Abstract
Intravaginal infection with plasmid-competent but not plasmid-free Chlamydia muridarum induces hydrosalpinx in mouse upper genital tract, indicating a critical role of the plasmid in chlamydial pathogenicity. To evaluate the contribution of the plasmid to chlamydial ascension and activation of tubal inflammation, we delivered plasmid-free C. muridarum directly into the endometrium by intrauterine inoculation. We found that three of the six mouse strains tested, including CBA/J, C3H/HeJ, and C57BL/6J, developed significant hydrosalpinges when 1 × 107 inclusion-forming units (IFU) of plasmid-free C. muridarum were intrauterinally inoculated. Even when the inoculum was reduced to 1 × 104 IFU, the CBA/J mice still developed robust hydrosalpinx. The hydrosalpinx development in CBA/J mice correlated with increased organism ascension to the oviduct following the intrauterine inoculation. The CBA/J mice intravaginally infected with the same plasmid-free C. muridarum strain displayed reduced ascending infection and failed to develop hydrosalpinx. These observations have demonstrated a critical role of the plasmid in chlamydial ascending infection. The intrauterine inoculation of the CBA/J mice with plasmid-free C. muridarum not only resulted in more infection in the oviduct but also stimulated more inflammatory infiltration and cytokine production in the oviduct than the intravaginal inoculation, suggesting that the oviduct inflammation can be induced by plasmid-independent factors, which makes the hydrosalpinx induction in CBA/J mice by intrauterine infection with plasmid-free C. muridarum a suitable model for investigating plasmid-independent pathogenic mechanisms.
INTRODUCTION
Hydrosalpinx can be induced by lower genital tract (LGT) infection with either Chlamydia trachomatis in women or Chlamydia muridarum in mice, leading to tubal factor infertility in both women (1, 2) and mice (3, 4). Thus, intravaginal inoculation of mice with C. muridarum has been used to study the mechanisms of C. trachomatis pathogenesis and immunity (3, 5–7). We recently optimized the C. muridarum mouse model by visually detecting long-lasting hydrosalpinges 8 weeks after infection (8–12). The C. muridarum murine model-based studies have led us to hypothesize that both adequate ascension to and induction of the appropriate inflammatory responses in the upper genital tract (UGT) are necessary for hydrosalpinx development. However, the virulence factors required for the C. muridarum pathogenicity that lead to tissue inflammation and pathology have not been identified.
C. trachomatis contains a highly conserved plasmid containing 8 open reading frames designated plasmid-encoded glycoprotein 1 to 8 (Pgp1 to -8, respectively) (13–16). This plasmid may play a significant role in C. trachomatis pathogenesis since plasmid-free C. trachomatis exhibited significantly reduced pathogenicity in ocular tissues of primates (17) and genital tract tissues of mice (18). These findings are consistent with a previous observation that induction of hydrosalpinx in mice by intravaginal infection with C. muridarum was plasmid dependent (19). We have recently confirmed the plasmid-dependent pathogenicity of C. muridarum in multiple strains of mice (9). Although the plasmid is required for overall chlamydial pathogenicity, it remains unclear whether the plasmid contributes to the promotion of chlamydial ascending infection, exacerbation of host inflammation in the oviduct, or both. It has been shown that intrauterine inoculation of mice with plasmid-competent C. muridarum can significantly enhance C. muridarum pathogenicity because intrauterine inoculation can bypass the cervical barrier and directly deliver C. muridarum organisms to the endometrial epithelia, leading to more efficient chlamydial ascension to the oviduct (20). When 11 different strains of mice were compared for their susceptibility to hydrosalpinx induction by intravaginal infection with plasmid-competent C. muridarum (10), we showed that CBA/J and SJL/J mice were highly susceptible and BALB/c, C57BL/6J, C57BL/10J, C3H/HeJ, and C3H/HeN mice were moderately susceptible, while the remaining mice, including the NOD/ShiLtJ, DBA/1J, DBA/2J, and A/J strains, were relatively resistant. Despite the diverse range of mouse susceptibilities to hydrosalpinx induction by intravaginal infection, higher incidence and more severe hydrosalpinges were induced in most mice when the 11 strains were infected via intrauterine inoculation. Thus, intrauterine infection can consistently enhance C. muridarum pathogenicity. We hypothesized that intrauterine infection with plasmid-free C. muridarum may lead to hydrosalpinx, especially in highly susceptible mouse strains, such as CBA/J and SJL/J. Comparison of hydrosalpinx induction between intravaginal and intrauterine infections with plasmid-competent C. muridarum has led us to demonstrate a critical role of ascending infection in chlamydial pathogenicity (10). We hypothesized that comparison of the upper genital tract pathology induced by plasmid-free C. muridarum inoculated intravaginally versus intrauterinally might allow us to determine the role of the plasmid in ascending infection.
Although the plasmid-dependent virulence factors are critical for chlamydial pathogenicity, plasmid-independent virulence factors may also play important roles in chlamydial pathogenesis. For example, plasmid-free Chlamydia caviae GPIC was reported to be as virulent as plasmid-competent GPIC (21). Mutations found in the chromosome-carried genes of C. trachomatis (CT135) and C. muridarum (TC0236) can enhance chlamydial infectivity independently of the plasmid (22, 23). Thus, both plasmid-dependent and -independent virulence factors can contribute to chlamydial pathogenicity. However, the failure of plasmid-free C. muridarum to induce hydrosalpinx when the organisms were delivered via intravaginal infection has made it difficult to use the mouse model for investigation of plasmid-independent pathogenicity. We hypothesized that intrauterine infection of susceptible mice with plasmid-free C. muridarum might lead to the induction of hydrosalpinx, which may provide an appropriate model for studying the pathogenic mechanisms of plasmid-free C. muridarum.
In the present study, we tested the above hypothesis by delivering plasmid-free C. muridarum cells directly into the upper genital tract of 6 different strains of mice by intrauterine inoculation. Three strains, including CBA/J, C3H/HeJ, and C57BL/6J, developed significant hydrosalpinges at a high infection dose. When the inoculum was reduced by 1,000-fold, CBA/J mice still developed significant hydrosalpinx. These observations have not only demonstrated a critical contribution of the plasmid to the chlamydial ability to overcome cervical barriers for ascension to the upper genital tract but also allowed us to use the intrauterine infection of CBA/J mice with plasmid-free C. muridarum to investigate the mechanisms of plasmid-independent pathogenicity.
MATERIALS AND METHODS
Chlamydia muridarum and mouse infection.
The Chlamydia muridarum organisms were propagated and purified in HeLa 229 cells (human cervical carcinoma epithelial cells; ATCC catalog no. CCL2), as described previously (24). The wild-type plasmid-competent Chlamydia muridarum (CMwt) strain Nigg was acquired from Robert Brunham (25). The plasmid-free C. muridarum CMUT3 strain was derived from the CMwt strain and plaque purified as described previously (9, 26). The plasmid-free C. muridarum CM972 strain was generously provided by C. M. O'Connell (27). For C. muridarum infection in mice, the organisms were used to infect 6- to 7-week-old female mice via either the intrauterine or intravaginal route with different amounts of live organisms (as indicated in individual experiments) following protocols described previously (10, 20, 28). The following 6 strains of mice used in the present study were all purchased from Jackson Laboratories (Bar Harbor, ME): CBA/J (stock no. 000656), C3H/HeJ (stock no. 000659), C57BL/6J SJL/J (stock no. 000686), DBA/2J (stock no. 000671), and A/J (stock no. 000646). Five days prior to infection, each mouse was injected with 2.5 mg medroxyprogesterone (Depo-Provera; Pharmacia Upjohn, Kalamazoo, MI) subcutaneously to increase mouse susceptibility to infection. After infection, mice were monitored for vaginal live organism shedding and sacrificed on day 60 postinfection for observation of gross genital tract pathologies. In parallel experiments, groups of mice were sacrificed on different days postinfection for quantitation of live organisms recovered from different segments of the genital tract.
For monitoring of live organism shedding from swab samples, vaginal/cervical swabs were taken every 3 to 4 days for the first 2 weeks and weekly thereafter until negative shedding was observed for 2 consecutive time points. To quantitate live chlamydial organisms, each swab was soaked in 0.5 ml of sucrose-phosphate-glutamic acid (SPG) and vortexed with glass beads, and the chlamydial organisms released into the supernatant were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with less than 1 inclusion-forming unit (IFU) per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFU per swab was calculated based on the mean IFU per view, the number of views per coverslip, the dilution factor, magnification, and inoculation volumes. Where possible, a mean IFU-per-swab value was derived from the serially diluted and duplicate samples for any given swab. The calculated total number of IFU per swab was converted into log10 and used to calculate the mean and standard deviation (SD) value across mice of the same group at each time point.
To monitor ascending infection, mice infected in parallel experiments were sacrificed on day 14 after infection. Whole genital tracts were sterilely harvested, and each tract was divided into three portions, including vagina/cervix (VC), uterine/uterine horn (UH), and oviduct/ovary (OV). VC was defined as the lower genital tract (LGT), while both UH and OV were defined as the upper genital tract (UGT). Tissue segments were homogenized in 0.2 ml cold SPG using a 2-ml tissue grinder (catalog no. K885300-0002; Fisher Scientific, Pittsburg, PA). After brief sonication and centrifugation at 3,000 rpm for 5 min to pellet large debris, the supernatants were titrated for live C. muridarum organisms on HeLa cells as described above. The results were expressed as log10 IFU per tissue segment.
Mouse genital tract pathology evaluation.
Mice were sacrificed 60 days after infection to evaluate urogenital tract tissue pathology. Before the genital tract tissues were removed from the mice, an in situ gross examination was performed under a stereoscope (Olympus, Center Valley, PA) for evidence of hydrosalpinx formation and any other gross abnormalities. The genital tract tissues were then excised in their entirety from the vagina to the ovary and laid on a blue photography mat for acquisition of digital images. The oviduct hydrosalpinges were visually scored based on their dilation size using a scoring system described previously (12). The presence of no oviduct dilation or swelling found by stereoscope inspection was defined as “no hydrosalpinx” and assigned a score of 0, hydrosalpinx only visible after amplification was given a score of 1, hydrosalpinx clearly visible to the naked eye but smaller than the ovary was given a score of 2, hydrosalpinx with a size similar to that of ovary was given a score of 3, and hydrosalpinx larger than the ovary was given a score of 4. Both the incidence and severity scores of oviduct hydrosalpinx were analyzed for statistical differences between groups of mice.
For histological scoring and inflammatory cell counting, the excised mouse genital tract tissues, after being photographed, were fixed in 10% neutral formalin, embedded in paraffin, and serially sectioned longitudinally at 5-μm widths. Efforts were made to include cervix, both uterine horns, oviducts, and lumenal structures of each tissue in each section. The sections were stained with hematoxylin and eosin (H&E) as described elsewhere (3). The H&E-stained sections were scored for severity of inflammation and oviduct dilation based on the modified schemes established previously (3, 29) by researchers who were blind to the mouse group designations. Scores from both sides of the oviducts were added to represent the oviduct pathology for a given mouse, and the individual mouse scores were calculated into medians for each group.
Inflammatory cell infiltration was scored as follows for the oviduct tissue: 0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; and 4, confluent infiltration.
Dilatation was scored as follows for the oviduct lumen: 0, no significant dilatation; 1, mild dilation of a single cross section; 2, one to three dilated cross sections (with the largest diameter smaller than that of the ovary on the same side); 3, more than three dilated cross sections (with the largest diameter equal to that of the ovary on the same side); and 4, confluent pronounced dilation (with the largest diameter larger than that of the ovary on the same side).
Immunofluorescence assay.
HeLa cells grown on coverslips were fixed with 4% (wt/vol) paraformaldehyde (Sigma) dissolved in phosphate-buffered saline (PBS) for 30 min at room temperature, followed by permeabilization with 2% (wt/vol) saponin (Sigma) for an additional 30 min. After washing and blocking, the cell samples were subjected to antibody and chemical staining. Hoechst 33342 (Sigma) was used to visualize DNA. A rabbit anti-chlamydial organism antibody plus a goat anti-rabbit IgG secondary antibody conjugated with Cy2 (green) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to visualize chlamydial organism-containing inclusions. Immunofluorescence images were acquired using an Olympus AX-70 fluorescence microscope equipped with multiple filter sets and Simple PCI imaging software (Olympus) as described previously (24). The images were processed using the Adobe Photoshop program (Adobe Systems, San Jose, CA). For titration of the live organisms recovered from a given sample, the mean number of inclusions per view was derived from counting five random views. The total number of live organisms recovered from a given sample was calculated based on the mean inclusions per view, number of views per coverslip under a given magnification, dilution factor, and inoculum volume and expressed as log10 IFU per sample.
Multiplex array for profiling cytokines in oviduct tissue.
Oviduct/ovary tissues harvested from mice infected with C. muridarum were homogenized as described previously (20, 29). The homogenates were used for simultaneous measurements of 32 mouse cytokines (23 of plex group I [catalog no. M60-009RDPD] plus 9 of plex group II [catalog no. MD0-00000EL]) using a multiplex bead array assay (Bio-Plex 200 system; all from Bio-Rad, Hercules, CA) by following the manufacturer's instructions. All cytokines are expressed in picograms per milliliter. The mean concentrations from mice infected intravaginally or intrauterinally were used for calculating the ratios, and the differences in cytokine concentrations between the two groups of mice were analyzed using Student's t test.
RESULTS
CBA/J mice are most susceptible to hydrosalpinx induction by intrauterine infection with plasmid-free C. muridarum.
The failure of intravaginal infection with plasmid-free C. muridarum to induce hydrosalpinx in mice (9, 19) makes it difficult to use the murine model to study plasmid-independent pathogenicity. Since intrauterine infection with plasmid-competent C. muridarum significantly enhanced the chlamydial pathogenicity in mouse upper genital tract (10, 20), we used an intrauterine route to infect 6 different strains of mice with plasmid-free C. muridarum and monitored the mice for hydrosalpinx development (Fig. 1A). We chose these 6 strains because each two strains represented one of the three categories of mouse susceptibility to the induction of hydrosalpinx by intravaginal infection with plasmid-competent C. muridarum (10). CBA/J and SJL/J mice were the only two strains found to be in the “highly susceptible” category. C3H/HeJ and C57BL/6J were 2 of the 5 strains in the category “susceptible,” while DBA2/J and A/J were 2 of the 4 strains in the category “resistant.” We found that CBA/J, C3H/HeJ, and C57BL/6J mice all developed significant hydrosalpinges when each mouse was intrauterinally inoculated with 1 × 107 IFU of the plasmid-free C. muridarum CMUT3 organisms. However, when the inoculum size was reduced to 2 ×105 IFU, only CBA/J (but not C3H/Hej or C57BL/6J) mice developed hydrosalpinx. Thus, CBA/J mice appeared to be most susceptible to the pathogenic effects of plasmid-free C. muridarum. This finding was somewhat surprising to us since SJL/J mice were also highly susceptible to the induction of hydrosalpinx by intravaginal infection with plasmid-competent C. muridarum (10) but failed to respond to the intrauterine infection with plasmid-free C. muridarum. We further titrated the infection doses required for hydrosalpinx induction in CBA/J mice (Fig. 1B). We found that 100% of CBA/J mice developed significant hydrosalpinx when the inoculum was either 1 × 106 or 2 × 105 IFU. Even an inoculum of 1 × 104 IFU induced 60% of CBA/J mice to develop hydrosalpinx. We then compared the plasmid-free CMUT3 strain generated in the authors' laboratory with the plasmid-free CM972 strain derived from a different laboratory (27) for their abilities to induce hydrosalpinx in CBA/J mice. The wild-type plasmid-competent C. muridarum strain was used as a positive control. We found that intrauterine infection with either the plasmid-free C. muridarum CMUT3 or CM972 organisms induced significant hydrosalpinx with a severity score of around 3 and incidence rates of 80% or higher, while the plasmid-competent C. muridarum strain induced a severity score of ∼7 and a 100% incidence rate. The gross pathology was also confirmed under microscopy (Fig. 2). Intrauterine but not intravaginal infection with the plasmid-free CMUT3 or CM972 organisms induced significant oviduct lumenal dilation. As a control, the plasmid-competent C. muridarum organisms induced severe oviduct dilation regardless of the infection routes. These observations have demonstrated that CBA/J mice are highly susceptible to hydrosalpinx induction via intrauterine infection with plasmid-free C. muridarum, which may be used for investigation of plasmid-independent pathogenic mechanisms.
FIG 1.
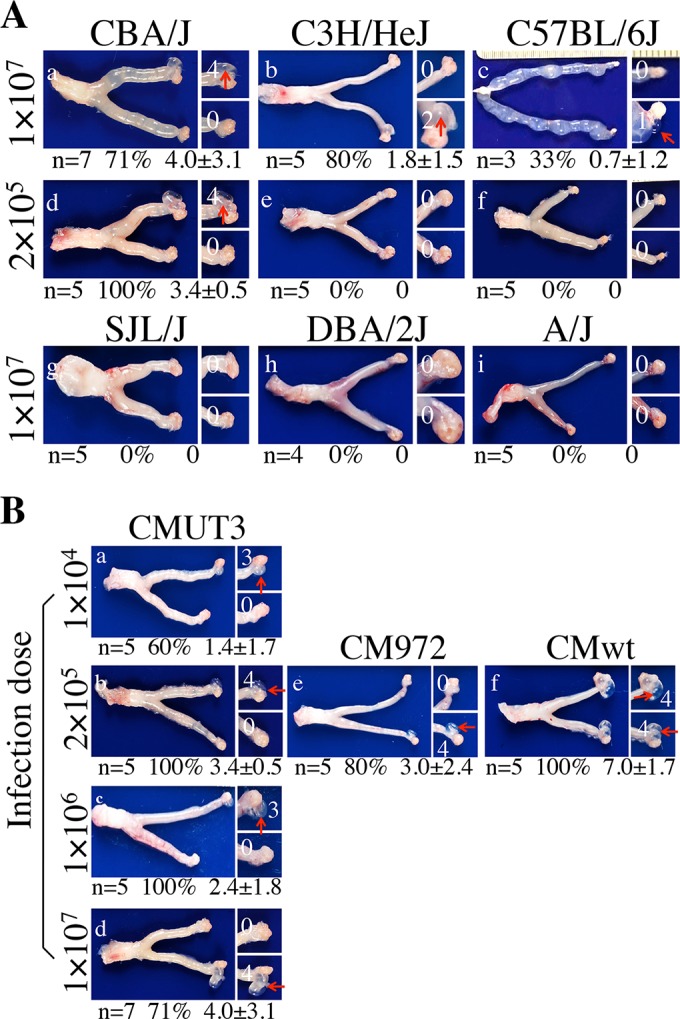
Oviduct gross pathology induced by plasmid-free C. muridarum. (A) CBA/J (a and d), C3H/HeJ (b and e), C57BL/6J (c and f), SJL/J (g), DBA/2J (h), and A/J (i) mice were infected intrauterinally with 1 × 107 (a to c and g to i) or 2 × 105 (d to f) IFU of plasmid-free C. muridarum CMUT3 and sacrificed 60 days after infection for observation of hydrosalpinx. A representative image of the whole genital tract from each group is presented in the left columns, with the vagina oriented on the left and the oviduct/ovary on the right. Images for the areas covering the oviduct/ovary portions are amplified and shown to the right of the corresponding whole genital tract images, with hydrosalpinx indicated by red arrows and hydrosalpinx severity scores indicated by white numbers. The sample size, hydrosalpinx incidence rates, and severity scores (means ± standard errors) from each group are listed under the corresponding images. (B) CBA/J mice were infected intrauterinally with plasmid-free C. muridarum CMUT3 at 1 × 104 (a), 2 × 105 (b), 1 × 106 (c), or 1 × 107 (d), plasmid-free C. muridarum CM972 at 2 × 105 (e), or plasmid-competent C. muridarum (CMwt) at 2 × 105 (f). These groups were also sacrificed 60 days after infection for observation of hydrosalpinx. The images and data are presented in the same way as described in the legend to panel A.
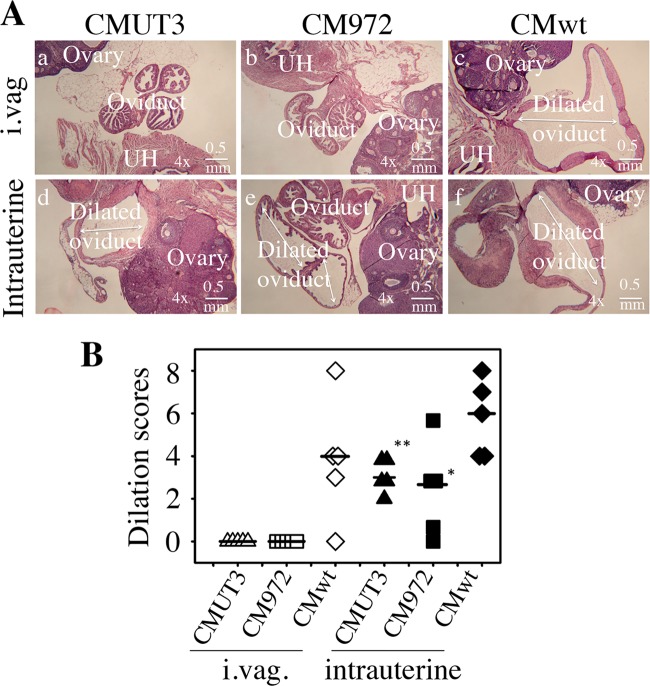
FIG 2.
Microscopic observation of oviduct dilation induced by plasmid-free C. muridarum. (A) The oviduct tissues of CBA/J mice infected intravaginally (i.vag. [a to c]) or intrauterinally (d to f) with 2 × 105 IFU of plasmid-free C. muridarum CMUT3 (a and d) or CM972 (b and e) or plasmid-competent wild-type C. muridarum (CMwt [c and f]) were harvested and subjected to H&E staining for microscopic evaluation of oviduct dilation. Representative images from each group taken under a 4× objective lens are shown. Uterine horn (UH), oviduct, and ovary tissues are marked in white letters. Dilated oviducts are indicated with white lines with arrowheads at both ends. The horizontal bar at the right bottom of each image represents a physical distance of 0.5 mm. (B) Severity of lumenal dilation was scored as described in Materials and Methods and listed along the y axis. With intravaginal infection (i.vag.), open triangles represent CMUT3, open squares represent CM972, and open diamonds represent the wild-type plasmid-competent C. muridarum strain, while with intrauterine infection, the corresponding solid symbols were used. Note that both the plasmid-free CMUT3 and CM972 organisms induced significant oviduct dilation with intrauterine but not intravaginal infection. *, P < 0.05; **, P < 0.01 (Wilcoxon rank sum test).
The ascension of plasmid-free C. muridarum to mouse oviduct is enhanced by intrauterine inoculation.
We first compared the time courses of live organism shedding from the lower genital tract between mice infected with C. muridarum with or without plasmid either intravaginally or intrauterinally (Fig. 3). There was no significant difference in shedding courses between intravaginally and intrauterinally infected mice regardless of the organisms used. This observation suggests that C. muridarum organisms maintained similar levels of infectivity in mouse lower genital tracts, whether the initial infection site was in the lower genital tract vagina/cervix mucosal epithelial cells or the upper genital tract endometrial epithelial cells. We further compared the oviduct infections of the plasmid-free CMUT3 organisms inoculated via either the intravaginal or intrauterine route (Fig. 4). The numbers of live organisms recovered from the oviduct but not the uterine/uterine horn or vagina/cervix tissues were significantly higher in the intrauterinally infected mice than in the intravaginally infected mice. The increased oviduct infection correlated well with the induction of hydrosalpinx by the intrauterine inoculation with the plasmid-free C. muridarum in CBA/J mice. However, when the levels of organism recovery from oviduct tissues were compared between different strains of mice, no significant correlation was found between the oviduct infection and hydrosalpinx induction. As shown in Fig. 5, although the numbers of live plasmid-free C. muridarum organisms recovered from the oviducts of CBA/J and C57BL/6J (highly susceptible or susceptible to hydrosalpinx induction by plasmid-free organisms) appeared to be higher than those recovered from the SJL/J and DBA2/J mice (resistant to hydrosalpinx induction by plasmid-free organisms), there was no significant difference. The biggest difference was between the CBA/J and SJL/J mice (P = 0.076, Mann-Whitney test). Thus, besides ascending infection, mouse strain background genes may also affect how mice respond to the oviduct infection, leading to the varied outcomes in hydrosalpinx incidence and/or severity among different mouse strains.
FIG 3.
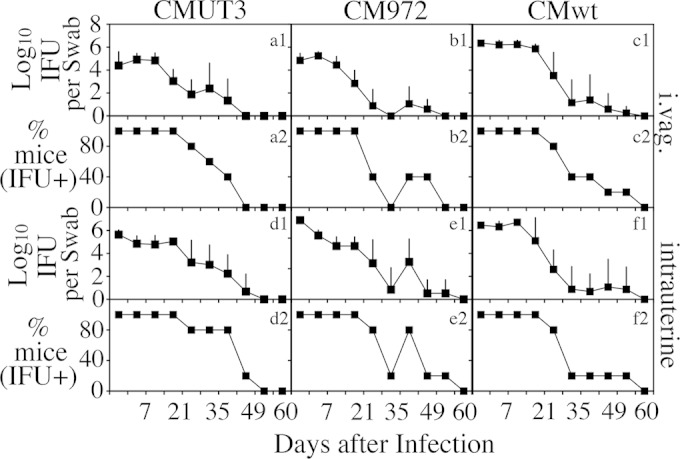
Live chlamydial organism shedding from mouse lower genital tract following C. muridarum infection. CBA/J mice were infected intravaginally (a1 to c1 and a2 to c2) or intrauterinally (d1 to f1 and d2 to f2) with plasmid-free C. muridarum CMUT3 (a1 and a2 and d1 and d2), plasmid-free CM972 (b1 and b2 and e1 and e2), or plasmid-competent wild type C. muridarum (CMwt [c1 and c2 and f1 and f2]) as described in the Fig. 2 legend. On different days after infection, as shown along the x axis, vaginal swabs were taken for titration of live organisms on HeLa cell monolayers. The live organisms recovered from swabs were expressed as log10 IFU along the y axis (a1 to c1 and d1 to f1). The percentage of mice remaining positive for shedding live organisms at each time point was plotted along the y axis (a2 to c2 and d2 to f2). Note that similar live organism shedding courses were found in all mouse groups.
FIG 4.
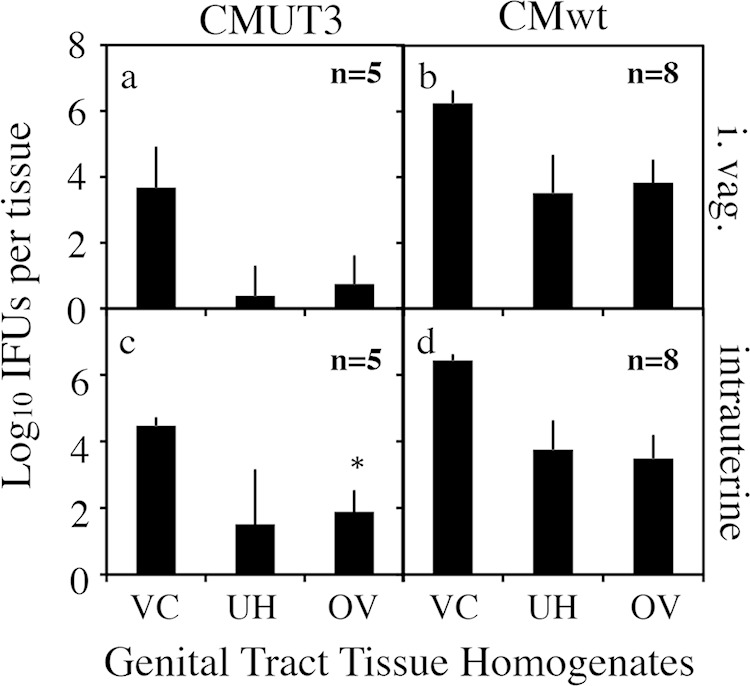
Live chlamydial organism recovery from genital tract tissues of CBA/J mice following C. muridarum infection. CBA/J mice were infected intravaginally (a and b) or intrauterinally (c and d) with plasmid-free C. muridarum CMUT3 (a and c) or plasmid-competent C. muridarum (CMwt [b and d]) at the dose of 2 × 105 IFU per mouse and sacrificed on day 14 after infection. Vaginal swabs were taken prior to mouse sacrifice. The entire genital tract tissue was harvested from each mouse and divided into the lower genital tract vagina/cervix (VC) and the upper genital tract uterus/uterine horn (UH) and oviduct/ovary (OV) sections as listed along the x axis. Each tissue section was homogenized for titration of live C. muridarum organisms. Log10 IFU were used to calculate mean and SD for each group as displayed along the y axis. Note that the number of live organisms recovered from the oviduct of mice infected intrauterinally with CMUT3 was significantly higher than that from intravaginally infected mice. *, P = 0.056 (two-tailed Mann-Whitney test).
FIG 5.
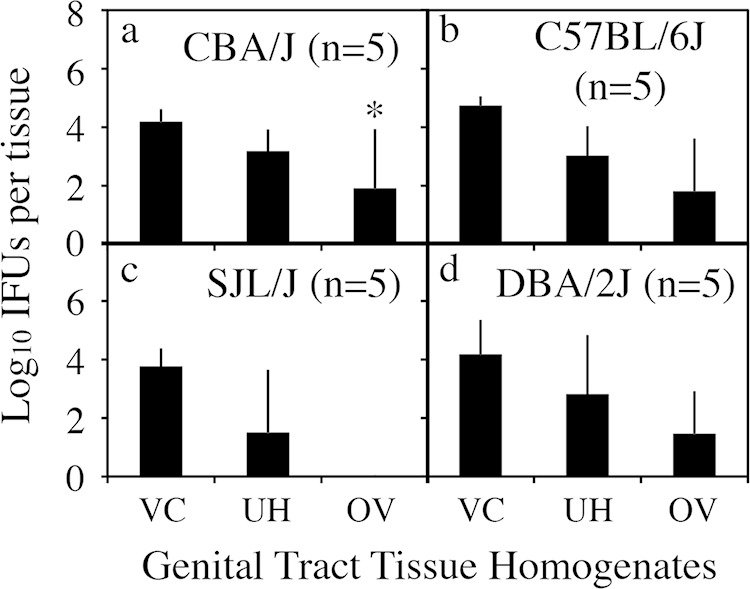
Live chlamydial organism recovery from genital tract tissues of 4 strains of mice following C. muridarum infection. Each mouse was infected intrauterinally with 1 × 107 IFU of plasmid-free C. muridarum CMUT3 (panels a for CBA/J, b for C57BL/6J, c for SJL/J, and d for DBA/2J). Mice were sacrificed on day 14 after infection to harvest the genital tract tissues, which were divided into the lower genital tract vagina/cervix (VC) and the upper genital tract uterus/uterine horn (UH) and oviduct/ovary (OV) sections, as listed along the x axis. Each tissue section was homogenized for titration of live C. muridarum organisms. Log10 IFU were used to calculate the mean and SD for each group as displayed along the y axis. *, P = 0.075 (two-tailed Mann-Whitney test).
Oviduct inflammatory responses are enhanced by intrauterine infection with plasmid-free C. muridarum.
When the oviduct tissues from CBA/J mice were evaluated for inflammatory histopathology under microscopy, we found that the inflammatory infiltration was significantly more severe in mice infected intrauterinally with either the plasmid-free CMUT3 or CM972 strain (Fig. 6). We further compared the cytokine levels in the CBA/J mouse oviduct tissue homogenates harvested on day 14 from mice infected with CMUT3 via either the intravaginal or intrauterine route (Table 1). We found that 12 out of the 32 cytokines measured were significantly higher in mice intrauterinally infected with CMUT3. These 12 cytokines include proinflammatory cytokines interleukin-1α (IL-1α) and IL-1β, Th1-promoting cytokine IL-12, Th17 cytokine IL-17, chemokines KC (keratinocyte chemoattractant), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α) and -β, RANTES, MIG (monokine induced by IFN-γ), leukemia inhibitory factor (LIF), and vascular endothelial growth factor (VEGF). Although there was no significant difference for the remaining cytokines, most of them displayed higher levels in the oviducts of mice infected intrauterinally. It is worth noting that the concentrations of most cytokines detected from the oviducts of plasmid-competent C. muridarum-infected mice were significantly higher than those from the plasmid-free C. muridarum-infected mice, which is consistent with the general concept that the plasmid-competent C. muridarum organisms are more effective in both ascending infection and stimulating tubal inflammation. Nevertheless, the fact that the plasmid-free C. muridarum still stimulated significant cytokines in the oviduct tissues suggests that plasmid-independent chlamydial factors are capable of activating inflammatory pathways in the oviduct, which makes the CBA/J mouse model suitable for investigation of the chlamydial plasmid-independent pathogenic mechanisms.
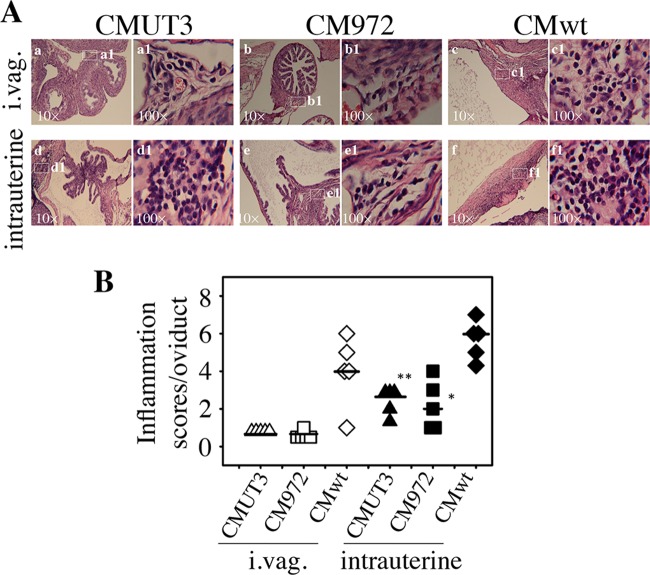
FIG 6.
Oviduct inflammatory infiltration induced by C. muridarum infection. (A) The oviduct tissues of CBA/J mice infected intravaginally (i.vag [a to c]) or intrauterinally (d to f) with CMUT3 (a and d), CM972 (b and e), or CMwt (c and f) were harvested and subjected to H&E staining for microscopic evaluation of inflammatory infiltration as described in the Fig. 2 legend. Representative images from each group taken under 10× (left [a to f]) and 100× (right [a1 to f1]) objective lenses, respectively, are shown. White rectangles in the 10× images indicate the same areas from which the right images were taken under a 100× lens. (B) The severity of inflammatory infiltration was semiquantitatively scored as described in Materials and Methods and listed along the y axis. Open triangles represent CMUT3, open squares represent CM972, and open diamonds represent plasmid-competent C. muridarum (CMwt), while with intrauterine infection, the corresponding solid symbols were used. Note that both the plasmid-free CMUT3 and CM972 organisms induced significantly more inflammatory infiltration when infected intrauterinally than intravaginally. *, P < 0.05; **, P < 0.01 (Wilcoxon rank sum test).
TABLE 1.
Cytokines from oviduct homogenates harvested 14 days after intravaginal or intrauterine infection with C. muridarum with or without plasmid
| Cytokinea | Cytokine concn (pg/ml)b |
Ratio of intrauterine to intravaginalc | P valued | ||
|---|---|---|---|---|---|
| Plasmid-competent C. muridarum, intravaginal (n = 8) | Plasmid-free C. muridarum |
||||
| Intravaginal (n = 5) | Intrauterine (n = 5) | ||||
| IL-1α | 3,917.9 ± 2,760.8 | 7.1 ± 4.5 | 50.4 ± 25.2 | 7.1 | 0.01 |
| IL-1β | 5,359.7 ± 3,966.1 | 35.5 ± 22.9 | 151.8 ± 64.1 | 4.3 | 0.01 |
| IL-2 | 12.9 ± 10.8 | 0.9 ± 1.4 | 0 | 0.16 | |
| IL-3 | 9.7 ± 7.4 | 0.5 ± 0.7 | 0.6 ± 0.8 | 1.2 | 0.85 |
| IL-4 | 30.4 ± 21.1 | 1.8 ± 2.2 | 2.1 ± 1.8 | 1.2 | 0.82 |
| IL-5 | 22.0 ± 18.7 | 0 | 2.5 ± 5.5 | 0.35 | |
| IL-6 | 201.5 ± 155.4 | 0 | 0 | ||
| IL-9 | 309.0 ± 257.9 | 20.0 ± 37.9 | 0 | 0.35 | |
| IL-10 | 57.4 ± 28.0 | 14.1 ± 8.2 | 22.9 ± 6.5 | 1.6 | 0.1 |
| IL-12(p40) | 365.3 ± 229.4 | 35.0 ± 19.6 | 225.6 ± 143.4 | 6.4 | 0.02 |
| IL-12(p70) | 271.9 ± 179.0 | 1.5 ± 3.4 | 2.8 ± 4.0 | 1.9 | 0.59 |
| IL-13 | 650.1 ± 174.2 | 371.3 ± 243.7 | 594.0 ± 383.0 | 1.6 | 0.3 |
| IL-17 | 250.4 ± 179.0 | 6.0 ± 3.6 | 14.4 ± 2.7 | 2.4 | <0.01 |
| Eotaxin | 910.6 ± 609.6 | 944.1 ± 904.6 | 620.4 ± 850.7 | 0.7 | 0.58 |
| G-CSF | 6,016.4 ± 6,218.6 | 115.8 ± 66.7 | 18.7 ± 82.0 | 1.6 | 0.2 |
| GM-CSF | 108.4 ± 91.2 | 0 | 11.3 ± 25.3 | 0.35 | |
| IFN-γ | 154.5 ± 111.2 | 3.4 ± 7.7 | 0 | 0.35 | |
| KC | 790.7 ± 633.3 | 2.9 ± 4.3 | 21.9 ± 15.3 | 7.5 | 0.03 |
| MCP-1 | 4,401.2 ± 3,033.3 | 136 ± 88.6 | 942.7 ± 674.8 | 6.9 | 0.03 |
| MIP-1α | 401.7 ± 297.3 | 3.2 ± 7.2 | 34.4 ± 23.8 | 10.7 | 0.02 |
| MIP-1β | 220.7 ± 138.2 | 28.4 ± 16.4 | 53.6 ± 10.8 | 1.9 | 0.02 |
| RANTES | 581.9 ± 374.1 | 14.5 ± 5.8 | 181.3 ± 113.0 | 12.5 | 0.01 |
| TNF-α | 87.2 ± 75.7 | 0 | 0 | ||
| IL-15 | 404.0 ± 297.4 | 60.9 ± 32.0 | 57.8 ± 32.2 | 0.9 | 0.88 |
| IL-18 | 444.1 ± 343.2 | 14.7 ± 20.2 | 39.4 ± 29.6 | 2.7 | 0.16 |
| FGF-basic | 2,343.1 ± 651.0 | 1,422.7 ± 932.3 | 1,304.6 ± 391.8 | 0.9 | 0.8 |
| LIF | 140.6 ± 103.4 | 2.7 ± 3.1 | 10.4 ± 3.9 | 3.9 | 0.01 |
| M-CSF | 259.4 ± 164.0 | 30.8 ± 18.3 | 101.8 ± 117.4 | 3.3 | 0.22 |
| MIG | 149,151.1 ± 102,714.6 | 644.7 ± 327.5 | 13,791.8 ± 13,080.8 | 21.4 | 0.05 |
| MIP-2 | 3,961.5 ± 4,127.7 | 1.7 ± 1.3 | 31.6 ± 38.8 | 18.2 | 0.12 |
| PDGF-BB | 0 | 120.2 ± 268.8 | 0 | 0.35 | |
| VEGF | 3,446.0 ± 3,052.1 | 860.6 ± 292.0 | 1,270.5 ± 252.0 | 1.5 | 0.04 |
Cytokines were from oviduct tissues of mice infected with C. muridarum. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; TNF-α, tumor necrosis factor alpha; FGF-basic, basic fibroblast growth factor; PDGF-BB, platelet-derived growth factor with two B chains.
Oviduct tissue homogenates were produced from CBA/J mice infected intravaginally with plasmid-competent C. muridarum (n = 8) or plasmid-free C. muridarum CMUT3 (n = 5) or intrauterinally with CMUT3 (n = 5) on day 14 after infection for simultaneous measurement of 32 cytokines using a multiplex bead array assay. The infection dose was 2 × 105 IFU per mouse. Cytokine concentrations are listed as means ± standard deviations.
The means from the two groups of mice were used to calculate the ratio of intrauterine to intravaginal.
Student's t test was used for comparison between the plasmid-free C. muridarum-infected groups. Note that concentrations of 12 of the 32 cytokines were significantly higher in the oviducts of mice infected intrauterinally (P values highlighted in boldface).
DISCUSSION
The Chlamydia-induced pathology in the upper genital tract following lower genital tract infection is dependent on both adequate ascension of chlamydial organisms to the upper genital tract and activation of the appropriate inflammatory responses in the oviduct (8–11, 30). The chlamydial plasmid has been shown to be an essential determinant for chlamydial pathogenicity (9, 19). Although recent successes in transforming Chlamydia (26, 31–34) have made it possible to map the plasmid-dependent virulence factors, the relative contributions of the plasmid to the ascension of chlamydial organisms and the activation of host inflammation in the oviduct remain unclear. The data from the present study together with previous observations have led us to conclude that the plasmid plays a critical role in the ability of C. muridarum to overcome the cervical barrier to reach the upper genital tract. First, plasmid-competent C. muridarum induced hydrosalpinx (10) (Fig. 1B and 2), while plasmid-free C. muridarum failed to do so when inoculated intravaginally (9) (Fig. 2), demonstrating that the plasmid is required for hydrosalpinx induction. Second, although intravaginal infection with plasmid-free C. muridarum failed to induce hydrosalpinx, intrauterine inoculation did cause hydrosalpinx at least in some mouse strains (Fig. 1). Since the intrauterine inoculation bypassed the cervical barrier and directly delivered the chlamydial organisms into the upper genital tract, the above observation indicated a critical role of the plasmid in chlamydial ascension through the cervical barrier. Finally, the intrauterinally delivered plasmid-free C. muridarum strain was detected in the oviduct in significant amounts (Fig. 4) and induced significant levels of inflammatory cytokines in the oviduct (Fig. 6 and Table 1), suggesting that the plasmid-free C. muridarum organisms possess the ability to induce hydrosalpinx-causing inflammation in the oviduct as long as these plasmid-free organisms are enabled to overcome the cervical barrier.
Our conclusion on a critical role of the chlamydial plasmid in aiding chlamydial ascension does not necessarily suggest that the plasmid does not play a role in activating tubal inflammation. On the contrary, careful comparison between mice intrauterinally infected with the plasmid-free C. muridarum strains and those infected with plasmid-competent C. muridarum strain revealed an important role of the plasmid in the induction of tubal inflammation. First, hydrosalpinx induced by plasmid-competent C. muridarum was significantly more severe than that induced by plasmid-free C. muridarum (Fig. 1B, panel f versus panels b and e). Second, although the intrauterinally inoculated plasmid-free C. muridarum strains induced significantly higher levels of inflammatory cytokines in the oviduct than the intravaginally inoculated plasmid-free C. muridarum strains (Table 1), these cytokine levels remained significantly lower than those induced by the plasmid-competent C. muridarum strain (Table 1) (10, 30). Thus, the plasmid-dependent factors must play a significant role in activating tubal inflammation. The chlamydial plasmid not only contains 8 open reading frames (13–16) but also regulates several dozen genes in the chromosome (26, 32, 33, 35). The recent success in transforming Chlamydia (26, 31-33, 36) should facilitate the identification of the plasmid-dependent virulence factors. Indeed, the plasmid-encoded Pgp3, an immunodominant (37), trimeric (38, 39), and secretion (14) protein, has been shown to play a significant role in C. trachomatis L2 infection of the mouse genital tract (40) and C. muridarum induction of hydrosalpinx (41).
Screening of 11 strains of mice for their susceptibility to the induction of hydrosalpinx by intravaginal and intrauterine infection with the plasmid-competent C. muridarum strain (10) has led us to conclude that both the mouse's ability to prevent ascending infection/clear upper genital tract infection and the mouse's responsiveness in terms of the types of inflammation in response to the chlamydial infection in the oviduct may determine the mouse's susceptibility to hydrosalpinx induction. For example, the DBA/2J mice prevented hydrosalpinx mainly by controlling ascending infection since intrauterine infection converted this strain from the “resistant” to the “highly susceptible” phenotype, while the A/J mice blocked hydrosalpinx development by controlling the oviduct inflammation since even intrauterine infection failed to induce hydrosalpinx in this strain. However, in the present study, neither strain was induced to develop hydrosalpinx by intrauterine infection with plasmid-free C. muridarum, suggesting that these 2 strains either efficiently cleared the upper genital tract infection with plasmid-free C. muridarum or, more importantly, failed to respond to the stimulation with plasmid-independent virulence factors, although the DBA/2J mice were able to develop hydrosalpinx-causing inflammation to the upper genital tract infection with the plasmid-competent C. muridarum strains. Thus, we conclude that a plasmid-dependent factor is required for induction of hydrosalpinx in DBA/2J mice, while no C. muridarum factors coded in either the chromosome or plasmid can induce hydrosalpinx in A/J mice. The C57BL/6J and C3H/HeJ mice reduced hydrosalpinx by decreasing ascending infection since intrauterine infection converted these mice from the “susceptible” to “highly susceptible” phenotype. However, these two strains of mice only developed very low levels of hydrosalpinx with a mean severity score of <2 when the dose of the intrauterine infection with the plasmid-free C. muridarum strain was increased to 1 × 107 IFU, suggesting that these two mouse strains were relatively resistant but more responsive than the DBA/2J and A/J mice to the oviduct stimulation by the plasmid-free C. muridarum strain; The CBA/J and SJL/J mice developed maximal levels of hydrosalpinx following an infection with plasmid-competent C. muridarum regardless of the routes, suggesting that these two strains were both inefficient at preventing ascending infection and hyperresponsive to chlamydial stimulation in the oviduct. Surprisingly, only the CBA/J mice maintained the high susceptibility, while the SJL mice became highly resistant when these mice were intrauterinally challenged with the plasmid-free organisms, suggesting that the SJL/J mice are only responsive to plasmid-dependent virulence factors, while CBA/J mice can respond to the plasmid-independent organisms or both. Another possibility is that the SJL/J mice may be able to efficiently clear the upper genital tract infection of the plasmid-free but not plasmid-competent organisms, while CBA/J mice may fail to clear either. This hypothesis seemed to be supported by the finding that no significant live plasmid-free organisms were recovered from the SJL/J mouse oviducts, while obvious live organisms were detected in the oviducts of CBA/J mice following an intrauterine infection. Although the difference was not significant, probably due to the small size, the SJL/J mice obviously prevented or cleared the oviduct infection by the intrauterinally inoculated plasmid-free C. muridarum, while the CBA/J mice were more permissive to the plasmid-free C. muridarum infection in the oviduct, leading to the development of hydrosalpinx.
It is interesting that intrauterine inoculation with plasmid-free C. muridarum induced hydrosalpinx in CBA/J, C3H/HeJ, and C57BL/6J mice, while intrabursal inoculation with the same plasmid-free C. muridarum organisms failed to do so in SJL/J, C3H/HeJ, and BALB/c mice (9). The question is why the intrauterine inoculation appears to be more efficient than intrabursal inoculation in inducing hydrosalpinx. Three major possibilities are speculated below. The first possibility is a delivery efficiency issue. Intrauterine injection directly delivers the chlamydial organisms into the fresh sterile endometrial epithelia (without prior inflammation), which maximizes the chance for the delivered chlamydial organisms to find epithelial cells for infection. However, intrabursal injection only delivers the organisms into the bursal area surrounded by fatty tissues. This is the space between the tubal fimbriae and ovary. The chlamydial organisms need to pass through the fimbriae to enter the oviduct or move into the ovary in order to find cells for infection. Not all injected organisms can make the journeys in either direction. Thus, the net number of organisms that can find epithelial cells for infection is much lower than that delivered via intrauterine inoculation, even though the same numbers of organisms are injected in both methods. In addition, our final readout is hydrosalpinx (but not fertility), which requires the infection of oviduct cells, while the ovarian infection may not significantly affect the development of hydrosalpinx, although ovarian infection can cause infertility. Thus, when the same numbers of organisms are injected via the two different routes, intrauterine inoculation can maximize the chance for chlamydial organisms to infect host cells. After replication in the endometrial epithelial cells, the progenies may invade tubal epithelial cells and end up with higher numbers of organisms in the tube, leading to more severe hydrosalpinx. The second possibility is a local tissue environmental issue. The endometrial environment may be less detrimental than the intrabursal/tubal area for chlamydial survival. The constitutively expressed immune effectors in the tube may be able to inactivate live chlamydial organisms more efficiently than those in the endometrial tissue. More importantly, invasion of the tubal epithelial cells may represent a more severe danger for the host than the invasion of the endometrial epithelial cells. Thus, the host may mount more rapid and robust responses to eliminate the infection during intrabursal infection. The net result is less organism survival in the tube when the same number of organisms is injected. In contrast, following an intrauterine infection, more organisms are able to grow in the endometrial epithelial cells and the progenies may enter into the tube with higher numbers. The third possibility is the hypothesis that the endometrial epithelia may have selected the chlamydial organisms for adaptation to grow in the endometrial tissues and also allowed the chlamydial organisms to optimize their gene expression for invasion of tubal epithelial cells. During natural infection, after the chlamydial organisms are introduced into the lower genital tract, some can pass through the cervical barrier and replicate in the endometrial tissues. The progenies produced in the endometrial epithelial cells can both enter the tubal epithelia and descend back to the lower genital tract to spread to other individuals. This person-to-person spreading may allow the descended chlamydial organisms to accumulate mutations that favor their growth in the endometrial tissues. However, the chlamydial organisms that have replicated in the tubal epithelial cells may not be able to descend all the way into the lower genital tract to pass to the next person. The tubal infection may be a dead end for chlamydial infection. The limited chance for the tubal progenies to reach to the lower genital tract may be the reason why it is has been difficult to use the vaginal shedding to predict tubal pathology. Evolutionally, there is little chance for the chlamydial organisms produced in the tube to accumulate mutations that favor their growth in the fallopian tube or oviduct cells (because of lack of passage to the next person). As a result, the oviduct epithelial cells may always be a “new environment” to the chlamydial organisms. As a result, chlamydial organisms may have adapted to grow in the endometrial but not tubal epithelial cells. As stated above, after endometrial replication, more organisms may eventually end up in the tube, leading to more severe tubal pathology. We are in the process of testing the above hypotheses.
In the present study, we have demonstrated the contribution of plasmid-independent factors to C. muridarum induction of hydrosalpinx, which is consistent with our recent finding that correlated mutations in chromosomal genes with C. muridarum attenuation in mice (42). In some cases, chromosomal gene mutations can also lead to enhanced infectivity (22, 23). The observation that plasmid-free C. caviae GPIC was as virulent as plasmid-competent GPIC (21) suggests that the C. caviae species mainly depends on its chromosome-encoded factors to exert its pathogenicity. Thus, investigation of both plasmid-dependent and -independent virulence factors is required for full understanding of the chlamydial pathogenic mechanisms. However, due to the fact that intravaginal infection with plasmid-free C. muridarum often failed to induce hydrosalpinx in mice (9, 19), even in the most susceptible CBA/J mice (9, 10), it has been difficult to evaluate the plasmid-independent virulence factors by using the murine model. In the present study, we found that intrauterine infection with plasmid-free C. muridarum induced significant hydrosalpinx in 3 of the 6 tested mouse strains. The CBA/J mice still developed robust hydrosalpinx even when the inoculum was reduced to 1 × 104 IFU. Thus, we propose that intrauterine infection of CBA/J mice with plasmid-free C. muridarum can be a suitable model for investigating the plasmid-independent pathogenesis.
ACKNOWLEDGMENT
This work was supported in part by grants (to G. Zhong) from the U.S. National Institutes of Health.
REFERENCES
- 1.Centers for Disease Control and Prevention. 16 November 2009. 2008 sexually transmitted disease surveillance. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/stats08/toc.htm. [Google Scholar]
- 2.Land JA, Van Bergen JE, Morre SA, Postma MJ. 2010. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum Reprod Update 16:189–204. doi: 10.1093/humupd/dmp035. [DOI] [PubMed] [Google Scholar]
- 3.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 4.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs 3:980–986. [PubMed] [Google Scholar]
- 7.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong X, Liu Y, Chang X, Lei L, Zhong G. 2014. Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection. Infect Immun 82:1833–1839. doi: 10.1128/IAI.01668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng B, Lu C, Tang L, Yeh IT, He Z, Wu Y, Zhong G. 2011. Enhanced upper genital tract pathologies by blocking Tim-3 and PD-L1 signaling pathways in mice intravaginally infected with Chlamydia muridarum. BMC Infect Dis 11:347. doi: 10.1186/1471-2334-11-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci S, Ratti G, Scarlato V. 1995. Transcriptional regulation in the Chlamydia trachomatis pCT plasmid. Gene 154:93–98. doi: 10.1016/0378-1119(94)00825-D. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Chen D, Zhong Y, Wang S, Zhong G. 2008. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun 76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas NS, Lusher M, Storey CC, Clarke IN. 1997. Plasmid diversity in Chlamydia. Microbiology 143:1847–1854. doi: 10.1099/00221287-143-6-1847. [DOI] [PubMed] [Google Scholar]
- 16.Seth-Smith HM, Harris SR, Persson K, Marsh P, Barron A, Bignell A, Bjartling C, Clark L, Cutcliffe LT, Lambden PR, Lennard N, Lockey SJ, Quail MA, Salim O, Skilton RJ, Wang Y, Holland MJ, Parkhill J, Thomson NR, Clarke IN. 2009. Co-evolution of genomes and plasmids within Chlamydia trachomatis and the emergence in Sweden of a new variant strain. BMC Genomics 10:239. doi: 10.1186/1471-2164-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, Parnell MJ, Zhong G, Caldwell HD. 2011. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med 208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigar IM, Schripsema JH, Wang Y, Clarke IN, Cutcliffe LT, Seth-Smith HM, Thomson NR, Bjartling C, Unemo M, Persson K, Ramsey KH. 2014. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis 70:61–69. doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell CM, Ingalls RR, Andrews CW Jr, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazer LC, Darville T, Chandra-Kuntal K, Andrews CW Jr, Zurenski M, Mintus M, AbdelRahman YM, Belland RJ, Ingalls RR, O'Connell CM. 2012. Plasmid-cured Chlamydia caviae activates TLR2-dependent signaling and retains virulence in the guinea pig model of genital tract infection. PLoS One 7:e30747. doi: 10.1371/journal.pone.0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. 2010. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun 78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell M, Darville T, Chandra-Kuntal K, Smith B, Andrews CW Jr, O'Connell CM. 2011. Infectivity acts as in vivo selection for maintenance of the chlamydial cryptic plasmid. Infect Immun 79:98–107. doi: 10.1128/IAI.01105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res 28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connell CM, Nicks KM. 2006. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology 152:1601–1607. doi: 10.1099/mic.0.28658-0. [DOI] [PubMed] [Google Scholar]
- 28.Tang L, Yang Z, Zhang H, Zhou Z, Arulanandam B, Baseman J, Zhong G. 2014. Induction of protective immunity against Chlamydia muridarum intracervical infection in DBA/1j mice. Vaccine 32:1407–1413. doi: 10.1016/j.vaccine.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. 2010. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol 184:2602–2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. 2011. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog 7:e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song L, Carlson JH, Whitmire WM, Kari L, Virtaneva K, Sturdevant DE, Watkins H, Zhou B, Sturdevant GL, Porcella SF, McClarty G, Caldwell HD. 2013. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect Immun 81:636–644. doi: 10.1128/IAI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong S, Yang Z, Lei L, Shen L, Zhong G. 2013. Characterization of Chlamydia trachomatis plasmid-encoded open reading frames. J Bacteriol 195:3819–3826. doi: 10.1128/JB.00511-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H, Gong S, Tian Y, Yang Z, Brunham R, Zhong G. 2013. Transformation of sexually transmitted infection-causing serovars of Chlamydia trachomatis using blasticidin for selection. PLoS One 8:e80534. doi: 10.1371/journal.pone.0080534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson JH, Whitmire WM, Crane DD, Wicke L, Virtaneva K, Sturdevant DE, Kupko JJ III, Porcella SF, Martinez-Orengo N, Heinzen RA, Kari L, Caldwell HD. 2008. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun 76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Cutcliffe LT, Skilton RJ, Persson K, Bjartling C, Clarke IN. 2013. Transformation of a plasmid-free, genital tract isolate of Chlamydia trachomatis with a plasmid vector carrying a deletion in CDS6 revealed that this gene regulates inclusion phenotype. Pathog Dis 67:100–103. doi: 10.1111/2049-632X.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Zhong Y, Lei L, Wu Y, Wang S, Zhong G. 2008. Antibodies from women urogenitally infected with C. trachomatis predominantly recognized the plasmid protein pgp3 in a conformation-dependent manner. BMC Microbiol 8:90. doi: 10.1186/1471-2180-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. 2010. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol 192:6017–6024. doi: 10.1128/JB.00847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galaleldeen A, Taylor AB, Chen D, Schuermann JP, Holloway SP, Hou S, Gong S, Zhong G, Hart PJ. 2013. Structure of the Chlamydia trachomatis immunodominant antigen Pgp3. J Biol Chem 288:22068–22079. doi: 10.1074/jbc.M113.475012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey KH, Schripsema JH, Smith BJ, Wang Y, Jham BC, O'Hagan KP, Thomson NR, Murthy AK, Skilton RJ, Chu P, Clarke IN. 2014. Plasmid CDS5 influences infectivity and virulence in a mouse model of Chlamydia trachomatis urogenital infection. Infect Immun 82:3341–3349. doi: 10.1128/IAI.01795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 23 February 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]