Abstract
Bacteria possess signal transduction pathways capable of sensing and responding to a wide variety of signals. The Cpx envelope stress response, composed of the sensor histidine kinase CpxA and the response regulator CpxR, senses and mediates adaptation to insults to the bacterial envelope. The Cpx response has been implicated in the regulation of a number of envelope-localized virulence determinants across bacterial species. Here, we show that activation of the Cpx pathway in Vibrio cholerae El Tor strain C6706 leads to a decrease in expression of the major virulence factors in this organism, cholera toxin (CT) and the toxin-coregulated pilus (TCP). Our results indicate that this occurs through the repression of production of the ToxT regulator and an additional upstream transcription factor, TcpP. The effect of the Cpx response on CT and TCP expression is mostly abrogated in a cyclic AMP receptor protein (CRP) mutant, although expression of the crp gene is unaltered. Since TcpP production is controlled by CRP, our data suggest a model whereby the Cpx response affects CRP function, which leads to diminished TcpP, ToxT, CT, and TCP production.
INTRODUCTION
Vibrio cholerae, a curved Gram-negative bacterium, is the causative agent of the waterborne disease cholera. More than 200 V. cholerae serogroups have been identified, of which the O1 serogroup has been associated with the pandemic spread of cholera (1). Furthermore, the O1 serogroup is classified into two biotypes, classical and El Tor; the latter is responsible for the seventh ongoing cholera pandemic (2). Recent cholera outbreaks in Haiti, Cameroon, and Zimbabwe (3–5) suggest an increase in the incidence of cholera (6).
V. cholerae colonizes the human small intestine, where it produces its main virulence factors, toxin coregulated pilus (TCP) and cholera toxin (CT). TCP, a type IV pilus, enables small intestine colonization and the establishment of microcolonies (7–9). CT is produced and secreted in a folded state from the periplasm across the outer membrane (OM) by the type II secretion system (T2SS) (10). This AB5-type ribosylating enterotoxin (11) leads to the secretion of Cl− and water from intestinal epithelial cells, which is the hallmark of the watery diarrhea associated with V. cholerae (12, 13). Virulence gene expression in V. cholerae is controlled by a regulatory cascade known as the ToxR regulon (14–17). Two membrane-bound DNA binding proteins, TcpP and ToxR, are critical for coordinated expression of the master virulence regulator, ToxT, in response to different environmental stimuli (i.e., temperature and pH) (18–22). ToxT directly activates the expression of the genes responsible for the biosynthesis of CT and TCP (16, 23). In addition, tcpPH promoter activation is positively regulated by two cytoplasmic regulators, AphA and AphB, and negatively regulated by cyclic AMP (cAMP) receptor protein (CRP) and by quorum sensing via HapR, a LuxR homolog that represses aphA transcription (24–29). Finally, an additional regulator, TsrA, modulates the expression of CT, TCP, and another envelope-localized virulence factor, the type VI secretion system (T6SS) in V. cholerae (30). The T6SS plays an important role in cytotoxicity toward amoebae and mammalian macrophages (31), as well as inter- and intrabacterial interactions, by conferring toxicity toward other bacteria (32, 33).
Bacterial pathogens utilize envelope-localized signal transduction systems to sense and modulate expression of genes in response to environmental signals. The Cpx envelope stress response is controlled by a two-component regulatory system (TCS), which is composed of the sensor histidine kinase CpxA and the cytoplasmic response regulator CpxR (34). CpxA is an inner membrane (IM) protein that autophosphorylates upon detecting an inducing cue via its periplasmic sensing domain and then becomes a phosphodonor to its response regulator, CpxR, at a conserved aspartate phosphorylation site (34, 35). CpxR phosphorylation leads to upregulation and downregulation of multiple genes by direct binding of CpxR to DNA (35). Additionally, the Cpx pathway regulates and is regulated by a periplasmic protein, CpxP, which reduces CpxA autokinase activity (36, 37). The Cpx system senses bacterial cell envelope stress, such as misfolded proteins, and regulates the expression of diverse genes involved in maintaining cell envelope homeostasis (38).
In many Gram-negative pathogens, the Cpx pathway regulates virulence gene expression (for a recent review, see reference 38). For example, the Cpx pathway regulates the expression of a number of virulence determinants in enteropathogenic Escherichia coli (EPEC) (type III secretion system [T3SS], bundle-forming pili [BFP], and motility), uropathogenic E. coli (UPEC) (P pilus), Yersinia pseudotuberculosis (T3SS), Legionella pneumophila (type IV secretion system [T4SS]), Shigella sonnei (virulence regulators and T3SS), Salmonella enterica serovar Typhimurium (invasion factors), and Haemophilus ducreyi (flp-tad and lspB-lspA2 operons, dsrA, ncaA, and hgbA) (39–51).
Studies on the human pathogen V. cholerae have shown that the activation of the Cpx pathway in V. cholerae N16961 is required to mediate cellular responses to perturbations in the cell envelope and also to stresses related to salinity (52). Recently, we found that activation of the Cpx response in the V. cholerae El Tor strain C6706, which is closely related to the V. cholerae N16961 strain (53), leads to changes in the expression of genes involved in iron acquisition under virulence-inducing conditions (54). Additionally, we and others found that the Cpx pathway regulates genes involved in antimicrobial resistance (54, 55). Finally, our microarray analysis also suggested that the Cpx pathway positively regulates the expression of the toxR gene (54), which encodes an important virulence factor regulator in V. cholerae (56).
In the current study, we characterized the effect of the activation of the Cpx response on different envelope-localized virulence factors in the V. cholerae El Tor strain C6706. We confirmed that the Cpx pathway positively regulates the expression of the transcriptional regulator ToxR. Furthermore, we found that the Cpx pathway regulates the expression of one of the major outer membrane (OM) porins in V. cholerae, OmpT, in a ToxR-independent manner. Our results indicate that activation of the Cpx pathway negatively regulates the expression of virulence determinants such as CT and TCP at the transcriptional level. We show that activation of the Cpx pathway leads to downregulation of tcpP and toxT expression, primarily through changes in CRP-mediated gene regulation. Overall, our work suggests that the Cpx response in V. cholerae El Tor C6706 negatively regulates CT and TCP expression through the downregulation of the tcpPH promoter and via altered function of the catabolite activator protein.
MATERIALS AND METHODS
Growth conditions.
Bacteria were grown in Luria-Bertani (LB) broth with the appropriate antibiotics at 37°C with aeration unless otherwise noted and stored at −80°C in LB broth containing 20% glycerol. For V. cholerae in vitro virulence induction, AKI conditions were used as previously described (57). Briefly, cultures grown overnight in LB broth were inoculated into AKI medium at a 1:10,000 dilution. After 6 h of static growth at 37°C, the culture was transferred to a 125-ml flask and shaken (225 rpm) at 37°C for 16 h. Antibiotics (all from Sigma) were used at the following concentrations in selective media: ampicillin (Amp), 100 μg/ml; kanamycin (Kan), 50 μg/ml; and streptomycin (Sm), 100 μg/ml. l-Arabinose (Sigma) was added to growth media to a concentration of 0.1% for CpxR induction experiments.
In-frame deletions and plasmid construction.
All strains and plasmids used in this study are listed in Table 1. All primers used in this study are listed in Table S1 in the supplemental material. A streptomycin-resistant variant of V. cholerae El Tor C6706 was used as the parental strain to create all of the V. cholerae strains used in this study. In-frame deletion of toxR was performed as described by Metcalf et al. (59). Briefly, in-frame deletion mutants were constructed by an overlap extension PCR method. The first PCRs were performed using the primers F1 and R1 (see Table S1), which flank the upstream region of toxR, and F2 and R2 (see Table S1), which flank the downstream region of the toxR gene. The second PCR was performed using as a template the PCR products F1-R1 and F2-R2 for the toxR knockout construct. Amplified DNA was cut with BamHI and NotI and band purified using the GeneJET gel extraction kit (Thermo Scientific) by following the manufacturer's instructions. These DNA fragments were cloned into the BamHI and NotI restriction sites of the suicide vector pWM91 (59) and transformed into E. coli strain DH5α-λpir. E. coli strain SM10λpir then was used as the donor to mobilize the plasmid into V. cholerae El Tor C6706 via conjugation for the selection of recombinants carrying the toxR deletion mutation. V. cholerae El Tor C6706 ompU, ompT, ompR, and cpxR mutants used in this study were originated from a transposon insertion library (60).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| C6706 | V. cholerae El Tor biotype strain C6706; Smr | J. Mekalanos (Harvard Medical School) |
| V52 | V. cholerae O37 serogroup strain; ΔhapA ΔrtxA ΔhlyA; Smr | 31 |
| V52ΔvasK | V52 mutant lacking vasK gene, carrying pBAD24 plasmid; Smr Ampr | 31 |
| NA24 | V52 carrying pBAD24 plasmid; Smr Ampr | This study |
| NA3 | V52 carrying pCpxR plasmid; Smr Ampr | This study |
| NA45 | C6706 carrying pBAD24 plasmid; Smr Ampr | 54 |
| NA44 | C6706 carrying pCpxR plasmid; Smr Ampr | 54 |
| NA335 | C6706 mutant lacking toxR gene; Smr | This study |
| NA111 | NA44 carrying pJW15 plasmid; Smr Ampr Kanr | This study |
| NA70 | NA44 carrying pN3 plasmid; Smr Ampr Kanr | This study |
| NA102 | NA44 carrying pN4 plasmid; Smr Ampr Kanr | This study |
| NA103 | NA44 carrying pN5 plasmid; Smr Ampr Kanr | This study |
| NA107 | NA44 carrying pN7 plasmid; Smr Ampr Kanr | This study |
| NA115 | NA44 carrying pN9 plasmid; Smr Ampr Kanr | This study |
| NA109 | NA44 carrying pN10 plasmid; Smr Ampr Kanr | This study |
| NA339 | NA335 carrying pBAD24 plasmid; Smr Ampr | This study |
| NA343 | NA335 carrying pCpxR plasmid; Smr Ampr | This study |
| EC16554 | Derivative of C6706 strain carrying TnFGL3 insertion in the cpxR gene (VC2692); Smr Kanr | 60 |
| EC18098 | Derivative of strain C6706 carrying TnFGL3 insertion in the ompU gene (VC0633); Smr Kanr | 60 |
| EC4591 | Derivative of strain C6706 carrying TnFGL3 insertion in the ompT gene (VC1854); Smr Kanr | 60 |
| EC10705 | Derivative of strain C6706 carrying TnFGL3 insertion in the ompR gene (VC2714); Smr Kanr | 60 |
| EC14253 | Derivative of strain C6706 carrying TnFGL3 insertion in the crp gene (VC2614); Smr Kanr | 60 |
| NA242 | EC10705 carrying pCpxR plasmid; Smr Kanr Ampr | This study |
| NA410 | EC14253 carrying pBAD24 plasmid; Smr Kanr Ampr | This study |
| NA409 | EC14253 carrying pCpxR plasmid; Smr Kanr Ampr | This study |
| Escherichia coli MG1655R | F− λ− ilvG-rfb-50 rph-1, carrying pBAD24 plasmid; Rifr Ampr | T. L. Raivio (University of Alberta) |
| Escherichia coli DH5α λpir | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 ϕ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Daniel Provenzano (University of Texas at Brownsville) |
| Escherichia coli SM10λpir | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu pir; Kmr | J. Mekalanos (Harvard Medical School) |
| Plasmids | ||
| pBAD24 | pBAD vector, pBR322 ori, araC; Ampr | 58 |
| pCpxR | pBAD24 carrying cpxR of Vibrio cholerae C6706; Ampr | 54 |
| pJW15 | pNLP10 with p15 ori reporter vector; Kanr | 39 |
| pN3 | cpxP promoter cloned into luxCDABE reporter vector pJW15; Kanr | 54 |
| pN4 | toxR promoter cloned into luxCDABE reporter vector pJW15; Kanr | This study |
| pN5 | ctxA promoter cloned into luxCDABE reporter vector pJW15; Kanr | This study |
| pN7 | tcpA promoter cloned into luxCDABE reporter vector pJW15; Kanr | This study |
| pN9 | tcpP promoter cloned into luxCDABE reporter vector pJW15; Kanr | This study |
| pN10 | toxT promoter cloned into luxCDABE reporter vector pJW15; Kanr | This study |
| pWM91 | oriR6K mobRP4 lacI ptac tnp mini-Tn10Km; Kanr Ampr | 59 |
To construct toxR-lux, ctxA-lux, tcpA-lux, tcpP-lux, and toxT-lux reporter plasmids, the promoter regions of toxR, ctxA, tcpA, tcpP, and toxT were amplified by PCR using primers listed in Table S1 in the supplemental material and cloned between the EcoRI and BamHI sites of the pJW15 vector (39). The resulting plasmids were designated pN4, pN5, pN7, pN9, and pN10.
RNA analyses.
We used quantitative reverse transcription-PCR (qRT-PCR) to validate the effect of cpxR overproduction on toxR and toxS gene expression as previously described (54). For analysis of the Cpx regulation of virulence factors, wild-type V. cholerae El Tor C6706, the cpxR transposon insertion mutant, and strain C6706 carrying the overexpression plasmid pCpxR were evaluated using two independent RNA preparations. Total RNA was extracted from cultures under AKI conditions (as explained above). After the static growth at 37°C, cultures were transferred to shaking growth conditions, and 0.1% arabinose was added to induce the overproduction of cpxR. When cultures reached an optical density at 600 nm (OD600) of ∼0.8, 1 ml of culture was harvested and resuspended in 1 ml of TRIzol reagent (Ambion), and total RNA was extracted as previously described (54). For each target gene, specific primers (see Table S1 in the supplemental material) were designed to amplify nucleotide fragments of ≈100 bp. qRT-PCR was performed using a 7500 Fast real-time PCR system (Applied Biosystems) as previously described (54). As described in reference 54, the gyrA gene was used as the endogenous control for comparison, since it was unaffected by CpxR overexpression.
Luminescence assay.
The luminescence activity produced by the vector control (pJW15) and the toxR-lux, ctxA-lux, tcpA-lux, tcpP-lux, and toxT-lux reporter plasmids in wild-type V. cholerae El Tor C6706 carrying pCpxR were performed under AKI medium (57). Briefly, cultures grown overnight in LB were subcultured under AKI conditions (described above). After 6 h of static growth, 198 μl of culture was transferred to a 96-well microtiter plate, induced with 0.1% arabinose, and returned to 37°C with agitation. The OD600 and the luminescence counts per second (CPS) were read every hour for up to 4 h postinduction. For time course luminescence assay analysis, following 6 h of static growth at 37°C, cultures were transferred to shaking growth conditions (125-ml flask) and induced with 0.1% arabinose to overexpress cpxR. Every 2 h, 200 μl of sample was collected, and the OD600 and CPS were read for a period of 16 h postinduction. Measurements were done using a Wallac 1420 multilabel plate reader (Perkin-Elmer).
Detection of OM profile.
OM samples were collected by subculturing V. cholerae strains under AKI conditions as described above. After transference and induction of the strains carrying the pCpxR or pBAD24 plasmid with 0.1% arabinose, cells were harvested in volumes normalized by the OD600 when cultures reached an OD600 of ∼0.8. OM samples were collected and extracted as previously described (61). OM preparations were electrophoresed on an SDS–10% PAGE, followed by staining with Coomassie blue for visualization.
Western blot analysis.
Expression of CT, TCP, and T2SS was measured by Western blotting against the Ctx-B subunits TcpA, EpsL, and EpsG, respectively. Briefly, whole-cell lysates and supernatant were collected by subculturing V. cholerae strains under AKI conditions as described above. The strains carrying the pCpxR or pBAD24 plasmid were induced right after the transference to the flask with 0.1% arabinose to induce the overproduction of cpxR, and then samples were collected after 16 h of incubation at 37°C. For time course Western blot experiments, every 2 h, a 1-ml equivalent of sample was collected for a period of 16 h postinduction. The expression of the T6SS was measured by Western blotting against the Hcp protein. Briefly, whole-cell lysates and supernatant were collected by subculturing V. cholerae O37 serogroup strain V52 (1:100) in LB broth for 1.5 h at 37°C before being induced with 0.1% arabinose, followed by an additional incubation at 37°C until they reached an OD600 of ∼0.65, when samples were collected. For all Western blot analyses, supernatants were filtered through 0.22-μm low-protein-binding polyvinylidene fluoride (PVDF) syringe filters (Millipore) and concentrated with 20% trichloroacetic acid (TCA). Whole-cell lysates and supernatant were electrophoresed on SDS-PAGE (10 or 12%) gels and transferred to nitrocellulose membranes as previously described (62). The blots were incubated with a 1:5,000 dilution of anti-CtxB, a 1:100,000 dilution of anti-TcpA, a 1:10,000 dilution of anti-CpxREC, a 1:100,000 dilution of anti-EpsG, a 1:20,000 dilution of anti-EpsL, a 1:500 dilution of anti-Hcp (63), and a 1:25,000 dilution of anti-rabbit immunoglobulin G-alkaline phosphatase conjugates (Sigma). Blots were developed as previously described (40).
GM1-ELISA.
CT production was determined by a GM1-based enzyme-linked immunosorbent assay (ELISA) as described previously (64), using V. cholerae El Tor C6706 strains carrying either pBAD24 or pCpxR culture supernatants under AKI conditions (described above). GM1-ELISA was performed using a 1:2,000 dilution of anti-CtxB and a 1:2,000 dilution of anti-rabbit immunoglobulin G-alkaline phosphatase conjugates (Santa Cruz Biotechnology). A CT standard curve was generated to estimate the amount of CT in the supernatant samples. The color intensity was measured as the OD405 in a Bio-Rad xMark microplate spectrophotometer (Bio-Rad).
Bacterial killing assay.
To assess the effect of Cpx activation on the expression of T6SS components, the susceptibility of Escherichia coli MG1655R to T6SS-mediated killing by the predator strain V. cholerae O37 serogroup V52 was assessed as described previously (32). Briefly, predator and prey strains were grown as lawns on LB plates plus selective antibiotics and resuspended in LB broth. Prey and predator were mixed at a 1:10 ratio and spotted onto predried LB agar plates in the absence or presence of 0.1% arabinose to induce the Cpx pathway. After an incubation of 4 h at 37°C, each spot was harvested, serially diluted, and spotted onto LB plates plus selective antibiotics and then incubated overnight at 37°C. Surviving prey (CFU/ml) were enumerated.
Motility analysis.
Two microliters of overnight cultures of V. cholerae El Tor C6706 and V. cholerae O37 serogroup strain V52, which carry either pBAD24 or pCpxR plasmids, was inoculated onto 0.3% LB agar plates in the absence or presence of 0.1% arabinose to induce the overproduction of CpxR. The diameter of the swim zones was recorded after 16 h of growing with the appropriate antibiotics. All of the inoculations were made in triplicate.
RESULTS
The Cpx response positively regulates the toxRS operon.
We previously demonstrated that activation of the Cpx pathway in V. cholerae El Tor strain C6706 leads to an increase in expression of the toxS (VC0983) and toxR (VC0984) genes (54). ToxR is a transcriptional regulator located in the inner membrane that contains an N-terminal domain with strong homology to the OmpR/PhoB protein family (65). ToxR activity is enhanced by the presence of the transmembrane protein, ToxS (66). To verify the observed regulation of toxS and toxR transcription by the Cpx pathway, we performed a qRT-PCR analysis and observed an increase in expression of both genes when the Cpx response was activated by means of CpxR overexpression (Fig. 1A). For this experiment and all those in which the Cpx pathway was activated, we used a previously constructed CpxR overexpression vector (pCpxR) in V. cholerae (54). We have shown that this method of Cpx pathway activation recapitulates induction of the Cpx response using envelope stress signals (54). Although we have not uncovered any cases where CpxR overexpression results in regulation of “false” target genes yet, we note that since this is not a natural activation of the Cpx response, it is possible that induction of the intact Cpx signaling pathway by exogenous inducers leads to different effects on gene expression than those observed here. We are currently investigating the impact of newly identified Cpx-inducing signals on the V. cholerae virulence regulon.
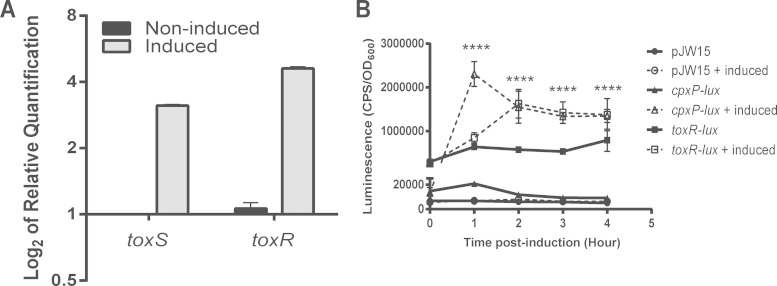
FIG 1.
Activation of the Cpx pathway regulates ToxR. (A) qRT-PCR analysis of toxS and toxR transcript levels. RNA was isolated from cultures of V. cholerae C6706, carrying the overexpression plasmid pCpxR, in the absence (noninduced; black bars) or presence of 0.1% arabinose (induced; gray bars) and converted to cDNA. The cDNA was subjected to qRT-PCR analysis as described in Materials and Methods. (B) Luminescence activity of V. cholerae C6706 carrying the overexpression plasmid pCpxR, transformed with the vector control (pJW15), cpxP-lux, or toxR-lux reporter. CpxR was overexpressed by adding 0.1% arabinose (induced). Reporter gene expression was measured as described in Materials and Methods and is reported as CPS corrected for cell density (OD600). Time zero represents the time when cells were shifted to shaking conditions and induced after the culture first was statically grown for 6 h. The overall averages and standard deviations resulting from two separate experiments performed in quintuplicate are shown. The asterisks indicate a statistically significant difference between induced and noninduced treatments for the toxR-lux reporter (P < 0.0001 by two-way ANOVA with Sidak's multiple-comparison test).
To study the temporal regulation of toxR transcription by the Cpx response, we constructed transcriptional fusions of the promoter region of toxR with the light-producing luxCDABE operon located on previously described reporter plasmid pJW15 (39). We used this reporter to measure expression of toxR when the Cpx pathway is activated. A cpxP-lux reporter was utilized as a positive control for the induction of the Cpx pathway using the same background strain and conditions. The cpxP-lux reporter was induced strongly by Cpx response activation, producing increased luminescence within the first hour after stimulation of CpxR overexpression from an arabinose-inducible promoter on the pBAD24 plasmid (Fig. 1B). As previously reported (67, 68), the activity of the toxR-lux reporter was mostly constant throughout growth under AKI conditions (Fig. 1B). When CpxR expression was induced, luminescence produced by the toxR-lux reporter increased steadily over 2 h to a level approximately twice that observed under noninduced conditions (Fig. 1B). After 2 h, toxR-lux transcription remained at high levels throughout the experiment (Fig. 1B). Although toxR expression generally is considered to be constitutive (69), our data suggest that CpxR, an envelope stress transcriptional regulator, positively regulates the expression of the toxRS operon. The delay in induction of toxR expression relative to that of the strongly regulated cpxP-lux reporter gene upon CpxR overexpression suggests that this effect is indirect (Fig. 1B).
The Cpx pathway regulates OmpT expression.
ToxR regulates the expression of two OM proteins in V. cholerae, OmpU and OmpT, by directly activating the ompU promoter and repressing the expression of the ompT promoter, which is important for bile resistance (70–73). In E. coli, the Cpx pathway directly and indirectly regulates the expression of OmpC and OmpF, the major porin constituents of the OM (74, 75). To test whether the activation of the Cpx pathway similarly regulates the expression of OmpU and OmpT in V. cholerae El Tor strain C6706, we examined the porin content in the OM in the absence and presence of CpxR overexpression. Wild-type C6706 carrying either the vector control (pBAD24) or the pCpxR plasmid was grown under AKI conditions in the presence of arabinose to induce CpxR expression. When OM preparations were analyzed by SDS-PAGE, OmpT levels were reduced when cpxR was overexpressed by addition of the inducer arabinose compared to levels under the noninducing conditions or in the vector control (Fig. 2A). The absence of this band in an ompT transposon insertion mutant confirmed that the protein diminished by CpxR overexpression was OmpT (Fig. 2A, compare lanes 4 and 6). No change in the level of the OmpU porin was observed upon CpxR overexpression (Fig. 2A, compare lanes 3 and 4).
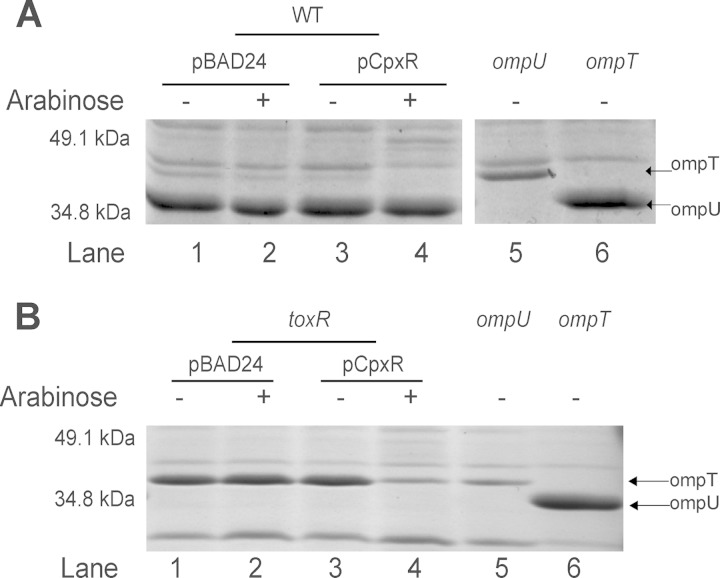
FIG 2.
Activation of the Cpx pathway downregulates OmpT expression. (A) Outer membrane (OM) protein profiles of V. cholerae C6706 carrying either pBAD24 (lane 1 and 2) or pCpxR (lane 3 and 4), ompU (lane 5), and ompT (lane 6). (B) OM protein profiles of the following deletion strains: a toxR mutant carrying either pBAD24 (lanes 1 and 2) or pCpxR (lanes 3 and 4), and ompU (lane 5) and ompT (lane 6) mutants. All strains were grown under AKI conditions at 37°C as described in Materials and Methods. OM proteins were resolved by 10% SDS-PAGE, followed by staining with Coomassie blue. The Cpx pathway was activated by inducing CpxR overexpression with 0.1% arabinose. Samples were collected from each strain at least three times; one representative SDS-PAGE is shown.
ToxR inhibits ompT expression, and the levels of OmpT in the OM are elevated in cells lacking ToxR (71, 72). To determine if the negative regulation of OmpT by CpxR is ToxR dependent, we examined the porin content in the OM from the wild-type C6706 strain and the toxR isogenic mutant under Cpx-activating conditions (i.e., overexpression of CpxR) and under AKI conditions. As expected, since ToxR is an ompU activator and ompT repressor (70, 71), in the toxR mutant there was a decrease in OmpU levels and an increase in OmpT levels in the OM (Fig. 2B, lanes 1 to 3). However, when the Cpx pathway was induced in the toxR mutant by the addition of arabinose to stimulate CpxR overexpression, OmpT levels still were diminished (Fig. 2B, compare lanes 3 and 4), although they were at higher levels than those observed under the same conditions in the wild-type strain. These data suggest that the negative regulation of OmpT by CpxR is mostly ToxR independent, although we cannot rule out that the positive regulation of ToxR by the Cpx response plays a small part in the inhibition of ompT expression.
In E. coli, the Cpx response controls porin expression by upregulating a small inner membrane protein called MzrA that stimulates the EnvZ-OmpR two-component system (75, 76). The response regulator OmpR in turn inversely regulates the expression of the two major OMPs in E. coli, upregulating OmpC production and inhibiting expression of OmpF (77). To assess if the OmpR (VC2714) homologue in V. cholerae El Tor C6706 was involved in the Cpx regulation of OmpT, we compared the porin content in OMs isolated from wild-type C6706 and an ompR transposon insertion mutant under Cpx-activating conditions. OmpT levels changed in the ompR transposon insertion mutant upon CpxR overexpression in a manner identical to that observed in the wild-type C6706 strain, which suggests that the negative regulation of OmpT by CpxR also is OmpR independent (see Fig. S1 in the supplemental material, compare lanes 3 and 4 to 7 and 8).
The V. cholerae Cpx response regulates the expression of CT and TCP.
Since CpxR overexpression resulted in increased levels of toxR transcription, a major virulence factor regulatory protein in V. cholerae, we sought to determine whether the Cpx system is involved in the regulation of virulence factors, as shown previously in other Gram-negative pathogens (38). We determined the effect of the activation of the Cpx pathway on the expression of cholera toxin and TCP, the two major virulence factors in V. cholerae El Tor strain C6706, in the presence of the pCpxR overexpression plasmid or the pBAD24 vector control and grown under AKI conditions (57). Production of CT was measured by Western blotting using antibodies directed against the Ctx-B subunit. As shown in Fig. 3A, secretion of cholera toxin was abolished upon the overexpression of CpxR in V. cholerae El Tor C6706 compared to that of the vector and uninduced controls (compare lanes 3, 4, and 5 to lane 6). To confirm the reduced levels of cholera toxin in the supernatant upon activation of the Cpx pathway, levels of cholera toxin in the culture medium of cells grown in the presence and absence of arabinose also were assessed using an ELISA. In agreement with the Western blot analysis (Fig. 3A), the results showed that the Cpx pathway has a large effect on cholera toxin production (Fig. 3B).
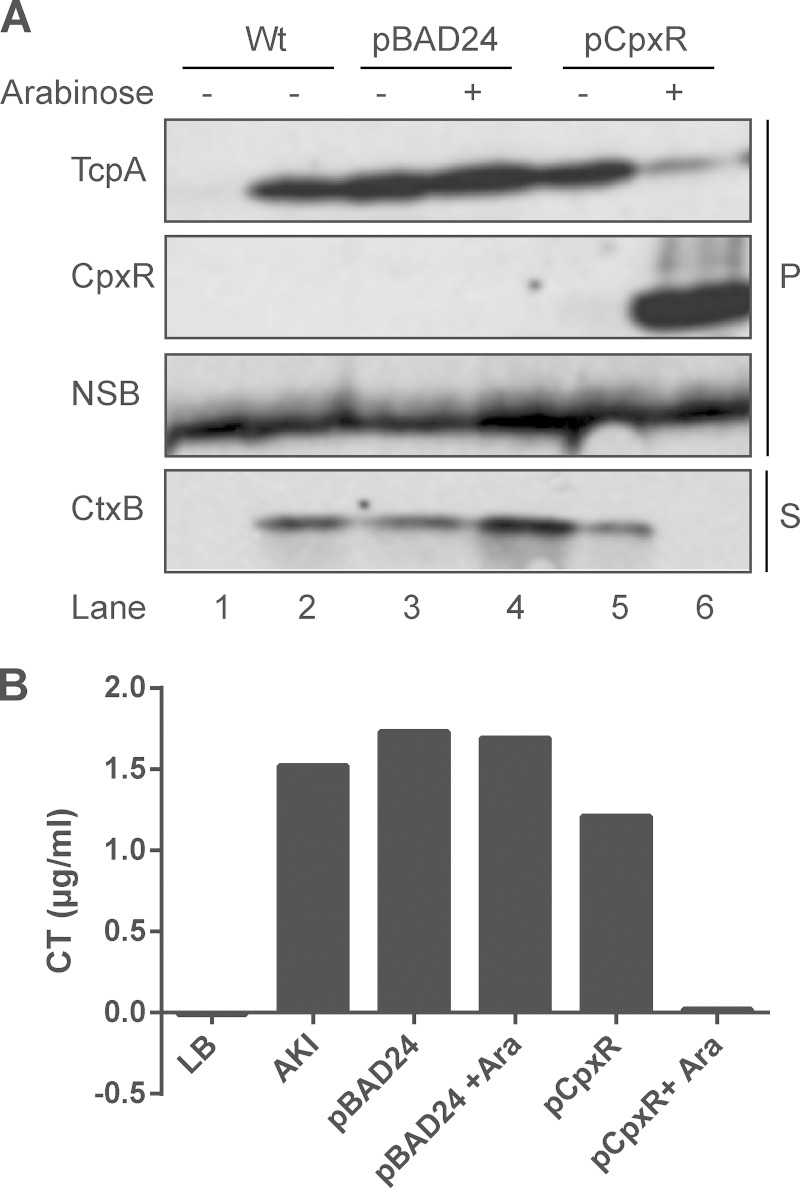
FIG 3.
Cholera toxin and TCP are reduced when the Cpx response is activated. Cell pellets (P) and supernatants (S) were collected from V. cholerae El Tor C6706 grown in LB (lane 1) or AKI medium (lanes 2 to 6) as described in Materials and Methods. Subcultures were grown for 6 h statically at 37°C before the addition of 0.1% arabinose to induce CpxR overexpression, followed by an additional 16 h of incubation at 37°C. Samples were analyzed by Western blotting against TcpA, CpxR, and CtxB (A) or ELISA (B). NSB, nonspecific band.
In V. cholerae, the type II secretion system (T2SS) is required for extracellular secretion of several proteins, including cholera toxin (78, 79). To test if the observed reduction of cholera toxin in the supernatant when the Cpx pathway was activated was due to a downregulation of the T2SS by the Cpx pathway, we measured the expression of EpsL and EpsG, both components of the T2SS in V. cholerae (80, 81). We determined that the activation of the Cpx pathway does not have any effect on the expression of the T2SS. No differences in the expression of EpsL and EpsG were observed in samples collected from the CpxR overexpression strain compared to the vector control strain (see Fig. S2 in the supplemental material).
To analyze TCP protein levels upon activation of the Cpx pathway, we examined the expression of TCP by measuring the expression of TcpA, the major subunit of TCP, in whole-cell lysates from cells grown under AKI conditions, which are favorable for TCP expression in V. cholerae (57) (see Materials and Methods). TcpA expression was reduced in wild-type C6706 carrying the pCpxR vector in the presence of arabinose compared to the uninduced sample and the vector control (Fig. 3A, compare lanes 3, 4, and 5 to lane 6), showing a trend similar to that seen with the expression of Ctx-B (Fig. 3A). These results suggest that the Cpx pathway negatively regulates expression of CT and the TCP in V. cholerae El Tor strain C6706.
To investigate if the Cpx pathway also plays a role in regulating the expression of other V. cholerae envelope-localized virulence factors, we determined if overexpression of CpxR had an effect on T6SS in V. cholerae O37 serogroup strain V52, which constitutively expresses an active T6SS (31). We also examined motility in V. cholerae El Tor strains C6706 and V52. Using Western blotting and a T6SS-mediated bacterial killing assay as previously described (32), we found that CpxR overexpression did not alter the synthesis and secretion of the hemolysin-coregulated protein (Hcp), the major T6SS component (see Fig. S3A in the supplemental material). These results suggest that the Cpx envelope stress response is not involved in regulating the biogenesis of the T6SS in V. cholerae V52, which is consistent with a previous study that showed that the elaboration and assembly of the T6SS is a dynamic process that occurs in the bacterial cytosol (82). We did notice that CpxR overexpression resulted in a 100-fold decrease in the ability of V. cholerae V52 to kill E. coli, however (see Fig. S3B), suggesting that the Cpx response somehow regulates the activity of the T6SS. In contrast to the case for E. coli (41), activation of the Cpx pathway did not impact motility in the V. cholerae strain El Tor C6706 or V52 (see Fig. S4).
Transcription of both ctxA and tcpA is inhibited by the Cpx pathway.
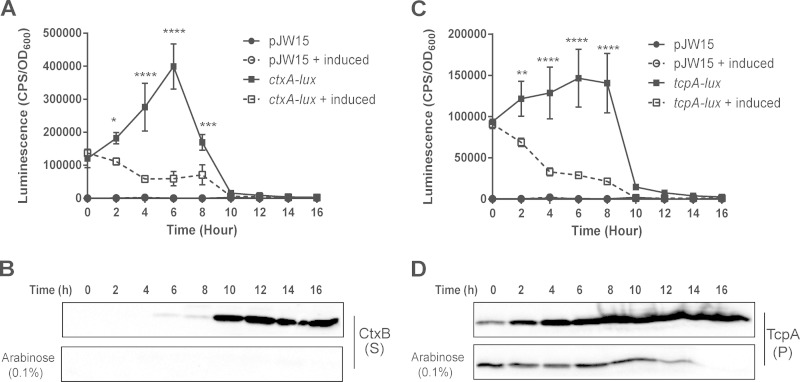
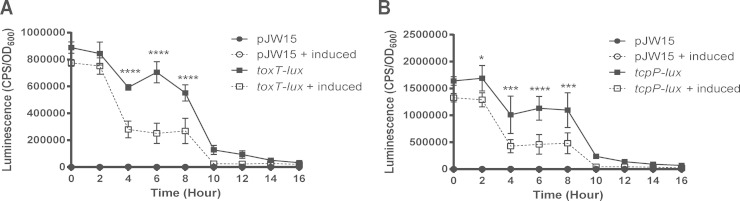
To determine if the negative regulation of CT and the TCP by CpxR occurred at the transcriptional level, we constructed ctxA-lux and tcpA-lux transcriptional reporter genes using the previously described reporter plasmid pJW15 (39). We used these reporters to measure ctxA and tcpA transcription when the Cpx pathway was activated by overexpressing CpxR under conditions shown to maximize CT production (57). As previously reported (68), expression of ctxA was detected after the AKI medium cultures were switched from static to shaking growth conditions (aerobic) and increased linearly over 6 h of growth (Fig. 4A). Consistent with these results, cholera toxin was detectable in the supernatants of cultures grown in AKI medium 6 h after the cultures were switched to aerating conditions and increased over 16 h of growth (Fig. 4B). Expression of ctxA was dramatically diminished when the Cpx pathway was activated, where the luminescence resulting from the ctxA-lux reporter decreased over time (Fig. 4A) and there was no detectable cholera toxin in the supernatant (Fig. 4B). Similarly, tcpA-lux expression was significantly lower in V. cholerae El Tor C6706 carrying the pCpxR overexpression plasmid in the presence of arabinose (Fig. 4C). Consistent with this finding, we observed a decrease in TcpA protein levels in the cell pellets of cultures when the Cpx pathway was activated (Fig. 4D). These findings suggest that the Cpx-mediated negative regulation of CT and TCP occurs at the transcriptional level.
FIG 4.
Activation of the Cpx pathway reduces ctxA and tcpA expression at the transcriptional level. Luminescence activity of V. cholerae C6706 carrying the overexpression plasmid pCpxR, transformed with either the vector control (pJW15) or ctxA-lux (A) and tcpA-lux (C) reporters. Reporter gene expression was measured as described in Materials and Methods and is reported as CPS corrected for cell density (OD600). The overall averages and standard deviations resulting from two separate experiments performed in triplicate are shown. Pellet (P) and supernatant (S) samples were collected from V. cholerae C6706 carrying the overexpression plasmid pCpxR and grown under AKI conditions as described in Materials and Methods. Samples were analyzed by Western blotting against CtxB (B) and TcpA (D). CpxR was overexpressed by adding 0.1% arabinose (induced). Time zero represents the time when cells were shifted to shaking conditions after cultures first were grown statically for 6 h under AKI conditions. The asterisks indicate a statistically significant difference between induced and noninduced treatments for the ctxA-lux and tcpA-lux reporters (P values by two-way ANOVA with Sidak's multiple-comparison test: *, 0.01 to 0.05; **, 0.001 to 0.01; ***, 0.0001 to 0.001; ****, <0.0001).
The Cpx pathway downregulates transcription of multiple regulators of virulence.
It is probable that the negative regulation of CT and TCP by the Cpx pathway is indirect, because a consensus CpxR binding site is not found in the promoter regions of the ctx or tcp gene by the Virtual Footprint tool (http://prodoric.tu-bs.de/vfp/) (83). Thus, we examined known regulators of CT and TCP in order to determine whether the Cpx pathway inhibits virulence factor production indirectly. Cholera toxin and TCP expression are controlled by a hierarchical regulatory system known as the ToxR regulon. ToxR, in conjunction with another inner membrane regulator, TcpP, positively controls the expression of the master virulence regulator ToxT (for a review, see reference 56). We were unable to assess whether the Cpx-mediated CT and TCP downregulation we observed occurs through ToxR, since those virulence factors were not detectable in a toxR mutant (data not shown).
To determine if CpxR affected the direct regulator of CT and TCP expression, ToxT, in V. cholerae El Tor strain C6706, we constructed a toxT-lux luminescent reporter gene as previously described (39). Expression of toxT was repressed upon activation of the Cpx pathway. The luminescence of the toxT-lux reporter decreased over time after the addition of the inducer arabinose to stimulate CpxR overproduction (Fig. 5A). Because toxT regulation is dependent on the expression of upstream regulators in the ToxR virulence cascade (15, 84), we also measured the expression of tcpP upon CpxR overexpression in the same fashion. The expression of the transcriptional regulator TcpP also was repressed when the Cpx pathway was activated under AKI conditions in a fashion similar to that of toxT (Fig. 5B). Thus, our data suggest that the Cpx response inhibits toxT transcription through its negative effect on transcription from the tcpPH promoter.
FIG 5.
Cpx pathway regulates expression of major virulence factor regulators. Luminescence activity of V. cholerae El Tor C6706 carrying the overexpression plasmid pCpxR and transformed with either the vector control (pJW15) or toxT-lux (A) and tcpP-lux (B) reporters. Reporter gene expression was measured as described in Materials and Methods and is reported as CPS corrected for cell density (OD600). The overall averages and standard deviations resulting from two separate experiments performed in triplicate are shown. The asterisks indicate a statistically significant difference between induced and noninduced treatments for the toxT-lux and tcpP-lux reporters (P value by two-way ANOVA with Sidak's multiple-comparison test: *, 0.01 to 0.05; **, 0.001 to 0.01; ***, 0.0001 to 0.001; ****, <0.0001).
The tcpPH operon is positively regulated by direct binding of the LysR type transcription factor AphB, in conjunction with the AphA protein, to its promoter region (24, 25, 28). To determine if Cpx regulation of aphA and/or aphB transcription is involved in the negative effect of the Cpx response on tcpPH, we used qRT-PCR to measure the expression of these genes when the Cpx response was activated by CpxR overexpression (Fig. 6A). We observed that while expression of the accessory regulator AphA was unaffected by Cpx pathway activation, there was a 2-fold decrease in the expression of aphB when cpxR was overexpressed (Fig. 6A). These data suggest that the Cpx response affects the expression of regulators upstream of the ToxR virulence cascade.
FIG 6.
Cpx pathway regulates transcript levels of virulence factors and regulators. qRT-PCR analysis of aphA, aphB, crp, ctxB, tcpA, tcpP, and toxR transcript levels. RNA was isolated from cultures of wild-type V. cholerae C6706 carrying the overexpression plasmid pCpxR, in the absence (noninduced; black bars) or presence of 0.1% arabinose (induced; gray bars) (A), and of V. cholerae C6706 strain (black bars) and the cpxR transposon insertion mutant (gray bars) and converted to cDNA (B). The cDNA was subjected to qRT-PCR analysis as described in Materials and Methods. The asterisks indicate a statistically significant difference between induced and noninduced treatments (A) or between the wild-type and cpxR mutant strains (B) (P value by two-way ANOVA with Sidak's multiple-comparison test: *, 0.01 to 0.05; **, 0.001 to 0.01; ***, 0.0001 to 0.001; ****, <0.0001).
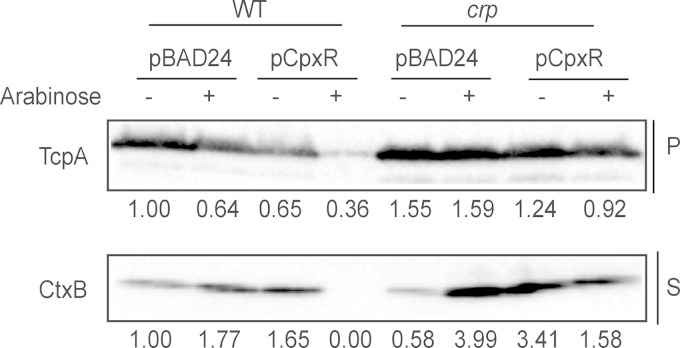
In both classical and El Tor V. cholerae strains it has been demonstrated that CRP has a negative effect on expression of CT and TCP, and this appears to be due to its ability to repress expression of the tcpPH operon (26, 27). Accordingly, we sought to determine if CRP could be involved in the inhibition of CT and TCP production by the Cpx response. To do this, we examined CT and TCP production in a crp transposon insertion mutant when the Cpx pathway was activated (Fig. 7). As previously reported (85), in the absence of CRP, levels of both CT and TCP were elevated (Fig. 7). While activation of the Cpx response by CpxR overexpression in the absence of CRP resulted in a small decrease in TCP and CT levels, the magnitude of this change obviously was smaller than that seen in a wild-type C6706 strain background (Fig. 7). These data suggest that Cpx-mediated inhibition of CT and TCP production is, in large part, dependent on CRP.
FIG 7.
Cpx-mediated inhibition of CT and TCP production is diminished in the absence of cAMP receptor protein (CRP). Cell pellets (P) and supernatants (S) were collected from wild-type V. cholerae C6706 and crp strains grown in AKI medium as described in Materials and Methods. Subcultures were grown for 6 h statically at 37°C before the addition of 0.1% arabinose to induce CpxR overexpression, followed by an additional 16 h of incubation at 37°C. Samples were analyzed by Western blotting using antibodies directed against TcpA and CtxB. The numbers below each lane indicate the intensity of either the TcpA or the CtxB band in each sample relative to the wild-type strain.
Negative regulation of virulence by CpxR overexpression is not a pleiotropic phenotype.
All of our studies examining Cpx regulation involved overexpression of the regulator CpxR. Although we have previously published that this condition accurately recapitulates activation of the Cpx response by envelope stress signals sensed via CpxA (54), we wanted to confirm that the negative effect of the Cpx response on virulence factor production was not an artificial effect of CpxR overexpression. Accordingly, we examined the transcript levels of virulence-related genes in the wild-type C6706 strain and the cpxR transposon insertion mutant under AKI virulence-inducing conditions. In spite of the fact that we did not detect CpxR protein by Western blotting under the same conditions used here (Fig. 1), there was an increase in the expression of ctxB, tcpA, and tcpP in the cpxR transposon insertion mutant and a decrease in the toxR transcript level in the same background (Fig. 6B), consistent with our finding that CpxR overexpression leads to inhibition of transcription of all of these genes. We conclude that some basal level of CpxR must be present in V. cholerae under these conditions that exerts a negative effect on expression of the virulence cascade. One strong possibility is that we are unable to detect basal levels of CpxR with our antiserum, which was raised against an E. coli MBP-CpxR fusion protein of which the CpxR portion is only 60% identical to that of V. cholerae. These data support our finding that the Cpx response negatively regulates virulence through the ToxT regulatory cascade, even in the absence of ectopic overexpression of CpxR.
DISCUSSION
Several studies have proposed that the Cpx pathway plays an important role in bacterial pathogenesis by regulating, positively and negatively, envelope-localized virulence factors (38). Based on these studies, we hypothesized that the Cpx pathway also regulates envelope-localized virulence factors in V. cholerae. In support of this hypothesis, we recently reported that the Cpx response regulates the expression of the toxRS operon (54). Expression of the toxRS operon has been considered to be constitutively active in LB broth or under virulence-inducing conditions (69, 86). However, we confirm here via qRT-PCR and luminescent reporter gene assays that activation of the Cpx response leads to upregulated transcription from the toxRS promoter (Fig. 1). The mechanism by which the Cpx response regulates the expression of the toxRS operon remains unclear; however, this regulation appears to be independent of the growth conditions (it occurs in both LB and AKI conditions) (data not shown). In addition, we think that this regulation is indirect, since there was a delay in the induction of toxR expression when the Cpx response is activated, and there is not a putative CpxR binding site in the promoter region of toxR.
Previous studies on E. coli have reported that the Cpx pathway regulates the expression of OM proteins (74), and some of its regulon members are required for their proper assembly (87). Since we found that CpxR positively regulates the expression of toxR, which regulates the expression of the two major OM porins in V. cholerae (70–72), we assessed whether the activation of the Cpx pathway also impacted OmpU and OmpT levels. We found that activation of the Cpx pathway leads to a decrease in the expression of the OmpT porin. A decrease in OmpT protein levels was detected in OM preparations when CpxR was overexpressed (Fig. 2). Similarly, a recent microarray study showed that activation of the Cpx response in the V. cholerae El Tor strain N16961, which is closely related to the V. cholerae El Tor strain C6706 used in this study (53), also resulted in downregulation of ompT expression (55). Although ToxR negatively regulates the production of OmpT (71, 72) and we found that the Cpx pathway activates the expression of the toxRS operon, the negative regulation of ompT expression by the Cpx pathway appears to be ToxR independent (Fig. 2B). The expression of OmpT also is regulated by CRP (71, 88), whose activity was found to be regulated by the Cpx response in this study (see below). Thus, the possibility remains that the Cpx response turns off OmpT production via a stimulatory effect on CRP rather than through direct regulation, since we did not find a putative CpxR binding site in the promoter region of ompT. At this point we cannot distinguish between these possibilities. Thus, regulation of OMP expression appears to be conserved between relatively diverse enteric pathogens, although it occurs via different regulatory circuitries. We previously showed that the Cpx pathway also regulates the expression of another important OMP, TolC (54). Together, these observations suggest an important role for the Cpx response in the trafficking of toxic components across the OM in V. cholerae El Tor strain C6706.
Our analyses showed that the activation of the Cpx response led to a decrease in the expression of some of the major virulence factors in V. cholerae, CT and TCP (Fig. 3A). Most likely, this negative regulation is at the transcriptional level, since we found a decrease in the expression of transcriptional reporters for both the ctxA and tcpA genes when the Cpx response was activated (Fig. 4). This observation is in apparent contradiction to our finding that when cpxR is overexpressed in V. cholerae El Tor strain C6706, it upregulates the expression of the virulence regulator ToxR (54), an activator of ctxA and tcpA expression (16, 56). In this regard, a recent study showed for the first time that ToxR can negatively regulate the production of CT and TCP in response to cyclic dipeptides (CDPs) (89). Bacterial production of cyclo(Phe-Pro) leads to ToxR-mediated activation of the LysR transcription factor LeuO, which in turn represses CT production (89). At this point, we cannot say whether Cpx-mediated activation of ToxR production is involved in the inhibition of CT and TCP via CDPs or some other mechanism (Fig. 8). A future goal of this work will be to examine this question by determining if ToxR-dependent regulation of leuO expression is altered when the Cpx response is induced. Interestingly, CDPs have been shown to accumulate in a growth-dependent manner in V. cholerae, specifically at stationary phase (90), where the Cpx response also is enhanced in E. coli (91).
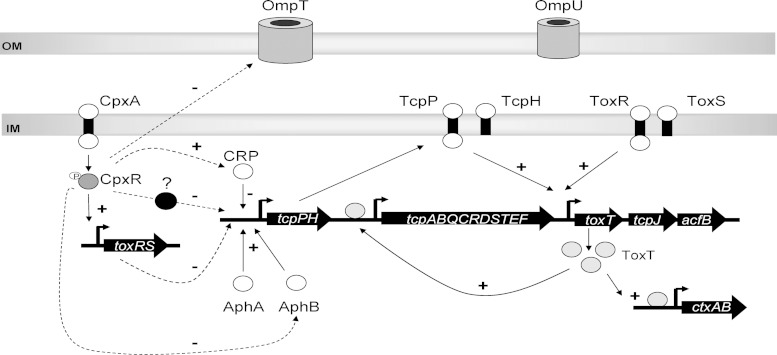
FIG 8.
Cpx pathway and virulence factor regulation in V. cholerae. Activation of the Cpx response leads to downregulation of some virulence factors, CT and TCP, in V. cholerae at the transcriptional level. This negative regulation of CT and TPC by the Cpx response is mediated mainly by changing the activity of the negative regulator of the tcpPH promoter, CRP. Therefore, this leads to a reduction of the expression of tcpP and the downstream regulator of the ToxR regulatory cascade, ToxT. In addition, CpxR may regulate CT and TCP through the regulation of the virulence regulators ToxR and AphB. Finally, activation of the Cpx response mediated changes in the outer membrane (OM) composition by repressing the expression of the OM porin OmpT in a ToxR-independent manner. IM, inner membrane.
CT, TCP, and other virulence-associated genes are controlled by a regulatory network known as the ToxR regulon (15, 84), in which the primary direct transcriptional regulator of virulence gene expression is the ToxT protein (for a review, see reference 69). We found that activation of the Cpx response downregulates the expression of toxT (Fig. 5). This suggests that the negative regulation of CT and TCP by the Cpx response is due indirectly to a decrease in toxT expression. In support of this model, we found that CpxR negatively regulates the transcription of additional genes in the virulence regulatory hierarchy, upstream of toxT, including the transmembrane response regulator TcpP (Fig. 5). We suspect that this is also indirect, since we were not able to identify a potential CpxR binding site in the tcpPH promoter region. We note that although CpxR overexpression resulted in diminished expression of several virulence regulators, their temporal pattern of expression was not altered (Fig. 5). This observation would be consistent with a dampening effect of the Cpx response at the top of the virulence hierarchy that nonetheless does not alter other activating inputs. The tcpPH locus is controlled by environmental signals (i.e., temperature and pH) and the transcriptional regulators AphA, AphB, and CRP (22, 24–27). AphA and AphB interact and bind directly upstream of the tcpPH operon to activate transcription (24, 25, 28). CRP binds to DNA sequences that overlap the AphA and AphB binding site and is thought to repress tcpPH transcription, perhaps partly by interfering with AphA/B-mediated activation (26). It has been proposed that CRP-mediated repression of CT and TCP expression, which occurs in both classical and El Tor biotype strains of V. cholerae (27), is mediated by its negative effect on AphA and AphB activity (26). We found here that the inhibition of CT and TCP expression was greatly reduced (although not abolished) in the crp mutant upon activation of the Cpx response, suggesting that the negative regulation of tcpPH expression by the Cpx response is modulated mainly by the regulation of CRP (Fig. 8). The fact that a small decrease in CT and TCP production still was observed upon CpxR overexpression in the crp mutant suggests that additional Cpx-regulated inputs independent of Crp also are at play.
At present, we cannot say exactly how the Cpx response regulates CRP. In our previous microarray study (54), crp was not found to be part of the Cpx regulon, and we confirmed here that overexpression of CpxR has no effect on crp transcript levels (Fig. 6A). These data indicate that the Cpx response stimulates CRP activity posttranscriptionally. We do not know how this occurs. One possibility is that a Cpx-regulated sRNA affects the translation or stability of the CRP-encoding mRNA. In this regard, we have shown that the Cpx response controls several known sRNAs in E. coli (92); however, it is not known whether this is true in V. cholerae, and no sRNAs have been described to date that regulate CRP expression. An alternative explanation is that the Cpx response alters the expression of sugar transporters that in turn affect levels of cAMP and CRP function. In support of this model, it has been demonstrated that mutation of cpxR in E. coli leads to changes in the expression of the CRP-regulated sRNA gene cyaR in a manner that is dependent on the Cpx-regulated glucose permease PtsG (92). We previously found that activation of the Cpx response impacted sugar transporters in V. cholerae as well, including the genes involved in galactose uptake (VC1325, VC1327, and VC1328) and utilization (VC1594, VC1595, and VC1596) (54). Based on these data, a plausible model is that the Cpx response negatively regulates CT and TCP expression indirectly by changing the activity of CRP in V. cholerae El Tor strain C6706, which in turn results in alterations in expression of activator proteins found at the top of the virulence regulatory hierarchy (Fig. 8).
In contrast to our results, Taylor and collaborators (55) recently reported that activation of the Cpx response had no impact on the expression of virulence factors (i.e., CT and TCP) in V. cholerae O1 El Tor strain N16961. At this point, we do not know the reason for this discrepancy, but it could be related to strain differences. For example, it is known that in V. cholerae O1 El Tor strain N16961 there is a frameshift mutation in the hapR gene (93); therefore, the quorum-sensing circuitry involved in the regulation of virulence factors is not intact in this strain. We also observed differences in the members of the Cpx regulon identified by Taylor and colleagues (55) compared to our study (54), further supporting the supposition that important Cpx-related regulatory network differences exist between V. cholerae El Tor strains C6706 and N16961.
The impact of the Cpx response on virulence gene regulation raises the question of when the Cpx pathway is important in the life cycle of V. cholerae. It was previously reported that there is an absence of intestinal growth defects of cpx mutants (i.e., cpxR, cpxA, cpxP, and cpxA*) using the suckling mouse model of V. cholerae (52). In spite of this, cpxP expression is induced in the small intestine of the mouse (94), and some Cpx regulon members (54) are enriched in transcriptome data sets described for V. cholerae isolated from human volunteers, human stool, and vomitus samples and from animal models (i.e., rabbit intestinal loop model and mice) (17, 95–99). In addition, several studies have suggested that expression of virulence factors (e.g., CT and TCP) is reduced during late infection (17, 89, 96–99). We speculate that the Cpx pathway plays a role in the adaptation of V. cholerae during late infection. This may be required for the negative regulation of virulence factors during the transition between the host and the environment. The limitation of nutrients such as iron during late infection (17, 94, 96) possibly triggers the activation of the Cpx response in V. cholerae (54).
Collectively, the findings presented in our study support the conclusion that the Cpx pathway in V. cholerae El Tor C6706 is involved in virulence gene regulation. In general, it is likely that the activation of the Cpx response downregulates envelope appendages in order to decrease unessential envelope protein traffic under envelope stress conditions (100). In pathogens, this may be an adaptive function associated with exit from the host. Taken together, our findings suggest that CpxR negatively regulates the two major virulence determinants of V. cholerae, CT and TCP, from the top of the ToxR regulatory cascade, in large part through regulating the activity of CRP, which impacts the expression of tcpPH and the downstream regulator toxT in V. cholerae El Tor C6706.
Supplementary Material
ACKNOWLEDGMENTS
We thank John J. Mekalanos for generously sharing some of the C6706 transposon mutants, Ronald K. Taylor for generously sharing the TcpA antibody, and Maria Sandkvist for generously sharing the EpsL and EpsG antibodies.
This work was funded by an operating grant from the Canadian Institute of Health Research (MOP 199847) and an Alberta Innovates Health Solutions Senior Scholar award to T.L.R. Work in the laboratory of S.P. is supported by a Canadian Institute of Health Research Operating Grant (MOP-84473), Alberta Innovates Health Solutions, and the Canadian Foundation for Innovation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.03056-14.
REFERENCES
- 1.Wachsmuth IK, Olsvik Ø, Evins GM, Popovic T. 1994. Molecular epidemiology of cholera, p 357–370. In Wachsmuth IK, Blake PA, Olsvik Ø (ed), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, DC. [Google Scholar]
- 2.Kaper JB, Morris JG, Levine MM. 1995. Cholera. Clin Microbiol Rev 8:48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mintz ED, Guerrant RL. 2009. A lion in our village–the unconscionable tragedy of cholera in Africa. N Engl J Med 360:1060–1063. doi: 10.1056/NEJMp0810559. [DOI] [PubMed] [Google Scholar]
- 4.Piarroux R, Barrais R, Faucher B, Haus R, Piarroux M, Gaudart J, Magloire R, Raoult D. 2011. Understanding the cholera epidemic, Haiti. Emerg Infect Dis 17:1161–1168. doi: 10.3201/eid1707.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, Lopez AL, Jiddawi MS, Clemens JD, von Seidlein L. 2011. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis 5:e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin C-S, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. 2011. The origin of the Haitian cholera outbreak strain. N Engl J Med 364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med 168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim MS, Ng D, Zong Z, Arvai AS, Taylor RK, Tainer JA, Craig L. 2010. Vibrio cholerae El Tor TcpA crystal structure and mechanism for pilus-mediated microcolony formation. Mol Microbiol 77:755–770. doi: 10.1111/j.1365-2958.2010.07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A 84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichow SL, Korotkov KV, Hol WG, Gonen T. 2010. Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol 17:1226–1232. doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill DM. 1977. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res 8:85–118. [PubMed] [Google Scholar]
- 12.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol 3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez J, Holmgren J. 2008. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci 65:1347–1360. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller VL, Taylor RK, Mekalanos JJ. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 15.Peterson KM, Mekalanos JJ. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun 56:2822–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bina J, Zhu J, Dziejman M, Faruque S, Calderwood S, Mekalanos J. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc Natl Acad Sci U S A 100:2801–2806. doi: 10.1073/pnas.2628026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Häse CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins DE, DiRita VJ. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol 14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 20.Krukonis ES, Yu RR, DiRita VJ. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol 38:67–84. doi: 10.1046/j.1365-2958.2000.02111.x. [DOI] [PubMed] [Google Scholar]
- 21.Murley YM, Carroll PA, Skorupski K, Taylor RK, Calderwood SB. 1999. Differential transcription of the tcpPH operon confers biotype-specific control of the Vibrio cholerae ToxR virulence regulon. Infect Immun 67:5117–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll PA, Tashima KT, Rogers MB, DiRita VJ, Calderwood SB. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol 25:1099–1111. doi: 10.1046/j.1365-2958.1997.5371901.x. [DOI] [PubMed] [Google Scholar]
- 23.Champion GA, Neely MN, Brennan MA, DiRita VJ. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol 23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 24.Kovacikova G, Skorupski K. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J Bacteriol 181:4250–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skorupski K, Taylor RK. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol 31:763–771. doi: 10.1046/j.1365-2958.1999.01215.x. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova G, Skorupski K. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol Microbiol 41:393–407. doi: 10.1046/j.1365-2958.2001.02518.x. [DOI] [PubMed] [Google Scholar]
- 27.Skorupski K, Taylor RK. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci U S A 94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacikova G, Lin W, Skorupski K. 2004. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol Microbiol 53:129–142. doi: 10.1111/j.1365-2958.2004.04121.x. [DOI] [PubMed] [Google Scholar]
- 29.Kovacikova G, Skorupski K. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol Microbiol 46:1135–1147. doi: 10.1046/j.1365-2958.2002.03229.x. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J, Shin OS, Cameron DE, Mekalanos JJ. 2010. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 107:21128–21133. doi: 10.1073/pnas.1014998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A 107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, Provenzano D, Pukatzki S. 2014. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 5:3549. doi: 10.1038/ncomms4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raivio TL, Silhavy TJ. 1999. The sigmaE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol 2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 35.Raivio TL, Silhavy TJ. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol 179:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raivio TL, Laird MW, Joly JC, Silhavy TJ. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the Cpx envelope stress response. Mol Microbiol 37:1186–1197. doi: 10.1046/j.1365-2958.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 37.Fleischer R, Heermann R, Jung K, Hunke S. 2007. Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J Biol Chem 282:8583–8593. doi: 10.1074/jbc.M605785200. [DOI] [PubMed] [Google Scholar]
- 38.Vogt S, Acosta N, Wong J, Wang JS, Raivio T. 2012. The CpxAR two-component system regulates a complex envelope stress response in Gram-negative bacteria, p 231–267. In Gross R, Beier D (ed), Two-component systems in bacteria. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 39.MacRitchie DM, Ward JD, Nevesinjac AZ, Raivio TL. 2008. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect Immun 76:1465–1475. doi: 10.1128/IAI.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogt SL, Nevesinjac AZ, Humphries RM, Donnenberg MS, Armstrong GD, Raivio TL. 2010. The Cpx envelope stress response both facilitates and inhibits elaboration of the enteropathogenic Escherichia coli bundle-forming pilus. Mol Microbiol 76:1095–1110. doi: 10.1111/j.1365-2958.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacRitchie DM, Acosta N, Raivio TL. 2012. DegP is involved in Cpx-mediated posttranscriptional regulation of the type III secretion apparatus in enteropathogenic Escherichia coli. Infect Immun 80:1766–1772. doi: 10.1128/IAI.05679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernday AD, Braaten BA, Broitman-Maduro G, Engelberts P, Low DA. 2004. Regulation of the pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol Cell 16:537–547. doi: 10.1016/j.molcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson KE, Liu J, Edqvist PJ, Francis MS. 2007. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect Immun 75:3913–3924. doi: 10.1128/IAI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altman E, Segal G. 2008. The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J Bacteriol 190:1985–1996. doi: 10.1128/JB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gal-Mor O, Segal G. 2003. Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J Bacteriol 185:4908–4919. doi: 10.1128/JB.185.16.4908-4919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama S, Watanabe H. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol 177:5062–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakayama SI, Watanabe H. 1998. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J Bacteriol 180:3522–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitobe J, Arakawa E, Watanabe H. 2005. A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J Bacteriol 187:107–113. doi: 10.1128/JB.187.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama S, Kushiro A, Asahara T, Tanaka R, Hu L, Kopecko DJ, Watanabe H. 2003. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology 149:2809–2817. doi: 10.1099/mic.0.26229-0. [DOI] [PubMed] [Google Scholar]
- 50.Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, Kormanec J, Roberts M. 2004. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun 72:4654–4661. doi: 10.1128/IAI.72.8.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gangaiah D, Zhang X, Fortney KR, Baker B, Liu Y, Munson RS, Spinola SM. 2013. Activation of CpxRA in Haemophilus ducreyi primarily inhibits the expression of its targets, including major virulence determinants. J Bacteriol 195:3486–3502. doi: 10.1128/JB.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slamti L, Waldor MK. 2009. Genetic analysis of activation of the Vibrio cholerae Cpx pathway. J Bacteriol 191:5044–5056. doi: 10.1128/JB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reimer AR, Van Domselaar G, Stroika S, Walker M, Kent H, Tarr C, Talkington D, Rowe L, Olsen-Rasmussen M, Frace M, Sammons S, Dahourou GA, Boncy J, Smith AM, Mabon P, Petkau A, Graham M, Gilmour MW, Gerner-Smidt P, V. cholerae Outbreak Genomics Task Force . 2011. Comparative genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg Infect Dis 17:2113–2121. doi: 10.3201/eid1711.110794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acosta N, Pukatzki S, Raivio TL. 2014. The Vibrio cholerae Cpx envelope stress response senses and mediates adaptation to low iron. J Bacteriol 197:262–276. doi: 10.1128/JB.01957-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor DL, Bina XR, Slamti L, Waldor MK, Bina JE. 2014. Reciprocal regulation of RND efflux systems and the Cpx two-component system in Vibrio cholerae. Infect Immun 82:2980–2991. doi: 10.1128/IAI.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Childers BM, Klose KE. 2007. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol 2:335–344. doi: 10.2217/17460913.2.3.335. [DOI] [PubMed] [Google Scholar]
- 57.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol 30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 58.Guzman L-M, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metcalf WW, Jiang WH, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 60.Cameron DE, Urbach JM, Mekalanos JJ. 2008. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci U S A 105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobos SR, Mora GC. 1991. Alteration in the electrophoretic mobility of OmpC due to variations in the ammonium persulfate concentration in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis 12:448–450. doi: 10.1002/elps.1150120615. [DOI] [PubMed] [Google Scholar]
- 62.Raivio TL, Popkin DL, Silhavy TJ. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol 181:5263–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. 2009. Translocation of a Vibrio cholerae type vi secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5:234–243. doi: 10.1016/j.chom.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardel CL, Mekalanos JJ. 1994. Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol 235:517–526. doi: 10.1016/0076-6879(94)35167-8. [DOI] [PubMed] [Google Scholar]
- 65.Martı′nez-Hackert E, Stock AM. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J Mol Biol 269:301–312. doi: 10.1006/jmbi.1997.1065. [DOI] [PubMed] [Google Scholar]
- 66.DiRita VJ, Mekalanos JJ. 1991. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell 64:29–37. doi: 10.1016/0092-8674(91)90206-E. [DOI] [PubMed] [Google Scholar]
- 67.DiRita VJ, Neely M, Taylor RK, Bruss PM. 1996. Differential expression of the ToxR regulon in classical and E1 Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci U S A 93:7991–7995. doi: 10.1073/pnas.93.15.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanjilal S, Citorik R, LaRocque RC, Ramoni MF, Calderwood SB. 2010. A systems biology approach to modeling Vibrio cholerae gene expression under virulence-inducing conditions. J Bacteriol 192:4300–4310. doi: 10.1128/JB.00182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matson JS, Withey JH, DiRita VJ. 2007. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun 75:5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford JA, Kaper JB, DiRita VJ. 1998. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol 29:235–246. doi: 10.1046/j.1365-2958.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- 71.Li CC, Crawford JA, DiRita VJ, Kaper JB. 2000. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol Microbiol 35:189–203. doi: 10.1046/j.1365-2958.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 72.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations–osmoregulation of outer-membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Provenzano D, Schuhmacher DA, Barker JL, Klose KE. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun 68:1491–1497. doi: 10.1128/IAI.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Batchelor E, Walthers D, Kenney LJ, Goulian M. 2005. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins ompF and ompC. J Bacteriol 187:5723–5731. doi: 10.1128/JB.187.16.5723-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. 2009. MzrA: a novel modulator of the EnvZ/OmpR two-component regulon. Mol Microbiol 72:1408–1422. doi: 10.1111/j.1365-2958.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai SJ, Inouye M. 2002. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem 277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 77.Slauch J, Garrett S, Jackson D, Silhavy T. 1988. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol 170:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overbye LJ, Sandkvist M, Bagdasarian M. 1993. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene 132:101–106. doi: 10.1016/0378-1119(93)90520-D. [DOI] [PubMed] [Google Scholar]
- 79.Sandkvist M, Morales V, Bagdasarian M. 1993. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene 123:81–86. doi: 10.1016/0378-1119(93)90543-C. [DOI] [PubMed] [Google Scholar]
- 80.Sandkvist M, Bagdasarian M, Howard S, DiRita V. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J 14:1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson TL, Abendroth J, Hol WGJ, Sandkvist M. 2006. Type II secretion: from structure to function. FEMS Microbiol Lett 255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 82.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, Jahn D. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187–4189. doi: 10.1093/bioinformatics/bti635. [DOI] [PubMed] [Google Scholar]
- 84.Skorupski K, Taylor RK. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol 25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 85.Liang W, Pascual-Montano A, Silva AJ, Benitez JA. 2007. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 153:2964–2975. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- 86.Goss TJ, Morgan SJ, French EL, Krukonis ES. 2013. ToxR recognizes a direct repeat element in the toxT, ompU, ompT, and ctxA promoters of Vibrio cholerae to regulate transcription. Infect Immun 81:884–895. doi: 10.1128/IAI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerken H, Leiser OP, Bennion D, Misra R. 2010. Involvement and necessity of the Cpx regulon in the event of aberrant β-barrel outer membrane protein assembly. Mol Microbiol 75:1033–1046. doi: 10.1111/j.1365-2958.2009.07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li CC, Merrell DS, Camilli A, Kaper JB. 2002. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae. Mol Microbiol 43:1577–1589. doi: 10.1046/j.1365-2958.2002.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bina XR, Taylor DL, Vikram A, Ante VM, Bina JE. 2013. Vibrio cholerae ToxR downregulates virulence factor production in response to cyclo(Phe-Pro). mBio 4:e00366–00313. doi: 10.1128/mBio.00366-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park D-K, Lee K-E, Baek C-H, Kim IH, Kwon J-H, Lee WK, Lee K-H, Kim B-S, Choi S-H, Kim K-S. 2006. Cyclo(Phe-Pro) modulates the expression of ompU in Vibrio spp. J Bacteriol 188:2214–2221. doi: 10.1128/JB.188.6.2214-2221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Wulf P, Kwon O, Lin EC. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J Bacteriol 181:6772–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vogt SL, Evans AD, Guest RL, Raivio TL. 2014. The Cpx envelope stress response regulates and is regulated by small non-coding RNAs. J Bacteriol 196:4229–4238. doi: 10.1128/JB.02138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mandlik A, Livny J, Robins WP, Ritchie JM, Mekalanos JJ, Waldor MK. 2011. RNA-Seq-based monitoring of infection-linked changes in Vibrio cholerae gene expression. Cell Host Microbe 10:165–174. doi: 10.1016/j.chom.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lombardo MJ, Michalski J, Martinez-Wilson H, Morin C, Hilton T, Osorio CG, Nataro JP, Tacket CO, Camilli A, Kaper JB. 2007. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc Natl Acad Sci U S A 104:18229–18234. doi: 10.1073/pnas.0705636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Q, Dziejman M, Mekalanos JJ. 2003. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc Natl Acad Sci U S A 100:1286–1291. doi: 10.1073/pnas.0337479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. 2007. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larocque RC, Harris JB, Dziejman M, Li X, Khan AI, Faruque AS, Faruque SM, Nair GB, Ryan ET, Qadri F, Mekalanos JJ, Calderwood SB. 2005. Transcriptional profiling of Vibrio cholerae recovered directly from patient specimens during early and late stages of human infection. Infect Immun 73:4488–4493. doi: 10.1128/IAI.73.8.4488-4493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MacRitchie DM, Buelow DR, Price NL, Raivio TL. 2008. Two-component signaling and gram negative envelope stress response systems, p 80–110. In Utsumi R. (ed), Bacterial signal transduction: networks and drug targets, vol 631 Springer Sciences, New York, NY. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.