Abstract
Obesity and diabetes are among the greatest risk factors for infection following total joint arthroplasty. However, the underlying mechanism of susceptibility is unclear. We compared orthopedic implant-associated Staphylococcus aureus infections in type 1 (T1D) versus type 2 (T2D) diabetic mouse models and in patients with S. aureus infections, focusing on the adaptive immune response. Mice were fed a high-fat diet to initiate obesity and T2D. T1D was initiated with streptozotocin. Mice were then given a trans-tibial implant that was precoated with bioluminescent Xen36 S. aureus. Although both mouse models of diabetes demonstrated worse infection severity than controls, infection in T2D mice was more severe, as indicated by increases in bioluminescence, S. aureus CFU in tissue, and death within the first 7 days. Furthermore, T2D mice had an impaired humoral immune response at day 14 with reduced total IgG, decreased S. aureus-specific IgG, and increased IgM. These changes were not present in T1D mice. Similarly, T2D patients and obese nondiabetics with active S. aureus infections had a blunted IgG response to S. aureus. In conclusion, we report the first evidence of a humoral immune deficit, possibly due to an immunoglobulin class switch defect, in obesity and T2D during exacerbated S. aureus infection which may contribute to the increased infection risk following arthroplasty in patients with T2D and obesity.
INTRODUCTION
Primary total hip and knee arthroplasties are common procedures, with over 800,000 occurring in the United States in 2006 (1). This number is projected to climb to 4 million by 2030 (2). The infection rate following these procedures is currently ca. 1 to 3% in the general population (3, 4), with Staphylococcus aureus and Staphylococcus epidermidis being the most frequent and persistent infectious organisms (5–7). Diabetes and obesity are two of the greatest risk factors for developing osteoarthritis (8), as well as developing an infection after total joint replacement (4, 9). Recent clinical studies have shown at least a doubling of the infection rates following knee and hip arthroplasty in the diabetic and obese populations (1), with some studies showing a 3- to 5-fold increase in infection rates (10, 11). Two factors have increased the serious implications of these risks: the emergence and increasing numbers of methicillin-resistant S. aureus infections (12) and the obesity epidemic.
The underlying cause(s) of increased susceptibility to infection in type 1 (T1D) and type 2 (T2D) diabetics following total joint arthroplasty is not understood. One widely held view is that hyperglycemia is responsible (13). This, however, does not take into account the increased susceptibility of nondiabetic obese and prediabetic patients to S. aureus infection, which suggests alternate or additional risk factors for infection besides hyperglycemia. Although untreated T1D and T2D are hyperglycemic, the underlying pathology behind each disease differs significantly. T1D is associated with autoinflammation targeting the beta cells causing hypoinsulinemia, whereas T2D is associated with hyperinsulinemia (at least in the early stages), chronic low-grade inflammation, obesity, and insulin resistance. Elucidating the differences between these two diseases will facilitate discriminating the underlying mechanism(s) of susceptibility to infection in diabetics.
To date, the innate immune response to infection in diabetes has been more intensely studied than the adaptive immune response. Studies have indicated reduced monocyte function, with macrophages and neutrophils from diabetics showing an impairment in cytokine release, oxidative burst, and phagocytic capability (13–16). Despite evidence that cells involved in adaptive immunity are causative in the progression of both T1D and T2D (17–19), few have studied how this involvement may affect responses to infection. One clinical study demonstrated an impaired humoral immune response to influenza vaccination in obese patients (20). Another group demonstrated reduced titers to tetanus vaccine in obese children (21). Although these studies are valuable in assessing the immune response of obese patients, more studies must be completed to determine the mechanism behind altered adaptive immunity, as well as to determine the response of diabetics to live pathogens, such as S. aureus.
The overall goal of this study was to further define risk factors for infection in T1D and T2D, particularly infections associated with orthopedic implants. The specific aim was to identify potential defects in the adaptive immune response to S. aureus infection that may be unique to T2D. To address this aim, we studied human samples and used widely accepted models of T1D and T2D. The high-fat diet (HFD) mouse model of obesity and diabetes mimics many aspects of human T2D. After 3 months on an HFD, the genetically susceptible C57BL/6 mice become obese, insulin resistant, and glucose intolerant (22). The streptozotocin model of T1D has been used since the late 1960s due to its selective destruction of beta cells (23). More recently, it has been used in multiple low doses to induce mild hyperglycemia (24) with minimal insulinitis in the C57BL/6 background (25). Hyperglycemia with limited inflammation and auto-immunity makes the streptozotocin-treated mouse a useful model for comparing the effect of S. aureus infections in hyperglycemia alone (T1D mouse) to that of obesity-induced hyperglycemia (T2D mouse). By comparing two distinct but related models of diabetes, the role of hyperglycemia in altering S. aureus infections and humoral immunity can be defined.
MATERIALS AND METHODS
Animals.
Animal studies were performed in accordance with protocols approved by the University of Rochester's Committee on Animal Resources that maximize humane treatment and alleviation of suffering. Male C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME) were housed five per cage in one-way housing on a 12-h light/dark cycle. To model T2D, mice were placed on a high-fat (60% kcal, D12492) or low-fat (10% kcal, D12450J) diet at 5 weeks of age (Open Source Diets; Research Diets, Inc., New Brunswick, NJ). To model T1D, streptozotocin (S0130; Sigma) was administered to 12-week-old mice at 40 mg/kg in citrate buffer as described elsewhere (24) on 4 consecutive days. Vehicle control mice received citrate buffer alone. Streptozotocin and vehicle-treated mice were fed standard chow (Lab Diet, St. Louis, MO). After streptozotocin treatment, mice were allowed to recover for 2 weeks. Prior to infection, overnight fasting and nonfasting blood glucose levels were measured using OneTouch glucose meters (Lifescan, Inc., Milpitas, CA). Glucose tolerance testing was also performed on mice that had fasted overnight. Mice were injected intraperitoneally with a 300-mg/kg bolus of glucose in sterile saline. Glucose levels were measured at 15, 30, 60, and 90 min. Streptozotocin-treated mice with nonfasting blood glucose levels below 200 mg/dl were excluded from the present study.
Orthopedic implantation and infection.
Infected orthopedic implants were generated and placed as we have previously described (26). In short, a flat (cross-section, 0.2 by 0.5 mm), stainless steel surgical wire was cut to a 4-mm length and bent at 1 mm to form an L-shaped pin. The pins were then sterilized and placed for 20 min in an overnight culture of Xen36 S. aureus (Perkin-Elmer, MA) grown to log phase. The inoculation dose on the pins was determined to be 2 × 105 CFU by sonication of individual pins, serially diluting the extracts, and plating on tryptic soy agar plates.
Mice were anesthetized using intraperitoneal injections of ketamine at 60 mg/kg and xylazine at 4 mg/kg with a preoperative buprenorphine regimen. The right leg was shaved and washed, and a 3- to 5-mm incision was made on the medial surface below the knee. The tibia was then predrilled from medial cortex through lateral cortex using 30-gauge and 26-gauge needles successively. The S. aureus-coated implant was then placed in the defect. The surgical site was closed using 5 to 0 interrupted sutures. Bioluminescence was measured by using a Xenogen IVIS camera system (Alameda, CA) on indicated days.
Tissue harvesting and bacterial CFU counting.
Mice were sacrificed at day 7 and 14 under anesthesia. Blood was collected by cardiac puncture. The CFU of S. aureus in infected tibiae were quantified after infected and necrotic soft tissue surrounding the pin insertion site was harvested and weighed, and the remaining tissue was dissociated from the tibia. Tissues were homogenized using an IKA T-10 handheld homogenizer (Wilmington, NC). Tenfold serial dilutions were prepared in phosphate-buffered saline (PBS), 100-μl portions of each dilution were plated on tryptic soy agar plates, and the colonies were counted at 24 h.
Serum immunoglobulin and antibody assays. (i) Total IgG and IgM assays.
For mouse enzyme-linked immunosorbent assays (ELISAs), 2 μg of goat anti-mouse IgG and IgM (Southern Biotech, Birmingham, AL)/ml was coated onto 96-well plates (Nunc, catalog no. 442404). For human ELISAs, goat anti-human IgG and IgM were used. After blocking in 3% bovine serum albumin for 1 h, serum at 1:10,000 was added to the wells (100 μl), followed by incubation at 4°C for 1 h. After five washes with PBS-Tween (PBST), secondary antibody (anti-IgG or anti-IgM; Southern Biotech) conjugated to horseradish peroxidase was added at a dilution of 1:4,000, followed by incubation for 1 h at 4°C. Sureblue peroxidase was used as a substrate (catalog no. 52-00-01; KPL, Gaithersburg, MD).
(ii) Whole S. aureus extract assay.
S. aureus extracts were prepared by incubating 1 ml of an overnight culture of Xen36 S. aureus with 20 mg of lysozyme (Sigma) and 1 mg of lysostaphin (Sigma) in sterile water. Extract was then used as a coating antigen in an ELISA at 1:2,000 in PBS. After blocking and washing in PBST, serum was added at 1:10, followed by IgG or IgM secondary antibody. For ELISAs where no standard control was available, one mouse or patient was chosen as the standard and set to an arbitrary unit of 1 for subsequent assays.
(iii) S. aureus antigen-specific assays.
The titers of antibodies to eight S. aureus antigens were determined by Luminex-based assays using recombinant S. aureus antigen-tagged microbeads and a Bio-Rad BioPlex instrument as described by the manufacturer. Antigens were synthesized by and purchased from GenScript (Piscataway, NJ) and chosen based on their potential as markers for S. aureus infection. Consideration for inclusion took into account location within the organism (i.e., intracellular, cell wall, and excreted), as well as function (i.e., iron binding, cell adhesion, survival factors, etc.), and included Gmd, Amd, IsdA, IsdB, IsdH, ClfA, ClfB, and FnbA.
Human subjects.
All work with human samples and the protocol for specimen procurement was reviewed and approved by the Research Subjects Review Board at the University of Rochester Medical Center. Patients with positive cultures for S. aureus infections, as reported by the Microbiology Laboratory at the University of Rochester Medical Center, were identified through the laboratory information system. The data collected from these cases included height, weight, body mass index (BMI), diabetic status, HbA1c level, and infection site. Any preexisting serum samples collected for clinical analysis within the prior 4 days on each patient were located in the Clinical Laboratory, and one aliquot was retrieved. Deidentified sera and associated patient data were made available to the investigators. Exclusion criteria included pancreatic insufficiency, hemodialysis, AIDS/HIV infection, and a height of less than 4 feet. Sites of infection were heterogeneous in nature and consisted mainly of surgical sites, wound abscesses, and bone infections. Importantly, there were no significant differences in the types of infections between nondiabetic and diabetic patients. Patients with positive cultures from catheters, sputum, and skin infections were excluded.
Statistics.
Multiple analyte comparisons were measured using one- or two-way analysis of variance (ANOVA) and Bonferroni's posttest. An unpaired Student t test was used when two groups were compared, including area-under-the-curve measurements. Area-under-the-curve measurements were determined by counting the total area above zero. The data sets with non-normal distribution were compared by using the Mann-Whitney test. All statistics were analyzed using GraphPad Prism.
RESULTS
Impaired glucose tolerance in mouse models of type 1 and type 2 diabetes.
Both HFD-fed T2D mice and streptozotocin-treated T1D mice were hyperglycemic compared to matched controls. Although T2D mice were consistently hyperglycemic in the fasting state, T1D mice had only modestly elevated fasting glucose (Fig. 1A), a finding consistent with the reported lack of sensitivity of this parameter to modest loss of beta cells (27). Hyperglycemia in T1D mice was pronounced in nonfasting samples, while the levels in T2D mice were not significantly higher than in vehicle control mice (Fig. 1B). Using a glucose tolerance test, both the T1D and the T2D mice displayed similar glucose intolerance (Fig. 1D and E). As expected, HFD-induced T2D mice gained significantly more weight than mice on a low-fat diet. Streptozotocin-treated T1D mice weighed significantly less than vehicle control mice, likely due to their impaired ability to produce insulin and resulting catabolic state (Fig. 1C).
FIG 1.
T2D and T1D models are both associated with hyperglycemia and impaired glucose tolerance. C57BL/6 mice at 5 weeks of age were placed on an HFD (T2D model) or lean diet for 12 weeks. Similarly paired 15-week-old mice were treated with streptozotocin at 40 mg/kg (T1D model) or vehicle on 4 consecutive days 2 weeks prior to testing. (A and B) Blood glucose measurements were made after an overnight fast (A) and in the nonfasting state (B). (C) Body weight was measured preinfection. (D and E) Glucose tolerance tests were performed on fasting mice (D), and the areas under the curve were analyzed based on data from individual mice (E). The results shown in panels A, B, and C were analyzed by unpaired Student t test; the results in panel D were analyzed by two-way ANOVA with Bonferroni's multiple-comparison test. No significant difference was measured between HFD- and streptozotocin-treated mice. The results shown in panel E were analyzed by one-way ANOVA with Bonferroni's multiple-comparison test. Error bars represent the standard errors of the mean. n > 5 for all. *, P < 0.05; **, P < 0.01; ***, P < 0.001. HF, high-fat diet; Veh., vehicle treated; Strep., streptozotocin treated.
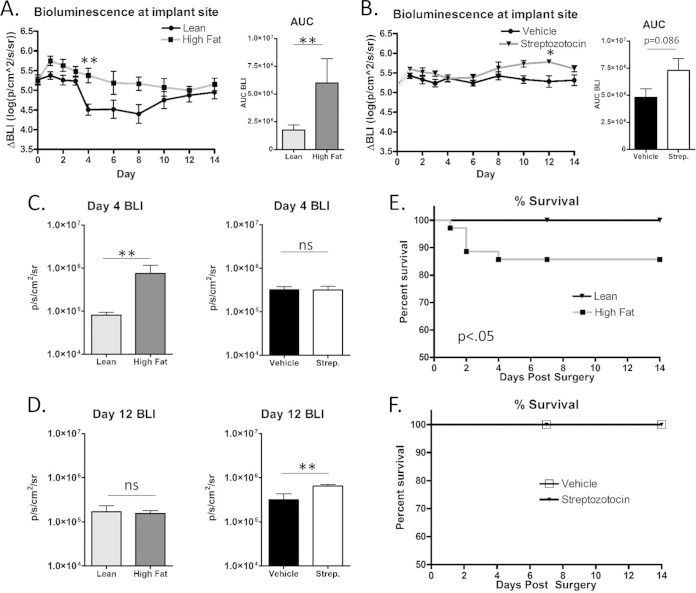
T2D mice have more severe S. aureus bone infection than T1D mice.
Mice were infected with methicillin-sensitive Xen36 S. aureus at the time of implant insertion through the medial tibia. Xen36 is a clinically isolated bioluminescent strain that enables burden of metabolically active bacteria to be studied longitudinally in vivo. Over a 14-day time course, only T2D mice had significantly increased total S. aureus (bioluminescence) at the implant site compared to control groups, as indicated by the area under the curve, although a trend was present in T1D mice (Fig. 2A and B). Interestingly, this increased bacterial burden was most apparent at different time points in the infection process, with T2D affecting the early course of infection (Fig. 2C), while T1D affected later stages of infection (Fig. 2D). Furthermore, 15% of the T2D mice died within 4 days of placement of the infected implant, whereas no T1D or control mice died (Fig. 2E and F).
FIG 2.
T2D mice have more severe S. aureus infections than T1D mice. Xen36 S. aureus-derived bioluminescence was measured at the site of the infected tibiae of T2D versus lean control mice (A) and T1D versus vehicle control mice (B). Area-under-the-curve measurements represent the average bioluminescence above zero in each group based on individual mice. (C) Day 4 bioluminescence in T2D mice versus lean control and T1D mice versus vehicle control. (D) Day 12 bioluminescence. (E and F) Percentages of T2D and lean controls (E) and T1D and vehicle controls (F) that survived over a 14-day infection. Two-way ANOVA was used to assess longitudinal bioluminescence (P < 0.0001 for panels A and B, as determined by Bonferroni's multiple-comparison test). An unpaired Student t test was used for all other comparisons. A log-rank test was used to assess survival. *, P < 0.05; **, P < 0.01. n > 7.
To confirm the results of the bioluminescence assay, the S. aureus CFU were quantitated from the infected tibia and adjacent necrotic, infected soft tissues at 7 and 14 days postinfection. At day 7, significantly more colonies of S. aureus were isolated from the tibias of infected T2D mice (Fig. 3A) than from controls. This is consistent with the bioluminescence data (Fig. 2A and C). T2D mice also showed a trend toward increased CFU from the surrounding necrotic soft tissue (Fig. 3B) and increased wet weight of this necrotic tissue (data not shown). No differences were observed in the CFU from bone or soft tissue of T1D mice at day 7 (Fig. 3A and B). This is also consistent with the bioluminescence data. At day 14, no differences in CFU were observed in the bones of T2D mice or T1D mice compared to control groups (Fig. 3C). However, an increase in CFU of S. aureus in the soft tissue abscess of T1D but not T2D mice was observed (Fig. 3D). This confirms the bioluminescence data and may indicate that the later rise in infection severity in T1D mice may reflect increased soft tissue infection rather than bone infection.
FIG 3.
Temporal differences in S. aureus infection between T1D and T2D mice. S. aureus CFU were isolated from infected tibias and adjacent infected soft tissues at days 7 and 14 after implant placement in T1D, T2D, and control mice. (A) CFU isolated at day 7 from tibias. (B) CFU isolated at day 7 from soft tissues. (C) CFU isolated from tibias at day 14. (D) CFU isolated from soft tissues at day 14. *, P < 0.05 (unpaired Student t test). n > 7.
Bone infection in T2D mice may be more susceptible to hematogenous spread.
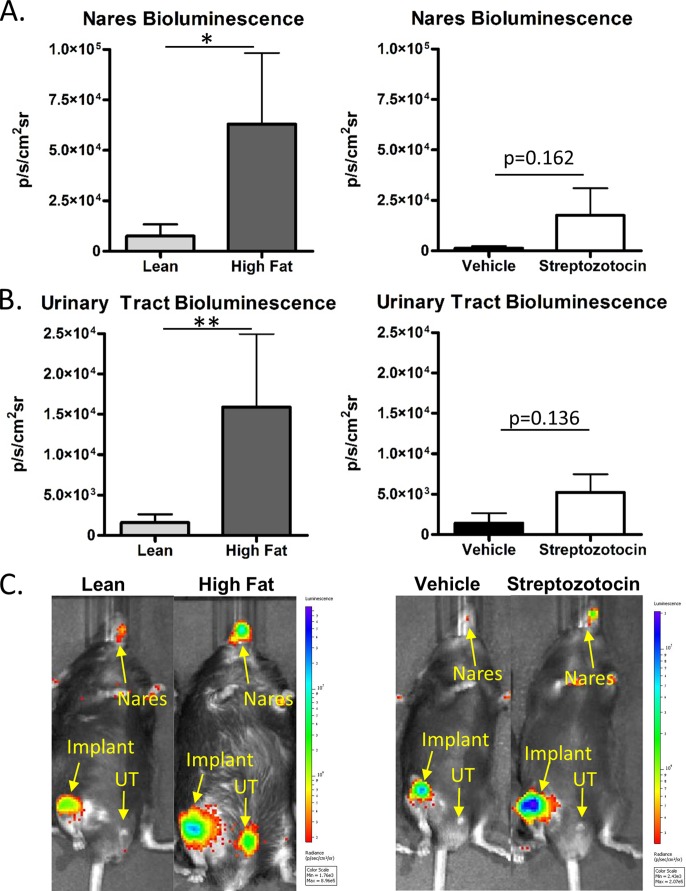
S. aureus infections that cannot be controlled by the immune response at the original site of infection are likely to spread hematogenously or nonhematogenously to colonize other body sites. The bioluminescence results indicated active infection or colonization in the nares and in the urinary tracts of mice with implant-associated infections at day 14. The mean S. aureus bioluminescence in the nares of T2D mice was significantly greater than in lean controls. The number of T2D mice with bioluminescence in the nares (6/10) was also higher than that in controls (2/10). Only one T1D mouse and no vehicle controls displayed significant bioluminescence in the nares (Fig. 4A and C). In the urinary tract, bioluminescence again indicated more active infection in T2D mice (Fig. 4B). T1D mice only trended toward an increase in urinary tract bioluminescence compared to controls (Fig. 4B and C). Thus, T2D mice demonstrated an apparent increase in S. aureus colonization in the urinary tract and nares, a result that was not noted in T1D mice.
FIG 4.
The spread of S. aureus infection to urinary tracts and nares is more frequent and severe in T2D mice. The spread of Xen36 S. aureus infection in T1D and T2D mice was monitored by bioluminescence (BLI). (A) BLI was measured in the nares of T2D mice and lean controls and of T1D mice and vehicle controls by using area of interest gating. (B) BLI in the urinary tract was measured and compared between T2D and T1D mice and their respective controls. (C) Representative full-body scans of mice infected with Xen36 S. aureus. All measurements were made at day 14. n = 10. *, P < 0.05; **, P < 0.01 (Mann-Whitney test).
T2D but not T1D mice display an altered adaptive immune response.
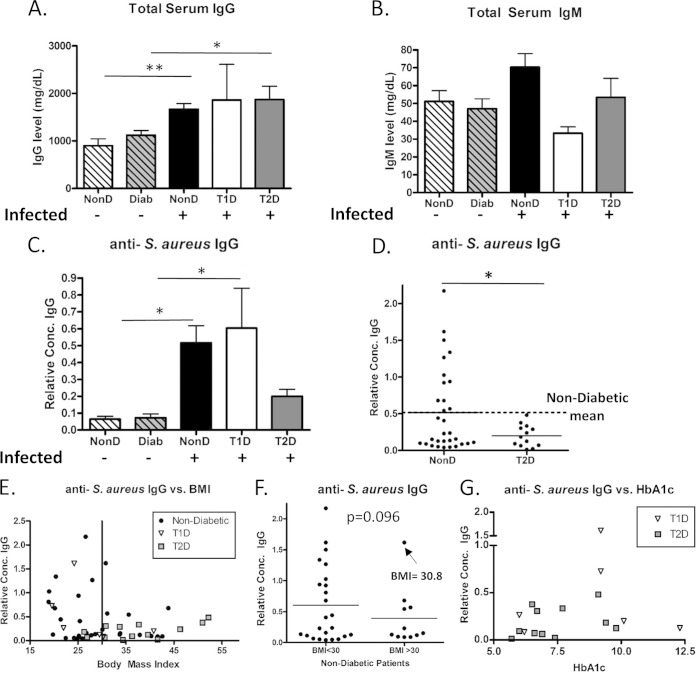
Although the impaired innate immune response in diabetes models has been relatively well characterized (13–16), the adaptive immune response, particularly in the obese, diabetic HFD model, has not been well studied. We observed here that serum IgG levels in HFD-induced T2D mice were less elevated than in controls at 14 days after S. aureus infection (Fig. 5A). No differences were observed between the groups prior to infection. In contrast, uninfected streptozotocin-treated mice had a significant increase in total IgG prior to infection, likely reflecting residual immune response to beta cell apoptosis as a result of the drug. However, no differences in IgG levels were observed between T1D mice and controls at day14 (Fig. 5B). Serum IgM levels, which are the first immunoglobulins released in response to infection, were elevated in the T2D mice compared to controls (Fig. 5C). T1D mice had similar IgM levels to controls, both prior to and after infection (Fig. 5D).
FIG 5.
T2D mice display a blunted immunoglobulin response to S. aureus infection. At day 14 postinfection, blood was collected from infected and uninfected T1D and T2D mice and matched controls. Serum immunoglobulin levels were measured by ELISA in each group. (A and B) Total IgG; (C and D) total IgM. Using S. aureus extracts as the coating antigen in an ELISA, immune responses directed against S. aureus were quantitated in samples from infected mice. (E and F) IgG antibody responses; (G and H) IgM antibody responses. (I) Antibody titers in infected mice were measured against eight selective S. aureus antigens using a Luminex-based ELISA. (J and K) Antibodies directed against the S. aureus antigen IsdB were measured by a single-analyte ELISA. Plots represent the means ± the SEM of normalized absorbance at 450 nm. n > 8. *, P < 0.05; **, P < 0.01. The results in panels A to D and panel I were assessed by one-way ANOVA with Bonferroni's multiple-comparison test. The results in panels E to H were assessed by an unpaired Student t test. The results in panels J to K were assessed by a Mann-Whitney test. HF, high-fat diet; Veh., vehicle treated; Strep., streptozotocin treated.
Using an ELISA against extracted S. aureus proteins, IgG antibody concentrations were reduced in infected T2D mice (Fig. 5E). An increase in IgM antibody concentration against S. aureus-specific antigens was also observed in T2D mice (Fig. 5G), paralleling the global increase in IgM. These reciprocal changes in S. aureus-specific IgG and IgM antibodies were not observed in serum from T1D mice (Fig. 5F and H). This response was further evaluated using an eight-S. aureus-antigen Luminex-based ELISA. Antibody levels against iron-regulated surface determinant B (IsdB) were highly sensitive to the blunting effect of T2D (Fig. 5I). Furthermore, antibodies to six of the seven remaining antigens demonstrated a similar but nonsignificant trend. To confirm the results, anti-IsdB levels were measured in a second mouse cohort using a single-analyte ELISA. Anti-IsdB antibody levels were again reduced in T2D mice (Fig. 5J). As with other humoral immune responses, no difference in anti-IsdB antibody levels was observed between T1D mice and controls (Fig. 5K).
Infected T2D patients have reduced anti-S. aureus antibody levels.
Serum samples were obtained at the time of culture confirmation of active S. aureus infection from local wound swabs in control patients and patients with T1D and T2D. Mean total IgG levels were higher in each infected patient category compared to uninfected controls regardless of diabetic status (Fig. 6A). No consistent trends in total IgM levels were apparent (Fig. 6B). Anti-S. aureus IgG levels were significantly increased in infected nondiabetic and T1D patients compared to noninfected controls, but no significant increase was seen in the T2D patients (Fig. 6C). None of the T2D patients had anti-S. aureus levels as high as the mean for the nondiabetic group (Fig. 6D). With obesity being strongly associated with T2D, the relationship between body mass and anti-S. aureus levels were examined. As shown in Fig. 6E, all infected patients stratified into two groups, one that contained all of the robust antibody responders to infection, and a second that contained only low responders. The former were localized almost exclusively at or below the BMI cutoff for obesity of 30. One robust nondiabetic responder had a BMI of 30.8. Most T2D patients had BMIs above 30 (Fig. 6E and Table 1). Only one T1D patient had a BMI significantly higher than 30. Nondiabetic patients with BMIs above 30 had low anti-S. aureus levels (Fig. 6F), with the exception of the one patient noted above. Furthermore, when plotted as a function of HbA1c, representing the average plasma glucose concentration over a 6- to 12-week period, there was no correlation between HbA1c levels and anti-S. aureus IgG in either type 1 or type 2 diabetics (Fig. 6G). This further indicates that the blunted immunoglobulin response is independent of hyperglycemia. Taken together, both T2D and obese nondiabetics display blunted IgG responses to S. aureus infection.
FIG 6.
Diabetic patients with active S. aureus infections have blunted IgG-specific humoral responses to S. aureus. Human samples were collected from S. aureus-infected patients or control patients. Serum immunoglobulin levels and selected antibody concentrations were measured by ELISA in each group. (A) total serum IgG; (B) total serum IgM. Using S. aureus extracts as the coating antigen in an ELISA, IgG immune responses directed against S. aureus were quantitated in all groups (C), and nondiabetic infected and T2D infected patient concentrations were compared (D). The dashed line in panel D represents the mean IgG response to S. aureus in nondiabetic controls. (E) Anti-S. aureus IgG response as a function of BMI. The vertical line at a BMI of 30 represents the cutoff for obesity. (F) Nondiabetic patients were stratified into two groups based on BMIs of <30 and ≥30, and their anti-S. aureus IgG responses were compared. (G) Anti-S. aureus IgG levels from type 1 and type 2 diabetic patients were plotted as a function of their HbA1c level at the time of clinical identification of S. aureus infection. Comparisons between more than two groups used one-way ANOVA with Bonferroni's multiple-comparison test. Comparisons between two groups were made using an unpaired Student t test. *, P < 0.05; **, P < 0.01.
TABLE 1.
Demographic data for S. aureus-infected patientsa
| Parameter | Mean ± SD (range) |
Pb | ||
|---|---|---|---|---|
| Nondiabetic | Type 1 diabetic | Type 2 diabetic | ||
| No. of patients | 31 | 6 | 13 | |
| Wt (kg) | 83.0 ± 25.7A (36.3–168.4) | 80.2 ± 15.4 (68.0–104.3) | 106.1 ± 34.8A (65.7–179.4) | <0.05A |
| Ht (m) | 1.71 ± 0.10 (1.45–1.88) | 1.67 ± 0.17 (1.37–1.88) | 1.66 ± 0.14 (1.44–1.85) | 0.67 |
| BMI | 27.8 ± 6.7B (18.9–43.8) | 27.7 ± 7.6C (19.6–40.8) | 37.5 ± 8.4B,C (26.3–52.2) | <0.001B; <0.05C |
| HbA1c (% [mmol/mol]) | NAc | 8.8 ± 2.4 (6.0–12.3) | 7.6 ± 1.4 (5.7–9.8) | 0.2 |
| 73 ± 26 (42–111) | 60 ± 15 (39–84) | |||
Deidentified serum samples from patients with culture-positive S. aureus infections were collected and grouped based by diabetic status. Each value represents the mean of each group ± the standard deviation. P values were determined by one-way ANOVA with Bonferroni's post hoc test.
P values in the last column correspond to the matching superscript capital letters in columns 2 to 4.
NA, not applicable.
DISCUSSION
In this study, a mouse model of T2D, the classic HFD mouse, developed more severe implant-associated S. aureus bone infections than the streptozotocin, T1D mouse model. This conclusion is based on elevated bioluminescence at the implant site (reflecting proliferation of the bioluminescent Xen36 strain of S. aureus) and increased CFU isolated from the tibia of the T2D mice compared to controls and T1D mice. T2D mice were also more susceptible to death following S. aureus infection. In most cases, death correlated closely with exceptionally high levels of bioluminescence (data not shown). Death was likely due to bacterial sepsis, but this was not confirmed. Furthermore, attrition of T2D mice from the data set likely causes an underestimation of the differences in response to S. aureus infection between T2D and controls. Notably, no mice in the streptozotocin, vehicle, or lean-fed groups died. It should be noted that Xen36 S. aureus, while a clinical isolate, has been less extensively characterized than more frequently used strains such as UAMS-1 or USA300. Thus, little is known of its virulence factors. Nonetheless, our data are consistent with clinical studies indicating obese and T2D patients are at increased risk for infection following total joint arthroplasty (1, 10). It is clear, however, that T1D mice also had more severe infections than their vehicle-treated controls at day 14. This agrees with studies indicating that T1D is also a risk factor for S. aureus infection (28). Thus, both T1D and T2D mouse models showed increased S. aureus infection severity relative to controls, though the course of the infections differed considerably between the two groups. Since both T1D and T2D are characterized by hyperglycemia, it is likely that hyperglycemia is a contributing factor to infection progression. However, other disease characteristics that distinguish these two types of diabetes likely contribute to the distinct features that define their differing courses of infection in the present study.
Bacteria that are not contained at the original infection site are likely to spread to other areas of the body and can be excreted or shed through the urinary tract. Only the T2D mice showed a significantly increased susceptibly to hematogenous spread of Xen36 S. aureus, as indicated by elevated bioluminescence within the urinary tract. There was also an increase in bioluminescence in the nares of mice with T2D. This could be due to higher levels of S. aureus excretion in T2D mice and subsequent colonization or to hematogenous spread. Regardless of the mechanism, the susceptibility of mice with T2D to nasal colonization by S. aureus was increased. Clinical studies have indicated that both T2D and obesity increase the rate of S. aureus colonization in the nares (29, 30). Thus, the T2D mouse simulates the human disease state.
S. aureus growth at the infection site in T2D mice was greater than that in controls at days 4 to 7 but was reduced to that of control mice at later time points (Fig. 2 and 3). Decreased planktonic growth and increased biofilm formation may contribute to this decreased bioluminescence. Since this reduction correlates with the increase in bioluminescence in other body sites, it is feasible that T2D mice do not clear the infection, but rather the infection spreads to other body sites. Supporting this interpretation, in many instances, S. aureus was isolated from the kidneys, bladder, and blood of T2D mice (data not shown). Thus, increased bacterial load and colonization indicate that T2D mice have an increased severity of infection compared to both lean-fed control and T1D mice.
While mouse models of diabetes have been used to show impaired cellular immunity in both macrophages (31) and neutrophils (13, 14), humoral immunity has not been well studied in T2D models. In this report, we demonstrate an impaired adaptive immune response in mice with obesity-associated T2D. In agreement with our results, clinical studies have shown that obesity is associated with an impaired humoral immune response to influenza vaccination (20), and obese children have reduced titers to the tetanus vaccine (21). A genetic model of T2D also displayed an impaired immunoglobulin response to West Nile virus (32). Although T2D mice have an impaired adaptive immune response to S. aureus infection, T1D mice do not, indicating that this effect is T2D specific. It cannot be ruled out that impaired cellular defects that have been demonstrated elsewhere (14, 31), specifically upstream of antigen presentation, are causative in reduced antibody levels in T2D mice. However, increased total IgM levels in T2D mice at day 14 postinfection provide support for a B cell defect, specifically an impairment in class switching as opposed to a defect in antigen presentation. This immunoglobulin class switching defect is of particular note, as patients with X-linked agammaglobulinemia or Bruton agammaglobulinemia are at increased risk for S. aureus infections (33, 34). In addition, cutaneous S. aureus granulomatosis (botryomycosis) has also been reported in mice with non-sex-linked agammaglobulinemia (35). Regardless of mechanism, an IgG production defect is very likely to increase susceptibility to and severity of infections.
S. aureus-infected patients with T2D consistently demonstrated a markedly reduced antibody response to S. aureus antigen, mirroring the results from the mouse model of T2D. T1D patients were indistinguishable from nondiabetics. This further indicates that characteristics specific to T2D impair the IgG response to S. aureus infection. Importantly, two distinct populations were apparent in the nondiabetic control patients when their antibody levels against S. aureus were plotted against BMI. All but one robust responder had BMIs less than 30, the cutoff for obesity. Since some of our T2D patients had BMIs of <30 and yet had low antibody levels, it cannot be concluded that obesity accounts for the low antibody response in this patient group. It appears that both T2D and obesity are factors in the blunted humoral immune response to S. aureus. Importantly, there was no correlation between HbA1c levels and anti-S. aureus IgG response in type 1 or type 2 diabetics. This further indicates a mechanism other than hyperglycemia that drives the impaired humoral immune response. There were no consistent differences in sites of infection in the patient populations to account for the differences in response. Taken together, our data indicate that both obesity and T2D result in a blunted anti-S. aureus antibody response to infection. Since type 1 diabetic patients do not share in this adaptive immune defect, it is unlikely that hyperglycemia is the driving effect behind this phenomenon.
Obesity and its associated low-grade chronic inflammation are known to be major drivers of T2D (36). Chronic inflammation has also been shown to impair B cell humoral immune responses in the context of aging (37). Obesity-associated T2D in the mouse is characterized by increased systemic tumor necrosis factor and chronic inflammation, similar to aged mice. The T2D mouse model of obesity and diabetes is also associated with increased interleukin-6 and gamma interferon production by B cells within the spleen (17), further indicating inflammation in a cell type that is crucial in mounting a humoral immune response to infection. Therefore, chronic inflammation associated with obesity and T2D may downregulate B cell responses, decrease B cell-driven antibody production, and increase infection rates in T2D. Although this hypothesis has yet to be tested in this model of obesity-induced type 2 diabetes and infection, it warrants further investigation.
In conclusion, we have demonstrated a blunted anti-S. aureus IgG antibody response to infection in obesity-associated T2D mice, as well as in obese and T2D patients. This reduction in antibody levels against S. aureus is a potential mechanism leading to increased susceptibility of obese, T2D patients to S. aureus infections such as those associated with total joint arthroplasty.
ACKNOWLEDGMENTS
This study was supported by the AOTrauma CPP Bone Infection, National Institutes of Health (NIH) P30 AR061307, and NIH T32 AR053459 grants.
We thank the staff of the Biochemical Genetics Lab of the University of Rochester Medical Center and University of Rochester students Brittany Garrison and Ainnie Dar for assistance with the clinical studies.
We confirm that there are no conflicts of interest.
REFERENCES
- 1.Jamsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. 2012. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am 94:e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. 2007. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. 2008. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. 2012. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA 308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JA, Nair SP. 2010. Interaction of staphylococci with bone. Int J Med Microbiol 300:193–204. doi: 10.1016/j.ijmm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 7.Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann Med 41:109–119. doi: 10.1080/07853890802337045. [DOI] [PubMed] [Google Scholar]
- 8.Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. 2011. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res Ther 13:R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Pozo JL, Patel R. 2009. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowsey MM, Choong PF. 2009. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res 467:1577–1581. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. 2014. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One 9:e95300. doi: 10.1371/journal.pone.0095300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein EY, Sun L, Smith DL, Laxminarayan R. 2013. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol 177:666–674. doi: 10.1093/aje/kws273. [DOI] [PubMed] [Google Scholar]
- 13.Yano H, Kinoshita M, Fujino K, Nakashima M, Yamamoto Y, Miyazaki H, Hamada K, Ono S, Iwaya K, Saitoh D, Seki S, Tanaka Y. 2012. Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect Immun 80:4409–4416. doi: 10.1128/IAI.00787-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Rich J, Hanses F, Lee JC. 2009. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun 77:1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. 2007. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A 104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, Kwon HJ, Kim HS. 2012. Macrophages from nonobese diabetic mouse have a selective defect in IFN-γ but not IFN-α/β receptor pathway. J Clin Immunol 32:753–761. doi: 10.1007/s10875-012-9682-3. [DOI] [PubMed] [Google Scholar]
- 17.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. 2013. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A 110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Hao L, Gill RG, Lafferty KJ. 1987. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes 36:535–538. doi: 10.2337/diab.36.4.535. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. 2012. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes 36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. 2006. Reduced tetanus antibody titers in overweight children. Autoimmunity 39:137–141. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. 1988. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167. doi: 10.2337/diabetes.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 23.Mansford KR, Opie L. 1968. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet i:670–671. [DOI] [PubMed] [Google Scholar]
- 24.Ventura-Sobrevilla J, Boone-Villa VD, Aguilar CN, Roman-Ramos R, Vega-Avila E, Campos-Sepulveda E, Alarcon-Aguilar F. 2011. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc 54:5–9. [PubMed] [Google Scholar]
- 25.Leiter EH. 1982. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci U S A 79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Gromov K, Soballe K, Puzas JE, O'Keefe RJ, Awad H, Drissi H, Schwarz EM. 2008. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res 26:96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junod A, Lambert AE, Stauffacher W, Renold AE. 1969. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest 48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovati AB, Drago L, Monti L, De Vecchi E, Previdi S, Banfi G, Romano CL. 2013. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS One 8:e67628. doi: 10.1371/journal.pone.0067628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen K, Danielsen K, Wilsgaard T, Sangvik M, Sollid JU, Thune I, Eggen AE, Simonsen GS, Furberg AS. 2013. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 8:e63716. doi: 10.1371/journal.pone.0063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipsky BA, Pecoraro RE, Chen MS, Koepsell TD. 1987. Factors affecting staphylococcal colonization among NIDDM outpatients. Diabetes Care 10:483–486. doi: 10.2337/diacare.10.4.483. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Leeman SE, Amar S. 2009. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A 106:10740–10745. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar M, Roe K, Nerurkar PV, Namekar M, Orillo B, Verma S, Nerurkar VR. 2012. Impaired virus clearance, compromised immune response and increased mortality in type 2 diabetic mice infected with West Nile virus. PLoS One 7:e44682. doi: 10.1371/journal.pone.0044682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried AJ, Bonilla FA. 2009. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev 22:396–414. doi: 10.1128/CMR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood P, UK Primary Immunodeficiency Network. 2009. Primary antibody deficiencies: recognition, clinical diagnosis and referral of patients. Clin Med 9:595–599. doi: 10.7861/clinmedicine.9-6-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridgeford EC, Fox JG, Nambiar PR, Rogers AB. 2008. Agammaglobulinemia and Staphylococcus aureus botryomycosis in a cohort of related sentinel Swiss-Webster mice. J Clin Microbiol 46:1881–1884. doi: 10.1128/JCM.01875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregor MF, Hotamisligil GS. 2011. Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 37.Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin AM, Riley RL, Blomberg BB. 2012. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol 188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]