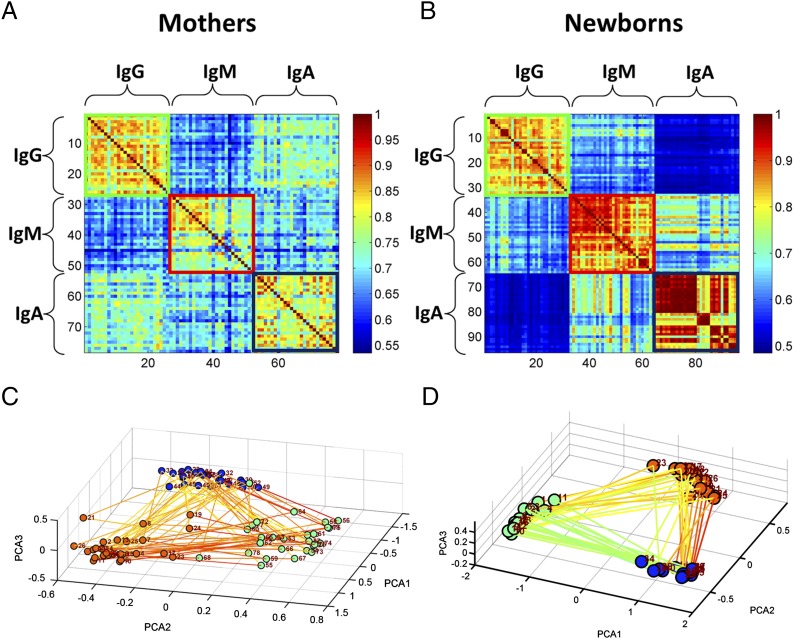
Correlations among IgG, IgM, and IgA isotype repertoires
We analyzed the correlations among the global Ab reactivities in the three isotype repertoires shared by all subjects: IgG, IgM, and IgA. In this analysis, we used only those subjects that had been tested for all three isotypes for both the mother and her offspring; this narrowed our subject dataset to 32 newborns and 26 mothers. Fig. 2 shows the correlations between the isotype repertoires in two formats: Pearson’s correlations (Fig. 2A, mothers; Fig. 2B, newborns) and three-dimensional PCA analysis (Fig. 2C, mothers; Fig. 2D, newborns). Each subject is represented three times in each panel of Fig. 2; the order of the individual subjects remains the same for each of the isotypes. Inspection of Fig. 2A and 2B shows that maternal IgG and IgA serum repertoires manifested a relatively high degree of correlation; newborn cord sera, in contrast, showed a relatively higher correlation between IgM and IgA, the two Ig isotypes that the newborns did not receive from the mothers. Fig. 2C and 2D show that individual newborn IgM and IgA repertoires, relative to the scatter of maternal repertoires, were much more tightly packed in repertoire space; thus, the IgM and IgA cord serum isotypes showed a much higher correlation between the repertoires of individual newborns than did the individual IgM and IgA isotypes of their mothers. In other words, the IgM and IgA Abs produced by developing babies in utero manifest relatively similar global repertoires (13, 14).
FIGURE 2.
The IgG, IgM, and IgA Pearson's correlation matrix for (A) 26 mothers and (B) 32 newborns. Each subject is represented three times in the figure; the order of the subjects remains the same in each of the isotypes. (C and D) PCA projection of the three isotypes of each sample for the (C) mothers and (D) newborns. The nodes are colored according to the isotype (IgG, green; IgM, red; and IgA, blue). The lines connecting the nodes were drawn between isotypes of the same subject. The lines are colored according to the normalized correlation between the samples.
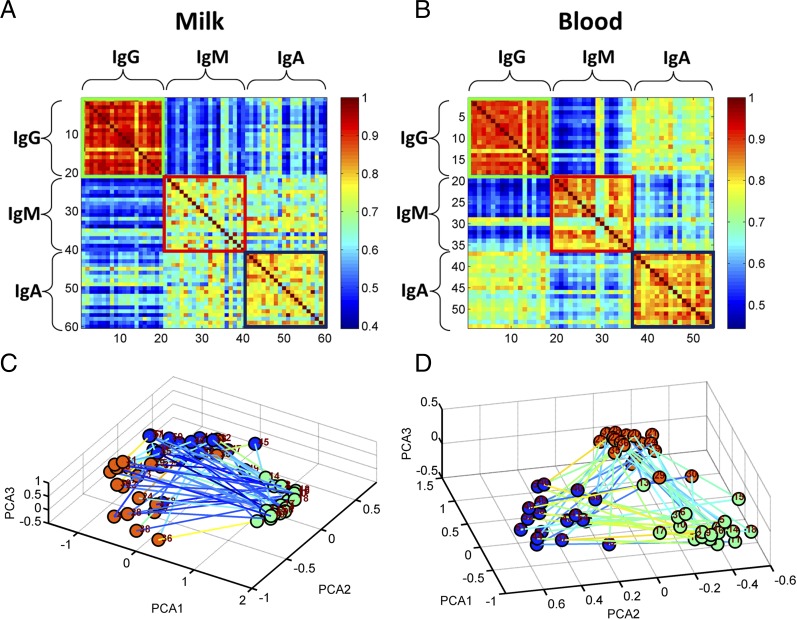
Fig. 3 shows the normalized repertoire correlations of IgG, IgM, and IgA in the milk and corresponding sera in mothers who had been tested for all three isotypes; the data are shown in two formats: Pearson’s correlations matrices and PCA projections. In the milk, Fig. 3A, the IgG repertoire was the most uniform between subjects, followed by IgA; the IgM repertoires were the least correlated between the individual mothers. Note that there were different correlations between the isotypes in milk, Fig. 3A, and serum, Fig. 3B. The serum IgA repertoire was more closely correlated with the serum IgG repertoire; in contrast, the milk IgA repertoire was more closely correlated to the milk IgM repertoire. The PCA projections, Fig. 3C and 3D, illustrate the different characters of the repertoires in milk and serum.
FIGURE 3.
Isotype normalized Pearson's correlation matrix and PCA projection of the milk and corresponding maternal serum samples. (A and B) show the Pearson’s correlation matrix, and (C and D) show the PCA. In (A and B), IgG is in the green bordered square 1–20; IgM in the red bordered square 21–40; and IgA in the blue bordered square 41–60. The matrix was ordered so that sample 1 corresponds to 21 and 41, 2 to 22 and 42, etc. In (C) and (D), the three isotypes of each sample are colored according to the isotype (IgG, green; IgM, red; and IgA, blue). The lines connecting the nodes were drawn between isotypes of the same subject. The lines are colored according to the normalized correlation between the samples.
Subject correlations among mother and newborn IgG, IgM, and IgA repertoires
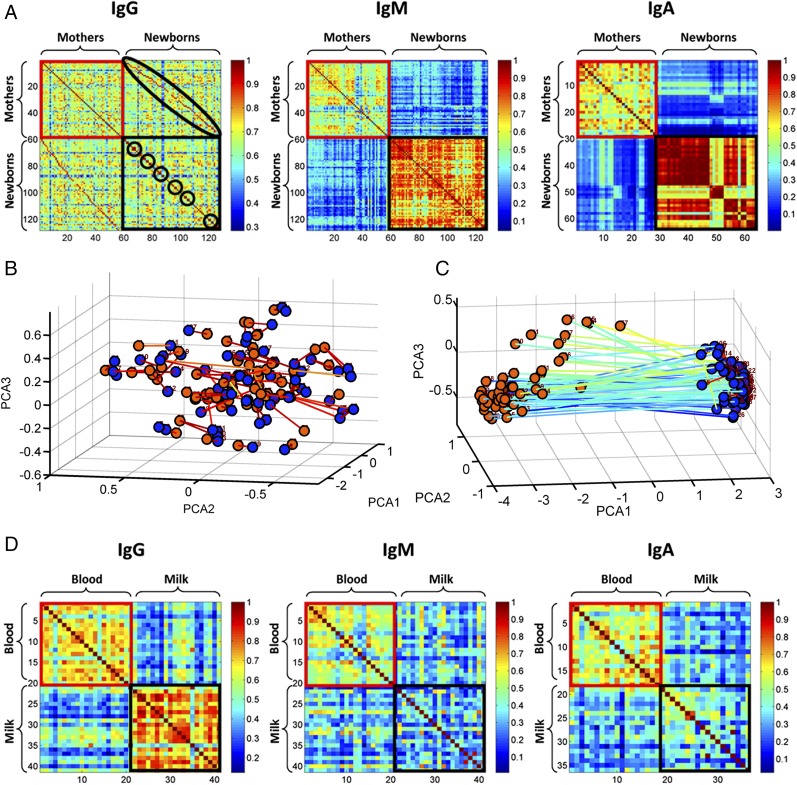
We analyzed the correlations between subject samples to detect relationships between particular Ag reactivities in the populations of maternal milk and serum and their newborn cord sera in their IgG, IgM, and IgA repertoires. The analysis included only paired data: maternal milk–serum pairs and mother–newborn serum pairs (or triplets); single (nonpaired) data were not included.
Pearson's correlation matrix (Fig. 4A) showed a high correlation (dark red in the off-diagonal) between the serum of each mother (red bordered square) and her offspring (black bordered square) in IgG repertoires. The small clusters of high correlation values within the newborns (black bordered squares) along the central diagonal reflect the similarity of the IgG autoantibody repertoire of twins and triplets. These close relationships of these related repertoires can also be observed in the PCA plot (Fig. 4B); note that each mother is closely positioned next to her offspring—singletons, twins, and triplets—in repertoire space. The high correlation between twins and triplets was significant (p value = 5.7e-19 using two-sample t test) compared with other newborns. The relatively low IgG correlation values between each of the adults indicate the individuality of the healthy IgG repertoire.
FIGURE 4.
Correlations between the repertoires of mother-cord samples. The three panels in (A) show the maternal-cord IgG, IgM, and IgA Pearson's correlation matrices. For the IgG and IgM isotypes, the matrix shows the correlations between 57 mothers (red bordered square) and their 71 newborns (black bordered square). For the IgA isotype, the matrix shows the correlations between 28 mothers (red bordered square) and their 36 newborns (black bordered square). In the IgG matrix, the off-diagonal, marked in the black ellipse, shows the strong correlations between each mother and her offspring. The high correlations between twins and triplets are marked by the circles along the main diagonal of the newborns’ correlation matrix. (B) and (C) show the PCA projection of 57 mothers (red nodes) and their 71 newborns (blue nodes) for (B) IgG and (C) IgM repertoires. The lines connecting the nodes were drawn between isotypes of the same subject. The lines connecting the nodes were drawn between each mother and her newborns and between siblings. The lines are colored according to the normalized correlation between the samples. The panels in (D) show the serum-milk IgG, IgM, and IgA Pearson's correlation matrices. The matrix shows the correlations between 21 milk (1–21 red bordered square) and the corresponding serum (22–40 black bordered square); the matrices are ordered so that milk sample 1 corresponds to serum sample 22, 2 to 23, and so forth.
It is known that active transfer of IgG from mother to fetus begins as early as 13 wk of gestation, and that transport takes place in a linear fashion as the pregnancy progresses; the largest amount of IgG is transferred in the third trimester (90). Total IgG concentrations in newborns, therefore, are directly related to the length of gestation, and infants born at <33 wk of gestation have substantially lower IgG levels than full-term babies (91). Nevertheless, we found no statistically significant differences between term and preterm babies in the correlation of their IgG repertoires with their mothers (data not shown); thus, there was no evidence of selective transport of certain IgG Abs related to gestational age.
The IgM and IgA subject correlation matrices (Fig. 4A) showed very weak correlation values between the serum repertoires of each mother (black bordered square) and her newborn’s cord serum (red bordered square); these two clusters manifested very different geometries, which are further amplified in the PCA plot (Fig. 4C), in which the newborns’ cluster was very compact, indicating that individual newborns shared very similar IgM and IgA repertoires; moreover, siblings were yet more similar to each other than were unrelated newborns (p value = 9.72e-05 using two-sample t test). No significant difference in correlation was detected between monozygotic compared with dizygotic twins. In contrast, the maternal cluster was relatively less correlated, indicating that each mother evolved individualized IgM and IgA repertoires.
Fig. 4D shows a lack of correlation between serum and milk in the IgG, IgM, and IgA isotype repertoires of each mother. Indeed, there appeared to be a greater variability of individual IgA and IgM repertoires in milk compared with serum, along with differences between milk and serum in the same woman.
Discussion
This study investigated repertoires of autoantibodies and anti-pathogen Abs present in the cord serum of newborn humans and in the blood serum and breast milk of their mothers; two features of these repertoires are reported. The first is an overview of the Ig isotypes and isotype correlations in the subjects, and the second is a closer look at the most highly reactive self-Ags and pathogen Ags bound by the Abs in each group. The basic question relates to the Abs made available to the newborn by its mother and the Abs produced by the newborn in utero; the maternal Ab endowment to baby can be viewed as an epigenetic heritage of part of mother’s immune experience; the Abs actively produced by baby in utero can be viewed as evolution’s way of priming baby’s immune system with a basic repertoire. T cell repertoires were not part of this Ab-binding microarray study, but the presence of IgG and IgA Abs would infer T cell help (92), In fact, a recent study of the CDR3 TCR types in a dataset of 28 healthy mice reports a subset of highly abundant shared TCRs that were found to be associated with autoimmune conditions, tumor immune responses, and allogeneic graft reactions (93); thus, the healthy T cell repertoire is also enriched in shared self-reactivity.
Note that we have designated as autoantibodies any Abs binding to self-Ags spotted on the microarray chip; however, we cannot know the immunogenic stimulus that initially induced such autoantibodies. Nevertheless, the IgM and IgA autoantibodies present in the cord bloods of newborn babies must have been induced in utero before birth and could reflect induction by immunogenic self-Ags present in the sterile fetus (13, 14). It is conceivable that mother’s IgG Abs transferred across the placenta could induce the fetus to produce IgM or IgA anti-idiotypic Abs (94), but the lack of correlation between cord and maternal IgM and IgA repertoires (Fig. 4A) suggests that the maternal IgG repertoire does not exert a strong influence on the Ag specificities of the newborn IgM and IgA repertoires.
The high correlation between IgM and IgA autoantibody repertoires in different cord bloods and the lack of correlation between cord blood autoantibody repertoires with the IgM and IgA autoantibody repertoires of their individual mothers suggest that these congenital autoantibody reactivities must be encoded in some presently unknown mechanism of positive B cell selection common to different individuals during their development (see Fig. 4A). Indeed, the increased correlation between the autoantibody repertoires of twins and triplets (Fig. 4A) suggests that there is some genetic basis for the congenital selection of autoantibodies binding to certain shared self-Ags (95, 96). It is reasonable to assume that the mothers of these newborns were also born with the common sets of shared IgM and IgA autoantibodies we detected in their babies; the divergence of the maternal serum IgM and IgA repertoires from the shared congenital repertoire indicates that autoantibody repertoires evolve after birth as a result of evolving individual immune experience (13). It has been reported that many natural autoantibodies are polyreactive and so the same autoantibody can bind a variety of different self molecules (97); however, the divergence of IgM and IgA repertoires between cord and maternal samples and within maternal samples (Fig. 4A) would suggest that most of these autoantibodies are not polyreactive to the same sets of self molecules.
The presence at birth of a shared set of IgM and IgA autoantibody reactivities in healthy infants would imply some evolutionary advantage, but, at present, we do not know what it might be. We have speculated that IgM autoantibodies might actually prevent autoimmune disease by blocking the access of potentially pathogenic, self-reactive T cells to key body molecules (98). It is also conceivable that autoantibodies to key body molecules might serve as sensors for biomarker molecules that disclose the needs of cells and tissues for immune maintenance (96). However they may function, healthy individuals express autoantibodies to a particular set of body molecules—we and others have referred to this phenomenon as constituting an immunological homunculus—an immunological representation of the body inscribed in the repertoires of both B cells and T cells (2, 95, 99, 100).
The high correlation between maternal and cord IgG repertoires can be explained by the active transport of maternal IgG Abs to the developing fetus (15). Most of the maternal IgG transmitted to the developing fetus takes place toward the end of gestation (91), but our finding of a high maternal-cord correlation even in premature births (Fig. 4A) indicates that there is probably no preference for specific Abs as global transport increases. One may wonder why evolution arranged for baby to receive passively mother’s blood IgG repertoire exclusively, whereas her IgA and IgM repertoires are transported only in mother’s milk. Only a small sample, if any, of the milk Abs are likely to be absorbed into the baby’s circulation, but mother’s milk Abs, obtained by nursing, could influence the development of baby’s gut microbiome and affect gut viruses (101); ingested Abs could also influence the absorption of ingested foods and affect oral tolerance (102). In any case, it is clear that mother makes different isotype repertoires available to baby in different anatomical compartments.
The list of autoantibodies binding to specific self-Ags, the homuncular Ags, disclosed in this study is interesting. The current version of the Ag chip included only 26 of the self-Ags that were most prevalent on the microarray of self-Ags spotted by Merbl et al. (14), who did not limit reactivity to the 95th percentile as we did in this work (see Table 2 in Ref. 14); nevertheless, 11 of the 26 prevalent autoantibody reactivities reported by Merbl et al. (14) also appeared in our more restricted list of highly reactive self-Ags. Autoantibodies to other self-Ags reported in the earlier study were also detected in this work, but these reactivities were excluded from Table I by the high reactivity threshold we used.
Similar to the results obtained by Merbl et al. (14), the list of highly reactive self-Ags in Table I includes hormones, enzymes, tissue molecules, and immune modulators. Many of the self-Ags among this highly reactive set are known to be associated with various autoimmune diseases such as myelin oligodendrocyte glycoprotein with multiple sclerosis (53, 103); MIF and CA125 with rheumatoid arthritis (104); glucagon with type 1 diabetes (105); and Laminin, low-density lipoprotein, and high-density lipoprotein with systemic lupus erythematosus (106, 107). Thus, the prevalence of some autoimmune diseases may be associated with the underlying prevalence in health of some autoantibody specificities; autoimmune disease, then, can be related to the loss of healthy regulation rather than to the accidental emergence of a forbidden clone (108).
Some self-Ags that were unique to milk appear to be involved in birth and development. For example, Atosiban (Tractocile, Antocin), which is an inhibitor of the hormones oxytocin and vasopressin, is used (tocolytic) to halt premature labor (24); stem cell factor, which plays an important role in the survival, proliferation, and migration of stem cells and melanoblasts during both development and maturation (109, 110); and EIF4G1, which is involved in the recognition of the mRNA cap, ATP-dependent unwinding of 5′-terminal secondary structure, and recruitment of mRNA to the ribosome (111).
Note that most of the highly reactive, prevalent autoantibodies shown in Table I have some association with cancer-related self-Ags, including BIRC2 (41), CA125 (43), MUC1 (54), stem cell factor (60), S-100 (61), myosin (56), GHRH (26, 27), glucagon (29), HGH (31), leptin (33), F3 coagulation factor III (36), EEF1A1 (45), fibronectin (48, 49), neurotrophin-3 (58), BCMO1 (63), citrate synthase (66), GST (68), PTGDS (70), laminin (83), and MIF (85). Indeed, we have found that dynamic changes in autoantibody repertoires mark the natural history in mice of variants of a syngeneic, transplantable tumor (112). The functions of these and other autoantibodies to tumor-associated and other self-Ags in healthy mothers and newborns need to be studied. It would be important to know whether the T cell repertoire in healthy subjects—the T cell homunculus—likewise contains clones potentially reactive to these particular self-Ags (23). In any case, the prevalence of highly reactive autoantibodies to tumor-associated self-Ags supports the idea that the healthy immune system is outfitted, even from birth, to express some form of tumor-associated immune reactivity. The recent reports that treatments with Abs to the immune suppressor molecules PD-1 and CTLA-4 can unleash antitumor immune rejection provide evidence that the immune system does contain latent tumor-associated effector autoimmunity that can be realized by depriving the tumor of protective immune downregulation (113–115). It would be interesting to test the effects of these treatments on the reactivity to the tumor-associated Ags manifested in the healthy repertoire.
Supplementary Material
This work was supported in part by the Maugy-Glass Chair in Physics of Complex Systems and the Tauber Family Foundation at Tel Aviv University and by National Science Foundation-sponsored Center for Theoretical Biological Physics Grants PHY-0216576 and 0225630.
The online version of this article contains supplemental material.
- PCA
- Principal Component Analysis.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cohen I. R. 1992. The cognitive principle challenges clonal selection. Immunol. Today 13: 441–444. [DOI] [PubMed] [Google Scholar]
- 2.Cohen I. R. 2000. Tending Adam’s Garden: Evolving the Cognitive Immune Self. Academic Press, London. [Google Scholar]
- 3.Cohen I. R. 2007. Real and artificial immune systems: computing the state of the body. Nat. Rev. Immunol. 7: 569–574. [DOI] [PubMed] [Google Scholar]
- 4.Janeway, C. A., P. Travers, M. Walport, and M. J. Shlomchik. 2005. Immunobiology: The Immune System in Health and Disease, 5th Ed. Garland Science, New York.
- 5.Perelson A. S. 1989. Immune network theory. Immunol. Rev. 110: 5–36. [DOI] [PubMed] [Google Scholar]
- 6.Tauber A. I. 1994. The Immune Self: Theory or Metaphor? Cambridge University Press, New York. [Google Scholar]
- 7.Nishio N., Ito S., Suzuki H., Isobe K. 2009. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology 128: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner S., Chen H., Tao S., Brenner H. 2015. Systematic review: serum autoantibodies in the early detection of gastric cancer. Int. J. Cancer 136: 2243–2252. [DOI] [PubMed] [Google Scholar]
- 9.Quintana F. J., Hagedorn P. H., Elizur G., Merbl Y., Domany E., Cohen I. R. 2004. Functional immunomics: microarray analysis of IgG autoantibody repertoires predicts the future response of mice to induced diabetes. Proc. Natl. Acad. Sci. USA 101(Suppl. 2): 14615–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerne N. K. 1974. Clonal selection in a lymphocyte network. Soc. Gen. Physiol. Ser. 29: 39–48. [PubMed] [Google Scholar]
- 11.Jerne, N. K. 1974. The immune system: a web of V-domains. Harvey Lect. 70 Series: 93–110. [PubMed]
- 12.Jerne N. K. 1974. Towards a network theory of the immune system. Ann. Immunol. 125C: 373–389. [PubMed] [Google Scholar]
- 13.Madi A., Hecht I., Bransburg-Zabary S., Merbl Y., Pick A., Zucker-Toledano M., Quintana F. J., Tauber A. I., Cohen I. R., Ben-Jacob E. 2009. Organization of the autoantibody repertoire in healthy newborns and adults revealed by system level informatics of antigen microarray data. Proc. Natl. Acad. Sci. USA 106: 14484–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merbl Y., Zucker-Toledano M., Quintana F. J., Cohen I. R. 2007. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J. Clin. Invest. 117: 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson L. A. 2000. The mother-offspring dyad and the immune system. Acta Paediatr. 89: 252–258. [PubMed] [Google Scholar]
- 16.Quintana F. J., Farez M. F., Viglietta V., Iglesias A. H., Merbl Y., Izquierdo G., Lucas M., Basso A. S., Khoury S. J., Lucchinetti C. F., et al. 2008. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 105: 18889–18894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson W. H., DiGennaro C., Hueber W., Haab B. B., Kamachi M., Dean E. J., Fournel S., Fong D., Genovese M. C., de Vegvar H. E., et al. 2002. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 8: 295–301. [DOI] [PubMed] [Google Scholar]
- 18.Pearson K. 1901. On lines and planes of closest fit to systems of points in space. Philos. Mag. 2: 559–572. [Google Scholar]
- 19.Van de Perre P. 2003. Transfer of antibody via mother’s milk. Vaccine 21: 3374–3376. [DOI] [PubMed] [Google Scholar]
- 20.Becquart P., Hocini H., Garin B., Sépou A., Kazatchkine M. D., Bélec L. 1999. Compartmentalization of the IgG immune response to HIV-1 in breast milk. AIDS 13: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 21.Berneman A., Belec L., Fischetti V. A., Bouvet J. P. 1998. The specificity patterns of human immunoglobulin G antibodies in serum differ from those in autologous secretions. Infect. Immun. 66: 4163–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boes M., Schmidt T., Linkemann K., Beaudette B. C., Marshak-Rothstein A., Chen J. 2000. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. USA 97: 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassilev T. L., Veleva K. V. 1996. Natural polyreactive IgA and IgM autoantibodies in human colostrum. Scand. J. Immunol. 44: 535–539. [DOI] [PubMed] [Google Scholar]
- 24.Reversi A., Rimoldi V., Marrocco T., Cassoni P., Bussolati G., Parenti M., Chini B. 2005. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J. Biol. Chem. 280: 16311–16318. [DOI] [PubMed] [Google Scholar]
- 25.Franzke A., Piao W., Lauber J., Gatzlaff P., Könecke C., Hansen W., Schmitt-Thomsen A., Hertenstein B., Buer J., Ganser A. 2003. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood 102: 734–739. [DOI] [PubMed] [Google Scholar]
- 26.Jaszberenyi M., Rick F. G., Popovics P., Block N. L., Zarandi M., Cai R. Z., Vidaurre I., Szalontay L., Jayakumar A. R., Schally A. V. 2014. Potentiation of cytotoxic chemotherapy by growth hormone-releasing hormone agonists. Proc. Natl. Acad. Sci. USA 111: 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kővári B., Rusz O., Schally A. V., Kahán Z., Cserni G. 2014. Differential immunostaining of various types of breast carcinomas for growth hormone-releasing hormone receptor: apocrine epithelium and carcinomas emerging as uniformly positive. APMIS 122: 824–831. [DOI] [PubMed] [Google Scholar]
- 28.Eskes S. A., Endert E., Fliers E., Wiersinga W. M. 2010. Prevalence of growth hormone deficiency in Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 95: 2266–2270. [DOI] [PubMed] [Google Scholar]
- 29.Zhao, H., L. Wang, R. Wei, D. Xiu, M. Tao, J. Ke, Y. Liu, J. Yang, and T. Hong. 2014. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes. Metab. 16: 850-860. [DOI] [PubMed]
- 30.Lee Y., Wang M. Y., Du X. Q., Charron M. J., Unger R. H. 2011. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handler M. Z., Ross A. L., Shiman M. I., Elgart G. W., Grichnik J. M. 2012. Potential role of human growth hormone in melanoma growth promotion. Arch. Dermatol. 148: 1179–1182. [DOI] [PubMed] [Google Scholar]
- 32.Llera A. S., Cardoso A. I., Stumpo R. R., Martinez A. S., Heinrich J. J., Poskus E. 1993. Detection of autoantibodies against hGH in sera of idiopathic hypopituitary children. Clin. Immunol. Immunopathol. 66: 114–119. [DOI] [PubMed] [Google Scholar]
- 33.Stefanou N., Papanikolaou V., Furukawa Y., Nakamura Y., Tsezou A. 2010. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer 10: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amarilyo G., Iikuni N., Shi F. D., Liu A., Matarese G., La Cava A. 2013. Leptin promotes lupus T-cell autoimmunity. Clin. Immunol. 149: 530–533. [DOI] [PubMed] [Google Scholar]
- 35.Moraes-Vieira P. M., Larocca R. A., Bassi E. J., Peron J. P., Andrade-Oliveira V., Wasinski F., Araujo R., Thornley T., Quintana F. J., Basso A. S., et al. 2014. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 44: 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regina S., Valentin J. B., Lachot S., Lemarié E., Rollin J., Gruel Y. 2009. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin. Chem. 55: 1834–1842. [DOI] [PubMed] [Google Scholar]
- 37.Chi P. D., Liu W., Chen H., Zhang J. P., Lin Y., Zheng X., Liu W., Dai S. 2014. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One 9: e91080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaji H. 2013. High-density lipoproteins and the immune system. J. Lipids 2013: 684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues Dos Santos C., Fonseca I., Dias S., Mendes de Almeida J. C. 2014. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer 14: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen C., Lu Y., Zhang B., Figueiredo D., Bean J., Jung J., Wu H., Barik A., Yin D. M., Xiong W. C., Mei L. 2013. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J. Clin. Invest. 123: 5190–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choschzick M., Tabibzada A. M., Gieseking F., Woelber L., Jaenicke F., Sauter G., Simon R. 2012. BIRC2 amplification in squamous cell carcinomas of the uterine cervix. Virchows Arch. 461: 123–128. [DOI] [PubMed] [Google Scholar]
- 42.Warford J., Robertson G. S. 2011. New methods for multiple sclerosis drug discovery. Expert Opin Drug Discov. 6: 689–699. [DOI] [PubMed] [Google Scholar]
- 43.Hall M. R., Petruckevitch A., Pascoe J., Persic M., Tahir S., Morgan J. S., Gourley C., Stuart N., Crawford S. M., Kornbrot D. E., et al. 2014. Using serum CA125 to assess the activity of potential cytostatic agents in ovarian cancer. Int. J. Gynecol. Cancer 24: 676–681. [DOI] [PubMed] [Google Scholar]
- 44.Basaran A., Zafer Tuncer S. 2007. Ascites is the primary cause of cancer antigen-125 (CA-125) elevation in systemic lupus erythematosus (SLE) patients with nephrotic syndrome. Med. Hypotheses 68: 197–201. [DOI] [PubMed] [Google Scholar]
- 45.Dahl L. D., Corydon T. J., Ränkel L., Nielsen K. M., Füchtbauer E. M., Knudsen C. R. 2014. An eEF1A1 truncation encoded by PTI-1 exerts its oncogenic effect inside the nucleus. Cancer Cell Int. 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tu L., Liu Z., He X., He Y., Yang H., Jiang Q., Xie S., Xiao G., Li X., Yao K., Fang W. 2010. Over-expression of eukaryotic translation initiation factor 4 gamma 1 correlates with tumor progression and poor prognosis in nasopharyngeal carcinoma. Mol. Cancer 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okazaki Y., Suzuki A., Sawada T., Ohtake-Yamanaka M., Inoue T., Hasebe T., Yamada R., Yamamoto K. 2006. Identification of citrullinated eukaryotic translation initiation factor 4G1 as novel autoantigen in rheumatoid arthritis. Biochem. Biophys. Res. Commun. 341: 94–100. [DOI] [PubMed] [Google Scholar]
- 48. Shuman Moss, L. A., and W. G. Stetler-Stevenson. 2013. Influence of stromal components on lung cancer carcinogenesis. J. Carcinog. Mutagen. DOI: 10.4172/2157-2518.S13-008. [DOI] [PMC free article] [PubMed]
- 49.Jia D., Yan M., Wang X., Hao X., Liang L., Liu L., Kong H., He X., Li J., Yao M. 2010. Development of a highly metastatic model that reveals a crucial role of fibronectin in lung cancer cell migration and invasion. BMC Cancer 10: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoffels J. M., de Jonge J. C., Stancic M., Nomden A., van Strien M. E., Ma D., Sisková Z., Maier O., Ffrench-Constant C., Franklin R. J., et al. 2013. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136: 116–131. [DOI] [PubMed] [Google Scholar]
- 51.Langereis J. D., Koenderman L., Huttenlocher A., Ulfman L. H. 2013. Gelsolin expression increases β1-integrin affinity and L1210 cell adhesion. Cytoskeleton 70: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biswas S., Sharma S., Saroha A., Bhakuni D. S., Malhotra R., Zahur M., Oellerich M., Das H. R., Asif A. R. 2013. Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach. PLoS One 8: e56246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reindl M., Di Pauli F., Rostásy K., Berger T. 2013. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat. Rev. Neurol. 9: 455–461. [DOI] [PubMed] [Google Scholar]
- 54.Nath, S., and P. Mukherjee. 2014. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 20: 332–342. [DOI] [PMC free article] [PubMed]
- 55.Yen J. H., Xu S., Park Y. S., Ganea D., Kim K. C. 2013. Higher susceptibility to experimental autoimmune encephalomyelitis in Muc1-deficient mice is associated with increased Th1/Th17 responses. Brain Behav. Immun. 29: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng D. E., Hung J. Y., Huang M. S., Hsu Y. L., Lu C. Y., Tsai E. M., Hou M. F., Kuo P. L. 2014. Myosin IIa activation is crucial in breast cancer derived galectin-1 mediated tolerogenic dendritic cell differentiation. Biochim. Biophys. Acta 1840: 1965–1976. [DOI] [PubMed] [Google Scholar]
- 57.Lv H., Lipes M. A. 2012. Role of impaired central tolerance to α-myosin in inflammatory heart disease. Trends Cardiovasc. Med. 22: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louie E., Chen X. F., Coomes A., Ji K., Tsirka S., Chen E. I. 2013. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 32: 4064–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moalem G., Gdalyahu A., Shani Y., Otten U., Lazarovici P., Cohen I. R., Schwartz M. 2000. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J. Autoimmun. 15: 331–345. [DOI] [PubMed] [Google Scholar]
- 60.Aguilar C., Aguilar C., Lopez-Marure R., Jiménez-Sánchez A., Rocha-Zavaleta L. 2014. Co-stimulation with stem cell factor and erythropoietin enhances migration of c-Kit expressing cervical cancer cells through the sustained activation of ERK1/2. Mol. Med. Rep. 9: 1895–1902. [DOI] [PubMed] [Google Scholar]
- 61.Kim W. T., Kim J., Yan C., Jeong P., Choi S. Y., Lee O. J., Chae Y. B., Yun S. J., Lee S. C., Kim W. J. 2014. S100A9 and EGFR gene signatures predict disease progression in muscle invasive bladder cancer patients after chemotherapy. Ann. Oncol. 25: 974–979. [DOI] [PubMed] [Google Scholar]
- 62.Kang K. Y., Woo J. W., Park S. H. 2014. S100A8/A9 as a biomarker for synovial inflammation and joint damage in patients with rheumatoid arthritis. Korean J. Intern. Med. 29: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pham D. N., Leclerc D., Lévesque N., Deng L., Rozen R. 2013. β,β-carotene 15,15′-monooxygenase and its substrate β-carotene modulate migration and invasion in colorectal carcinoma cells. Am. J. Clin. Nutr. 98: 413–422. [DOI] [PubMed] [Google Scholar]
- 64.Glorieux, C., J. Auquier, N. Dejeans, B. Sid, J. B. Demoulin, L. Bertrand, J. Verrax, and P. B. Calderon. 2014. Catalase expression in MCF-7 breast cancer cells is mainly controlled by PI3K/Akt/mTor signaling pathway. Biochem. Pharmacol. 89: 217–223. [DOI] [PubMed]
- 65.Mansour R. B., Lassoued S., Gargouri B., El Gaïd A., Attia H., Fakhfakh F. 2008. Increased levels of autoantibodies against catalase and superoxide dismutase associated with oxidative stress in patients with rheumatoid arthritis and systemic lupus erythematosus. Scand. J. Rheumatol. 37: 103–108. [DOI] [PubMed] [Google Scholar]
- 66.Schlichtholz B., Turyn J., Goyke E., Biernacki M., Jaskiewicz K., Sledzinski Z., Swierczynski J. 2005. Enhanced citrate synthase activity in human pancreatic cancer. Pancreas 30: 99–104. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L. Y., Lu Y. P., Yang L., Luo G. H., Song J., Li Y. P. 2009. Detection of citrate synthase autoantibodies in rats with chronic allograft nephropathy. Transplant. Proc. 41: 4366–4368. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira A. L., Oliveira Rodrigues F. F., Dos Santos R. E., Rozenowicz R. L., Barbosa de Melo M. 2014. GSTT1, GSTM1, and GSTP1 polymorphisms as a prognostic factor in women with breast cancer. Genet. Mol. Res. 13: 2521-2530. [DOI] [PubMed] [Google Scholar]
- 69.Ardesjö B., Hansson C. M., Bruder C. E., Rorsman F., Betterle C., Dumanski J. P., Kämpe O., Ekwall O. 2008. Autoantibodies to glutathione S-transferase theta 1 in patients with primary sclerosing cholangitis and other autoimmune diseases. J. Autoimmun. 30: 273–282. [DOI] [PubMed] [Google Scholar]
- 70.Wu C. C., Shyu R. Y., Wang C. H., Tsai T. C., Wang L. K., Chen M. L., Jiang S. Y., Tsai F. M. 2012. Involvement of the prostaglandin D2 signal pathway in retinoid-inducible gene 1 (RIG1)-mediated suppression of cell invasion in testis cancer cells. Biochim. Biophys. Acta 1823: 2227–2236. [DOI] [PubMed] [Google Scholar]
- 71.Pareek T. K., Belkadi A., Kesavapany S., Zaremba A., Loh S. L., Bai L., Cohen M. L., Meyer C., Liby K. T., Miller R. H., et al. 2011. Triterpenoid modulation of IL-17 and Nrf-2 expression ameliorates neuroinflammation and promotes remyelination in autoimmune encephalomyelitis. Sci. Rep. 1: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S., Kanda T., Nakamoto S., Imazeki F., Yokosuka O. 2012. Knockdown of receptor-interacting serine/threonine protein kinase-2 (RIPK2) affects EMT-associated gene expression in human hepatoma cells. Anticancer Res. 32: 3775–3783. [PubMed] [Google Scholar]
- 73.Guirado M., Gil H., Saenz-Lopez P., Reinboth J., Garrido F., Cozar J. M., Ruiz-Cabello F., Carretero R. 2012. Association between C13ORF31, NOD2, RIPK2 and TLR10 polymorphisms and urothelial bladder cancer. Hum. Immunol. 73: 668–672. [DOI] [PubMed] [Google Scholar]
- 74.Jun J. C., Cominelli F., Abbott D. W. 2013. RIP2 activity in inflammatory disease and implications for novel therapeutics. J. Leukoc. Biol. 94: 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu J., Yang Q. Y., Sai K., Chen F. R., Pang J. C., Ng H. K., Kwan A. L., Chen Z. P. 2013. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro-oncol. 15: 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown K. D. 2013. Transglutaminase 2 and NF-κB: an odd couple that shapes breast cancer phenotype. Breast Cancer Res. Treat. 137: 329–336. [DOI] [PubMed] [Google Scholar]
- 77.Szondy Z., Korponay-Szabó I., Király R., Fésüs L. 2011. Transglutaminase 2 dysfunctions in the development of autoimmune disorders: celiac disease and TG2-/- mouse. Adv. Enzymol. Relat. Areas Mol. Biol. 78: 295–345. [DOI] [PubMed] [Google Scholar]
- 78.Nomura T., Huang W. C., Zhau H. E., Josson S., Mimata H., Chung L. W. 2014. β2-Microglobulin-mediated signaling as a target for cancer therapy. Anticancer Agents Med. Chem. 14: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hermansen M. L., Hummelshøj L., Lundsgaard D., Hornum L., Keller P., Fleckner J., Fox B., Poulsen L. K., Jacobsen S. 2012. Increased serum β2-microglobulin is associated with clinical and immunological markers of disease activity in systemic lupus erythematosus patients. Lupus 21: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 80.Bodzek P., Partyka R., Damasiewicz-Bodzek A. 2014. Antibodies against Hsp60 and Hsp65 in the sera of women with ovarian cancer. J. Ovarian Res. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasperkiewicz M., Tukaj S., Gembicki A. J., Silló P., Görög A., Zillikens D., Kárpáti S. 2014. Evidence for a role of autoantibodies to heat shock protein 60, 70, and 90 in patients with dermatitis herpetiformis. Cell Stress Chaperones 19: 837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noonan E. J., Fournier G., Hightower L. E. 2008. Surface expression of Hsp70B’ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones 13: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ranjan A., Bane S. M., Kalraiya R. D. 2014. Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp. Cell Res. 322: 249–264. [DOI] [PubMed] [Google Scholar]
- 84.Páez M. C., Matsuura E., Díaz L. A., Shoenfeld Y., Serrano N. C., Anaya J. M. 2013. Laminin-1 (LM-111) in preeclampsia and systemic lupus erythematosus. Autoimmunity 46: 14–20. [DOI] [PubMed] [Google Scholar]
- 85.Kindt N., Lechien J. R., Nonclercq D., Laurent G., Saussez S. 2014. Involvement of CD74 in head and neck squamous cell carcinomas. J. Cancer Res. Clin. Oncol. 140: 937–947. [DOI] [PubMed] [Google Scholar]
- 86.Meza-Romero, R., G. Benedek, X. Yu, J. L. Mooney, R. Dahan, N. Duvshani, R. Bucala, H. Offner, Y. Reiter, G. G. Burrows, and A. A. Vandenbark. 2014. HLA-DRalpha1 constructs block CD74 expression and MIF effects in experimental autoimmune encephalomyelitis. J. Immunol. 192: 4164-4173. [DOI] [PMC free article] [PubMed]
- 87.Hsiao B. Y., Chang T. K., Wu I. T., Chen M. Y. 2014. Rad GTPase inhibits the NFκB pathway through interacting with RelA/p65 to impede its DNA binding and target gene transactivation. Cell. Signal. 26: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 88.Weng Z., Patel A. B., Vasiadi M., Therianou A., Theoharides T. C. 2014. Luteolin inhibits human keratinocyte activation and decreases NF-κB induction that is increased in psoriatic skin. PLoS One 9: e90739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boudoukha S., Cuvellier S., Polesskaya A. 2010. Role of the RNA-binding protein IMP-2 in muscle cell motility. Mol. Cell. Biol. 30: 5710–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saji F., Samejima Y., Kamiura S., Koyama M. 1999. Dynamics of immunoglobulins at the feto-maternal interface. Rev. Reprod. 4: 81–89. [DOI] [PubMed] [Google Scholar]
- 91.Malek A., Sager R., Kuhn P., Nicolaides K. H., Schneider H. 1996. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 36: 248–255. [DOI] [PubMed] [Google Scholar]
- 92.Parker D. C. 1993. T cell-dependent B cell activation. Annu. Rev. Immunol. 11: 331–360. [DOI] [PubMed] [Google Scholar]
- 93.Madi A., Shifrut E., Reich-Zeliger S., Gal H., Best K., Ndifon W., Chain B., Cohen I. R., Friedman N. 2014. T-cell receptor repertoires share a restricted set of public and abundant CDR3 sequences that are associated with self-related immunity. Genome Res. 24: 1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemke H., Coutinho A., Lange H. 2004. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 25: 180–186. [DOI] [PubMed] [Google Scholar]
- 95.Cohen I. R. 1992. The cognitive paradigm and the immunological homunculus. Immunol. Today 13: 490–494. [DOI] [PubMed] [Google Scholar]
- 96.Cohen I. R. 2007. Biomarkers, self-antigens and the immunological homunculus. J. Autoimmun. 29: 246–249. [DOI] [PubMed] [Google Scholar]
- 97.Notkins A. L. 2004. Polyreactivity of antibody molecules. Trends Immunol. 25: 174–179. [DOI] [PubMed] [Google Scholar]
- 98.Cohen I. R., Cooke A. 1986. Natural autoantibodies might prevent autoimmune disease. Immunol. Today 7: 363–364. [DOI] [PubMed] [Google Scholar]
- 99.Nobrega A., Haury M., Grandien A., Malanchère E., Sundblad A., Coutinho A. 1993. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological “homunculus” of antibodies in normal serum. Eur. J. Immunol. 23: 2851–2859. [DOI] [PubMed] [Google Scholar]
- 100.Poletaev A. B. 2002. The immunological homunculus (immunculus) in normal state and pathology. Biochemistry Mosc. 67: 600–608. [DOI] [PubMed] [Google Scholar]
- 101.Wold A. E., Adlerberth I. 2000. Breast feeding and the intestinal microflora of the infant—implications for protection against infectious diseases. Adv. Exp. Med. Biol. 478: 77–93. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto T., Tsubota Y., Kodama T., Kageyama-Yahara N., Kadowaki M. 2012. Oral tolerance induced by transfer of food antigens via breast milk of allergic mothers prevents offspring from developing allergic symptoms in a mouse food allergy model. Clin. Dev. Immunol. 2012: 721085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben-Nun A., Mendel I., Bakimer R., Fridkis-Hareli M., Teitelbaum D., Arnon R., Sela M., Kerlero de Rosbo N. 1996. The autoimmune reactivity to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis is potentially pathogenic: effect of copolymer 1 on MOG-induced disease. J. Neurol. 243(Suppl. 1): S14–S22. [DOI] [PubMed] [Google Scholar]
- 104.Szekanecz E., Sándor Z., Antal-Szalmás P., Soós L., Lakos G., Besenyei T., Szentpétery A., Simkovics E., Szántó J., Kiss E., et al. 2007. Increased production of the soluble tumor-associated antigens CA19-9, CA125, and CA15-3 in rheumatoid arthritis: potential adhesion molecules in synovial inflammation? Ann. N. Y. Acad. Sci. 1108: 359–371. [DOI] [PubMed] [Google Scholar]
- 105.Gergely A., Korányl L., Halmos T., Zsombók M., Péterfy F., Csizér F., Salamon F., Takó J. 1973. Anti-glucagon antibodies in diabetes mellitus. Ann. Immunol. Hung. 17: 231–233. [PubMed] [Google Scholar]
- 106.Ben-Yehuda A., Rasooly L., Bar-Tana R., Breuer G., Tadmor B., Ulmansky R., Naparstek Y. 1995. The urine of SLE patients contains antibodies that bind to the laminin component of the extracellular matrix. J. Autoimmun. 8: 279–291. [DOI] [PubMed] [Google Scholar]
- 107.Fesmire J., Wolfson-Reichlin M., Reichlin M. 2010. Effects of autoimmune antibodies anti-lipoprotein lipase, anti-low density lipoprotein, and anti-oxidized low density lipoprotein on lipid metabolism and atherosclerosis in systemic lupus erythematosus. Rev. Bras Reumatol. 50: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodnow C. C., Sprent J., Fazekas de St Groth B., Vinuesa C. G. 2005. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435: 590–597. [DOI] [PubMed] [Google Scholar]
- 109.Manova K., Nocka K., Besmer P., Bachvarova R. F. 1990. Gonadal expression of c-kit encoded at the W locus of the mouse. Development 110: 1057–1069. [DOI] [PubMed] [Google Scholar]
- 110.Orr-Urtreger A., Avivi A., Zimmer Y., Givol D., Yarden Y., Lonai P. 1990. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development 109: 911–923. [DOI] [PubMed] [Google Scholar]
- 111.Silvera D., Arju R., Darvishian F., Levine P. H., Zolfaghari L., Goldberg J., Hochman T., Formenti S. C., Schneider R. J. 2009. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell Biol. 11: 903–908. [DOI] [PubMed] [Google Scholar]
- 112.Merbl Y., Itzchak R., Vider-Shalit T., Louzoun Y., Quintana F. J., Vadai E., Eisenbach L., Cohen I. R. 2009. A systems immunology approach to the host-tumor interaction: large-scale patterns of natural autoantibodies distinguish healthy and tumor-bearing mice. PLoS One 4: e6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hodi F. S., O’Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., Gonzalez R., Robert C., Schadendorf D., Hassel J. C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., Powderly J. D., Carvajal R. D., Sosman J. A., Atkins M. B., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen I. R. 2014. Activation of benign autoimmunity as both tumor and autoimmune disease immunotherapy: a comprehensive review. J. Autoimmun. 54: 112–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.