Abstract
Listeria monocytogenes rhombencephalitis is a severe progressive disease despite a swift intrathecal immune response. Based on previous observations, we hypothesized that the disease progresses by intra-axonal spread within the central nervous system. To test this hypothesis, neuroanatomical mapping of lesions, immunofluorescence analysis, and electron microscopy were performed on brains of ruminants with naturally occurring rhombencephalitis. In addition, infection assays were performed in bovine brain cell cultures. Mapping of lesions revealed a consistent pattern with a preferential affection of certain nuclear areas and white matter tracts, indicating that Listeria monocytogenes spreads intra-axonally within the brain along interneuronal connections. These results were supported by immunofluorescence and ultrastructural data localizing Listeria monocytogenes inside axons and dendrites associated with networks of fibrillary structures consistent with actin tails. In vitro infection assays confirmed that bacteria were moving within axon-like processes by employing their actin tail machinery. Remarkably, in vivo, neutrophils invaded the axonal space and the axon itself, apparently by moving between split myelin lamellae of intact myelin sheaths. This intra-axonal invasion of neutrophils was associated with various stages of axonal degeneration and bacterial phagocytosis. Paradoxically, the ensuing adaxonal microabscesses appeared to provide new bacterial replication sites, thus supporting further bacterial spread. In conclusion, intra-axonal bacterial migration and possibly also the innate immune response play an important role in the intracerebral spread of the agent and hence the progression of listeric rhombencephalitis.
INTRODUCTION
Due to its resistance to hostile conditions, the Gram-positive bacterium Listeria monocytogenes is ubiquitously distributed in the environment and commonly contaminates food and animal feed (1–3). Upon oral uptake, L. monocytogenes may turn into an intracellular pathogen causing listeriosis, one of the deadliest foodborne infections in humans and ruminants (1, 4–6). Clinical syndromes associated with L. monocytogenes infection include febrile gastroenteritis, mastitis, septicemia, abortion, and central nervous system (CNS) infection, the latter accounting for the high mortality rates associated with listeriosis (1, 5–9). In most human patients, neurolisteriosis manifests as meningitis or meningoencephalitis, and L. monocytogenes is listed among the most common pathogens of bacterial meningitis (10–13). Further forms of neurolisteriosis include brainstem encephalitis (rhombencephalitis) occurring in 25% of cases and brain abscesses accounting for a further 10% of cases with CNS infection (11, 14). Neurolisteriosis in ruminants occurs almost exclusively as rhombencephalitis (1, 15–17) and mimics its human counterpart in many respects (17, 18), lending itself as a spontaneous animal model.
Although the intracellular life cycle of L. monocytogenes has been elegantly deciphered by in vitro studies (19), crucial steps in the infection process within the host are incompletely understood. In particular, one of the current challenges is to understand how L. monocytogenes invades the brain and spreads within the brain (20, 21). Listeric meningitis/meningoencephalitis and brain abscesses in humans likely result from hematogenous spread generally occurring against a background of other pathologies and physiological stress situations (6, 8, 13, 21). In rhombencephalitis, which frequently occurs in otherwise healthy human patients and ruminants (1, 9, 11, 21–23), there is strong evidence for the entry of the bacteria into the brainstem by the axonal migration of L. monocytogenes along various cranial nerves (17, 18, 24, 25). This includes localization of L. monocytogenes in cranial nerve axons (24–27), frequent involvement of cranial nerve nuclei (17, 18), isolation of L. monocytogenes from the brain but not from other organs (28), and demonstration of E-cadherin, a major receptor for L. monocytogenes entry, in cranial nerves of ruminants (27).
Despite swift and intense intrathecal responses of both the innate and adaptive immune systems against L. monocytogenes (29), L. monocytogenes is able to replicate and to propagate within the brain, causing severe, progressive, and frequently lethal disease. The efficient spread of L. monocytogenes could be explained by its ability to move from cell to cell, which permits the agent to multiply and diffuse within tissues protected from the host defenses by avoiding contact with the extracellular compartment (19). In view of the observed ability of L. monocytogenes to spread within cranial nerves (25, 26), our previous studies showing progression of the lesions within the brain (17) and the sporadic association of L. monocytogenes with axons in the hippocampal slice model (30), we hypothesized that intercellular spread of the agent in the CNS could also be intra-axonal. Moving within axons, a highly interconnected network with little or no major histocompatibility complex (MHC) expression (29, 31), would, at least temporarily, allow L. monocytogenes to escape immune detection while efficiently spreading to distant CNS regions.
To test our hypothesis, we performed neuroanatomical mapping combined with ultrastructural and immunofluorescence (IF) analyses of brain lesions in naturally infected ruminants and infection assays in bovine fetal brain cell cultures. Our results provide evidence that L. monocytogenes efficiently spreads within axons to rostral brain areas by employing its actin tail polymerization machinery. Furthermore, these results indicate that intra-axonal L. monocytogenes cells are closely chased by phagocytes invading the axonal space, thereby creating inflammatory foci with bacterial amplification along the trajectory of axonal migration and further contributing to the spread of infection.
MATERIALS AND METHODS
Animals and tissue preparation.
Paraffin blocks from 41 ruminants (19 sheep, 14 goats, and 8 cows) with L. monocytogenes rhombencephalitis extending into rostral brain regions were retrieved from the neuropathological archive at the Vetsuisse Faculty, University of Bern, Bern, Switzerland. Cases included in this study had occurred between 2000 and 2013 in various areas of Switzerland either in the context of herd outbreaks (defined by >1 case per herd) or as sporadic single cases (see Table S1 in the supplemental material). L. monocytogenes etiology had been confirmed by histopathology combined with immunohistochemistry (IHC), using a polyclonal rabbit antibody against L. monocytogenes serotypes 1 and 4 (1:200; Difco). In a subset of cases, the serotype group (determined by PCR) and genetic lineage of isolated L. monocytogenes strains were available from a previous study (32) (see Table S1 in the supplemental material). For histological analysis, cross-sectioned blocks of representative levels (medulla oblongata, pons, cerebellum, midbrain, thalamus, basal nuclei, and cerebral cortex, including hippocampus) were cut at 5 μm and stained with hematoxylin and eosin (H&E).
Topographical mapping of the lesions.
Exact topographical mapping of intracerebral microabscesses and abscesses, which generally coincide with the presence of bacteria (17), was performed on H&E-stained sections of representative brain levels of all 41 animals. Affected structures and localization of the lesions were identified and systematically recorded on neuroanatomical images derived from transverse sections of the respective species from an atlas (33) in each individual animal. The stage of the lesions (acute, subacute, or chronic) was recorded as well. Finally, these findings were summated in one image per species per representative brain level.
Immunofluorescence and confocal laser microscopy of brain tissue sections.
Sections from 11 animals (7 sheep and 4 goats) from the above-described series containing large numbers of bacteria in IHC slides and exhibiting acute microabscesses were selected. Transverse (11 animals) and longitudinal (1 animal) sections from the corresponding blocks were deparaffinized and pretreated with enzymatic epitope retrieval (immunofluorescence for L. monocytogenes and neurofilament or lysozyme) by using trypsin (1 mg/ml) and 2% calcium chloride (2 μl/ml) in phosphate-buffered saline supplemented with Tween (PBS-T) for 15 min at 37°C or with heat-induced epitope retrieval (immunofluorescence for L. monocytogenes and Iba-1 [ionized calcium binding adaptor molecule 1]) in citrate buffer (pH 6) at 99°C for 15 min by using a laboratory microwave. Sections were blocked with 5% normal goat serum for 30 min and then incubated with antibodies against L. monocytogenes serotypes 1 and 4 and neurofilament (monoclonal mouse anti-neurofilament antibody clone 2F11 at a 1:100 dilution; Dako) at room temperature for 4 h, with antibodies against Iba-1 (polyclonal rabbit antibody against Iba-1, catalog number 019-19741, at a 1:500 dilution; Wako) and neurofilament at 4°C overnight, or with antibodies against lysozyme (polyclonal rabbit anti-human lysozyme, catalog number A0099, at a 1:200 dilution; Dako) and neurofilament at room temperature for 4 h. Subsequently, tissue slides were incubated with the matching Alexa-Fluor 488- and 555-labeled anti-rabbit and anti-mouse IgG antibodies (1:100; Invitrogen) (1 h). Nuclei were stained with either TOTO-3 (1:500; Invitrogen) (30-min incubation) or 4′,6-diamidino-2-phenylindole (DAPI) (1:10,000; AppliChem) (30-min incubation). Confocal and zeta-stack images were acquired on an Olympus FluoView 1000 confocal microscope equipped with 488-nm, 555-nm, and 632-nm laser channels. Images were processed with Imaris 3D software.
Electron microscopy of rhombencephalitis.
Electron microscopy (EM) analysis was performed on brainstems from 5 animals (3 sheep, 1 goat, and 1 cow) and targeted at the periphery of acute microabscesses. Areas of interest were identified on paraffin sections. Using the paraffin slides as a reference, the corresponding tissue regions were then localized on the cut face of the adjacent brain blocks fixed in 4% formalin, and suitable samples were excised. The collected tissue samples were postfixed with 1% OsO4 (Chemie Brunschwig, Basel, Switzerland) in 0.1 M cacodylate buffer (pH 7.4) for 1 h at 4°C and embedded in Epon (Fluka, Buchs, Switzerland). Semithin sections were stained with toluidine blue and used to narrow down the regions of interest further. Resin blocks were trimmed accordingly, and ultrathin sections exhibiting silver interference were produced with diamond knives (Diatome, Biel, Switzerland) on a Reichert-Jung Ultracut E instrument (Leica, Heerbrugg, Switzerland). Sections were collected on collodion-coated 200-mesh copper grids (Electron Microscopy Sciences). Sections were double stained with 0.5% uranyl acetate for 30 min at 40°C (Sigma-Aldrich, Steinheim, Germany) and with 3% lead citrate for 10 min at 20°C (Laurylab, Saint Fons, France) by using an Ultrastain instrument (Leica, Vienna, Austria) and examined on a Philips CM12 transmission electron microscope (FEI, Eindhoven, Holland) at an accelerating voltage of 80 kV. Micrographs were captured with a Mega View III camera using iTEM software (version 5.2; Olympus Soft Imaging Solutions GmbH, Münster, Germany).
Bacterial strains.
Strain L146/2007 is a serotype 4b L. monocytogenes strain isolated from a case of bovine rhombencephalitis (32). The green fluorescent protein (GFP)-expressing strain L146/2007-GFP was generated by transformation of parental strain L146/2007 with pPL2-Phyper-GFP (34) (kindly provided by Martin Loessner, ETH Zürich) by electroporation in a 0.1-cm cuvette using a GenePuls apparatus (Bio-Rad, Hercules, CA, USA) set to 25 μF, 200 Ω, and 1.25 kV. For the generation of the actA deletion mutant (L146/2007-GFP-ΔactA), extracted DNA from L146/2007 was used as the template to amplify the up- and downstream regions of actA by PCR (Roche, Basel, Switzerland), using primer pairs ΔactA_1_fw_SalI (TCGGTCGACGTTCTGATGGTTACTTGACTG)/ΔactA_2_rv (GCCAATAGCTAATCCCACTTATACTCCCTCCTCG) and ΔactA_3_fw (GTGGGATTAGCTATTGGCGTGTTCTCTTTAGGG)/ΔactA_4_rv_XmaI (TTTTCCCGGGTTTAAATCCTTGAGCGAACTTAGG), respectively. The two amplicons were spliced together by PCR using primers ΔactA_1_fw_SalI and ΔactA_4_rv_XmaI, resulting in a deletion of 1,723 bp in the open reading frame (ORF) of actA. The fused DNA fragments were inserted into SalI and XmaI sites of plasmid pMAD (35) to create pMAD-ΔactA. Strain L146/2007-GFP was transformed with plasmid pMAD-ΔactA by electroporation with the same settings as those described above, and transformants were selected on brain heart infusion (BHI) agar plates with 5 μg/ml erythromycin (Sigma) and 50 μg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for 2 days at a permissive temperature for plasmid replication of 30°C. A blue colony was isolated and grown in BHI broth with 5 μg/ml erythromycin for 2 days at a nonpermissive temperature of 42°C, during which bacteria were passaged in fresh BHI broth twice a day to select for single-crossover integration of the plasmid. A 1:10 dilution was then plated onto BHI agar plates with erythromycin and X-Gal and grown at 42°C for 1.5 days. A light blue colony was transferred into BHI broth and grown at 30°C for 6 h, and 1 μl of culture was then transferred into fresh BHI broth and grown at 42°C for 3 h. Serial dilutions were plated onto BHI agar medium with X-Gal and grown at 42°C for 2 days. White colonies indicating a second crossover were tested for the loss of the integrated plasmid backbone by sensitivity to erythromycin. The actA deletion of the final clone was confirmed by PCR.
Generation of FBBC-1 cells constitutively expressing LifeAct-TagRFP for visualization of filamentous actin.
The LifeAct-TagRFP open reading frame was amplified by PCR (Roche, Basel, Switzerland) from the vector pCMV-LifeAct-TagRFP (Ibidi, Martinsried, Germany) with primers LifeAct_fw_RsrII (ATATATCGGACCGATGGGTGTCGCAGATTTGATC) and LifeAct_rv_RsrII (ATATATCGGTCCGTCAATTAAGTTTGTGCCCCAG). PCR products were digested with RsrII and ligated into a modified version of the pRRL vector (36) (kindly provided by Patrick Salomon, University of Geneva), which contains convenient RsrII restriction sites inserted into the multiple-cloning site (37). For lentivirus production, 5 × 106 HEK293T cells were grown in a 10-cm cell culture dish and transfected the following day with 5 μg of pRRL-LifeAct-TagRFP, 2 μg psPAX2, and 2 μg pMD2.G (36), using TransIT-LT1 transfection reagent (Mirus, Madison, WI, USA) according to the manufacturer's recommendations. At 24 and 48 h following transfection, supernatants of transfected cells were harvested and centrifuged. Supernatants containing lentivirus were used to infect FBBC-1 cells in three reiterative cycles.
Immunofluorescence and live-cell imaging of fetal bovine brain cells infected with L. monocytogenes.
Fetal bovine brain cells (FBBC-1) (38) and FBBC-1-LifeAct-TagRFP cells were grown in a mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium supplemented with 10% fetal calf serum (FCS), 50 ng/ml epithelial growth factor (EGF) (kindly provided by Eliane Müller, Institute of Animal Pathology, Vetsuisse Faculty Bern), 50 ng/ml recombinant human basic fibroblast growth factor (bFGF; Sigma, Buchs, Switzerland), and N2 supplement (Invitrogen, Zug, Switzerland).
For immunofluorescence, 2 × 105 cells per well were seeded onto coverslips in 24-well plates and treated with 100 μM forskolin (Merck-Millipore, Schaffhausen, Switzerland) for 2 days to induce neuronal differentiation. Prior to infection, cells were starved in DMEM without supplements for 1 h. Cells were then infected at a multiplicity of infection (MOI) of 5 to 10 with serotype 4b L. monocytogenes strain L146/2007 isolated from a case of bovine rhombencephalitis (32). At 1 h postinfection (p.i.), the medium was replaced with DMEM supplemented with 10% FCS and 50 μg/ml gentamicin in order to inhibit extracellular growth of L. monocytogenes. At 24 h p.i., coverslips were fixed in 4% paraformaldehyde (PFA) for 30 min at 37°C, subsequently washed with PBS-T, and blocked with 10% normal goat serum for 30 min. Coverslips were incubated with antibodies against L. monocytogenes (polyclonal rabbit antibody against L. monocytogenes serotypes 1 and 4 at a 1:200 dilution; Difco) and neurofilament (monoclonal mouse anti-neurofilament antibody at a 1:100 dilution; Dako) for 2 h, followed by matching Alexa-Fluor 488- and 555-labeled anti-rabbit and anti-mouse IgG antibodies (1:500; Invitrogen) (1 h). Filamentous actin was stained by incubation with Dylight633-labeled phalloidin (1:500; Life Technologies) at room temperature for 45 min. At least 3 independent experiments were performed. Images were obtained by using an Olympus FluoView 1000 confocal laser scanning microscope and analyzed by using ImageJ software (W. S. Rasband [http://imagej.nih.gov/ij/]).
For live-cell imaging, FBBC-1 and FBBC-1-LifeAct-TagRFP cells (2 × 104) were seeded onto a 14-mm microwell of a 35-mm petri dish (Mattek Co., Ashland, MA) and grown in DMEM–F-12 medium supplemented with 10% FCS, 50 ng/ml EGF, 50 ng/ml bFGF, N2 supplement (Invitrogen), and 100 μM forskolin (Merck-Millipore). The next day, cells were infected with L146/2007-GFP or L146/2007-GFP-ΔactA expressing green fluorescent protein at an MOI of 100 to facilitate the observation of motile bacteria. At 1 h p.i., the medium was replaced with medium supplemented with 50 μg/ml gentamicin. For imaging of infected FBBC-1 cells, 4 to 6 loci with L146/2007-GFP and axon-like processes were selected at different time points postinfection (2, 19, and 24 h), and 1 picture per locus was taken every minute for a total of 1 h by using a Nikon Eclipse TE2000-E microscope equipped with a Hamamatsu Orca-ER video camera. For live imaging of infected FBBC-1-LifeAct-TagRFP cells, time-lapse videos (1 picture every 15 s) were obtained with an Olympus FluoView 1000 confocal laser scanning microscope within 2 to 36 h following infection. At least 2 independent experiments were evaluated.
RESULTS
Neuropathology of rhombencephalitis.
As described previously (17), lesions were localized mainly in the medullary and pontine areas (Fig. 1; see also Table S2 in the supplemental material), frequently along the midline. These lesions consisted of microabscesses in association with areas of malacia and intense perivascular mononuclear cuffing, cardinal lesions of neurolisteriosis in ruminants (17, 24, 39). In agreement with data from previous studies (17, 29), no species differences were observed, except for a generally higher ratio of macrophages/microglia to neutrophils and a lower bacterial load in microabscesses of cattle. In the brainstem, microabscesses regularly coalesced into large areas of necrosis and suppuration, affecting both gray and white matter (Fig. 1). In all 3 ruminant species, microabscesses could be observed rostral to the medullary-pontine area (Fig. 1) throughout the capsula interna (13/41 animals) (Fig. 2a) and corona radiata (3/41). In the rostral areas as opposed to the brainstem, mainly the white matter was affected (Fig. 2). In addition to acute microabscesses, subacute to chronic lesions were found in the brainstem, while the most rostral lesions in the same animals were mainly acute (Fig. 2), reflecting the progression of the infection from the brainstem to the forebrain over time, as described previously (17). Occasionally, microabscesses in the white matter of the brainstem and rostral areas assumed a linear pattern along axonal tracts, as previously observed (Fig. 2c) (17).
FIG 1.

Specific topographical distribution pattern of microabscesses in listeric rhombencephalitis in 41 animals. Microabscesses are indicated as red dots in transverse sections at different levels of the brain, white matter is shown in dark gray, and gray matter is shown in light gray. Red circles indicate large areas of necrosis and suppuration. (a) Cerebral hemisphere with corpus striatum. Microabscesses are selectively located within white matter tracts of the capsula interna and corona radiata containing axonal fibers. (b) In the thalamus, microabscesses are located mainly within white matter tracts. (c) Midbrain. The commissura caudalis and the tegmental tract are predominantly affected. (d) Pons and middle cerebellar peduncle. (e) Rostral part of the medulla oblongata and caudal cerebellar peduncle. (f) Caudal part of the medulla oblongata rostral to the obex region. The fasciculus longitudinalis medialis, fasciculi tegmentalis, and caudal cerebellar peduncle are frequently affected. Note the severe lesions in the reticular formation with large areas of necrosis and suppuration.
FIG 2.
Specific topography of microabscesses in listeric rhombencephalitis. Shown are H&E-stained brain sections. Bar, 200 μm. (a) Microabscesses selectively affect the white matter of the capsula interna (arrows). (b) Large microabscess (M) in the white matter of the fasciculi tegmenti (Forel) of the midbrain. The contralateral fasciculi tegmenti (Forel) are unaffected (*). A, aqueduct. (c) Chain of microabscesses in the caudal commissure (CC).
Topographical mapping of microabscesses.
Mapping of the lesions revealed a consistent distribution pattern in all 3 animal species with preferential and almost selective affection of certain nuclear areas and white matter tracts. The formatio reticularis (39/41 animals) and white matter tracts such as the fasciculi tegmenti (Forel) (24/41) (Fig. 2b), fasciculus longitudinalis medialis (18/41), spinal tract of the trigeminal nerve (21/41), cranial cerebellar peduncle (14/41), internal capsule (13/41) (Fig. 2a), cerebellar medulla (19/41), and commissura caudalis (8/41) (Fig. 2c) were predominantly affected. Gray matter lesions were most frequently located in the vestibular nuclei (15/41), nucleus of the spinal tract of the trigeminal nerve (10/41), and thalamic nuclei (7/41) (Fig. 1; see also Table S2 in the supplemental material).
Immunofluorescence and confocal laser microscopy of brain tissue sections.
In samples from all 11 animals (7 sheep and 4 goats) for which immunofluorescence was performed the vast majority of L. monocytogenes bacteria were localized within microabscesses, as determined by IF and H&E staining of consecutive sections (Fig. 3). These microabscesses contained Iba-1 (Fig. 3c)- and lysozyme (data not shown)-positive macrophages/microglia. The bacteria were closely associated with nuclei of phagocytes, indicating their intracellular localization (Fig. 3b). In the investigated white matter tracts in the vicinity of the microabscesses, only relatively few L. monocytogenes bacteria could be found (Fig. 4), which were most frequently associated with nuclei, likely from microglia cells (Fig. 4a to c). Interestingly, in all animals, some of these bacteria were closely associated with axonal neurofilaments (Fig. 4). Such L. monocytogenes bacteria were frequently oriented in parallel to the axonal neurofilaments (Fig. 4a to c, e, and f), and some were colocalized with neurofilaments (Fig. 4e). In 3 cases, single L. monocytogenes bacteria, either associated with cells or apparently free in the extracellular space, were found in the Virchow-Robin spaces of blood vessels or within the lumen of blood vessels (data not shown).
FIG 3.
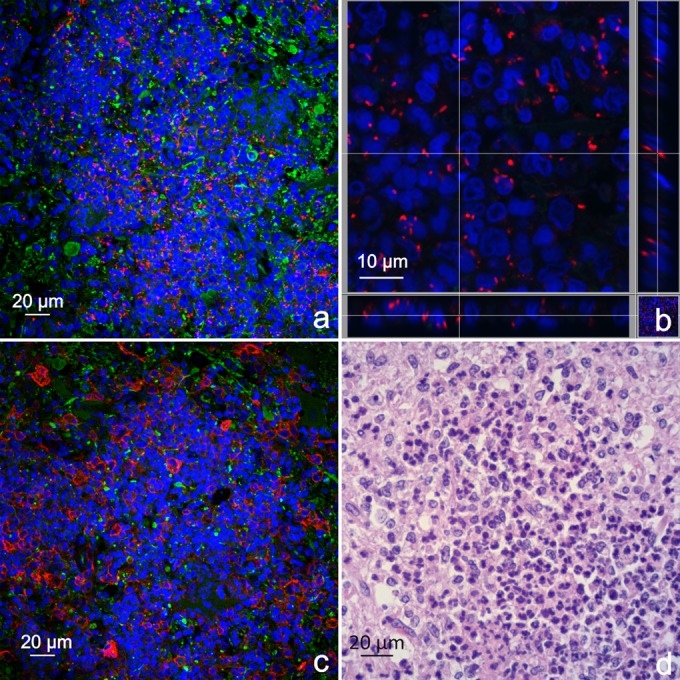
L. monocytogenes bacteria are predominantly associated with phagocytes within microabscesses. Shown are consecutive tissue sections of the midbrain area from a sheep containing a microabscess. (a) Immunofluorescence with antibodies against L. monocytogenes (red) and neurofilaments (green). Nuclei are stained in blue (DAPI). Bacteria are predominantly present in microabscesses, as indicated by agglomerations of nuclei. (b) Section view of the z-stack of high-magnification images from the same microabscess. L. monocytogenes bacteria are closely associated with segmented and unsegmented nuclei, indicating their intracellular localization within neutrophils and macrophages/microglia, respectively. (c) Immunofluorescence with antibodies against Iba-1 (red) and neurofilaments (green). Nuclei are stained in blue (DAPI). Cells with large oval nuclei are Iba-1 positive, indicating the presence of macrophages/microglia. The remaining cells have segmented nuclei, indicating the presence of neutrophils. (d) H&E staining of the corresponding area. Most cells within the microabscesses have condensed and segmented nuclei (neutrophils); the second population of cells have large round to oval nuclei (macrophages/microglia).
FIG 4.
L. monocytogenes bacteria are associated with neurofilaments and within or aligned along axons. Shown are immunofluorescence images of L. monocytogenes (red) and neurofilaments (green) in brains of 2 sheep affected by listeriosis. Nuclei are stained in blue (TOTO-3). (a to c) Beyond microabscesses, bacteria are closely associated with cellular nuclei (arrowheads) or neurofilaments (arrows), with the latter indicating their intra-axonal location. Such bacteria are frequently aligned with the axonal axis (b and c). (d) Multiple bacteria are found in a fragmented axon (arrow) and associated with a cellular nucleus (arrowhead) within a dilated myelin sheath. (e) Section view of the z-stack of images from multiple L. monocytogenes bacteria within an axon (arrows). The z-planes of the section view clearly show the bacteria within the axon. Some of the bacteria partially colocalize with the neurofilaments. (f) Section view of the z-stack of images of a bacterium in close contact with the axonal neurofilaments.
Electron microscopy of rhombencephalitis.
The brain areas of 5 animals (3 sheep, 1 cow, and 1 goat) selected for electron microscopy contained white matter and gray matter with neurons as well as numerous inflammatory cells, including macrophages and polymorphonuclear leukocytes.
In all 5 animals, myelinated axons were found to exhibit various degrees of degeneration, including axonal swelling, vacuolation, and accumulation of neurofilaments, mitochondria, and lysosomes (Fig. 5 and 6), with condensation and complete disintegration occurring in advanced stages (Fig. 5e and f). Swollen axons were surrounded by an attenuated thin, but mostly intact, myelin sheath (Fig. 5 and 6). A remarkable finding associated with the axonal changes in all 5 cases was the frequent presence of neutrophils and sometimes macrophages inside the axonal space surrounded by an intact myelin sheath (Fig. 5a and d to f). They were occasionally located between the myelin sheath and axolemma (Fig. 5d) but more often in axons at various stages of degeneration (Fig. 5d). For 2 animals, we observed neutrophils located between split myelin lamellae of otherwise intact myelin sheaths (Fig. 5c and e).
FIG 5.
Neutrophils migrate between myelin lamella into the axonal space. Shown are transmission electron microscopy images of the inflammatory reaction in the axonal space. (a) Semithin section of a myelinated area in the brainstem in the vicinity of a microabscess. Numerous axons are swollen and surrounded by a thinned myelin sheath. Several myelin sheaths contain neutrophils within the axonal space (arrows). (b) Transmission electron microscopy of swollen axons such as those shown in panel a, demonstrating axonal degeneration with accumulation of neurofilaments (asterisk), mitochondria, and several large vacuoles. The surrounding myelin sheath is thinner but intact. (c) Morphologically normal axon surrounded by an intact myelin sheath of an appropriate thickness. A neutrophil has migrated inside a split between myelin lamellae (arrow). (d) Axon in the early stage of degeneration with accumulating organelles but an intact myelin sheath of a normal thickness. One neutrophil (asterisk) invaded the axoplasma. A second neutrophil seems to be located in the adaxonal space (arrow). (e) Swollen dystrophic axon surrounded by a thinned myelin sheath, which is split and contains a neutrophil between myelin lamellae (arrows). An adjacent axonal space contains three neutrophils (asterisk). (f) The axon is completely destroyed, but the axonal space is still surrounded by an intact thinned myelin sheath and contains numerous neutrophils. The latter contain intravacuolar and intracytoplasmic L. monocytogenes bacteria (arrows).
FIG 6.
L. monocytogenes bacteria are located within axons and possess actin tails. (a) Semithin section in the periphery of a microabscess. The white matter is infiltrated by numerous inflammatory cells. Many axons are swollen and surrounded by a thinned myelin sheath. One severely swollen axon contains several bacteria (arrow). (b) Transmission electron microscopy image of a dystrophic axon containing one longitudinally sectioned and several cross-sectioned L. monocytogenes cells (arrows) below its axolemma. (c) Transmission electron microscopy image of a swollen axon with two intra-axonal L. monocytogenes cells, surrounded by a rim of electron-dense material consistent with an actin comet tail (arrows), amid bundles of neurofilaments. (d) Two cross-sectioned L. monocytogenes cells within an axon. One cell is clearly surrounded by a unipolar rim of electron-dense material consistent with an actin comet tail of condensed actin filaments (arrow). (e) High-magnification image of a cross-sectioned intra-axonal L. monocytogenes cell with the actin tail visible as a halo consisting of an electron-dense network of filamentous material. (f) High-magnification image of L. monocytogenes within an intra-axonal neutrophil. L. monocytogenes bacteria are within single-membrane-bound vacuoles. One L. monocytogenes cell is dividing (arrow).
L. monocytogenes bacteria with morphology as described previously in cell culture (40, 41) were found in 2 out of the 5 animals (1 sheep and 1 cow). These cigar-shaped bacteria had a cell wall overlaying a thin electron-lucent membrane adjacent to a thick electron-dense granular cytoplasm becoming less dense toward the center of the cell (Fig. 6c to f). Most bacteria were intracellular in macrophages and neutrophils, and some were observed inside neurons, dendrites, and terminal axons (data not shown). Bacteria were located free within the cytoplasm and frequently embedded in a thin layer of dense fibrillary matrix consistent with polymerized actin (Fig. 6c to e), as described previously (40, 41). Occasionally, dividing bacteria were observed, and actin-embedded bacteria were arranged in small chains protruding from one infected cell into a neighbor cell. In inflammatory cells, bacteria were found inside cytoplasmic vacuoles (Fig. 5e and 6f) and within the cytoplasm. In both animals, L. monocytogenes bacteria were unequivocally localized inside degenerating and intact axons (Fig. 6b to e). Some of these bacteria were located close to the axolemma (Fig. 6b to d), reflecting the superficial association with neurofilaments observed in immunofluorescence studies, and other bacteria were located in a more central position closely surrounded by neurofilaments (Fig. 6a and c to e). Similar to the immunofluorescence results, most of the bacteria were longitudinally oriented along the axonal axis. Intra-axonal bacteria were frequently surrounded by a unipolar halo of electron-dense actin interpreted as actin tails (Fig. 6c to e). Additionally, L. monocytogenes bacteria were observed inside neutrophils and macrophages within the axonal space (Fig. 5e and 6f) but not in such phagocytes that invaded the split myelin lamella.
Immunofluorescence and live-cell imaging of L. monocytogenes-infected fetal bovine brain cells.
Upon treatment with forskolin as described previously by Takenouchi et al. (38), a subpopulation of cells were neurofilament positive and developed long, fine, axon-like cellular processes, indicating partial neuronal differentiation of FBBC-1 cells. When inoculated with L. monocytogenes L146/2007, a field strain isolated from a case of cattle rhombencephalitis, bacteria were located predominantly in neurofilament-negative cells at 24 h postinoculation. However, in accordance with the in vivo findings, we also detected intracellular bacteria in neurofilament-positive cells and their axon-like processes (Fig. 7). Only a small proportion of L. monocytogenes bacteria either were surrounded by actin clouds or had actin tails, as visualized with phalloidin staining. Time-lapse images from FBBC-1 cells infected with L146/2007-GFP showed dispersed infection of cells. Most bacteria located within the central cytoplasm showed very limited motility, and only a few bacteria moved rapidly within the cytoplasm (see Videos S1 to S3 in the supplemental material). In contrast, conspicuous motility was observed in bacteria that caused bulging of the cellular membranes, forming elongated cellular protrusions. Axon-like processes predominantly contained bacteria that either were immotile or formed cellular protrusions. Occasionally, bacteria moved over considerable distances within these processes (Fig. 8; see also Videos S1 and S2 in the supplemental material). When FBBC-1 cells constitutively expressing LifeAct-TagRFP (42) were infected with the L146/2007-GFP strain, time-lapse images showed that the majority of immotile bacteria were not surrounded by actin. When present, actin was predominantly arranged in a bipolar cloud around dividing bacteria within cells and their processes (see Videos S2 and S3 in the supplemental material). Motility in the cytoplasm, within cellular protrusions, and in axon-like processes was associated with actin tail formation at one polar end of the bacteria (see Video S2 in the supplemental material). Most of these actin tails were short and were associated with slowly moving bacteria. Long actin tails were observed only occasionally at the polar end of rapidly moving bacteria in the cytoplasm and in cellular protrusions. When FBBC-1 cells were infected with the actA deletion mutant of L146/2007 (L146/2007-GFP-ΔactA), bacterial infection appeared to be more restricted, and cells were fully packed with bacteria. In time-lapse images, formations of cellular protrusions by bulging bacteria were not observed (see Video S4 in the supplemental material). Intracellular movement of L146/2007-GFP-ΔactA bacteria was rarely observed and occurred in association with the retraction of cellular processes preceding cellular death (see Video S4 in the supplemental material). Actin cloud and tail formation was absent in FBBC-1-LifeAct-TagRFP cells infected with L146/2007-GFP-ΔactA (see Video S4 in the supplemental material).
FIG 7.

L. monocytogenes bacteria are found in axon-like processes of fetal bovine brain cells (FBBC-1) with neuronal differentiation. Shown are IF images of L. monocytogenes-infected FBBC-1 cells at 24 h p.i., with L. monocytogenes (L146/2007) in green, neurofilaments in red, and actin in white. (a and b) L. monocytogenes bacteria without an actin tail colocalize with neurofilaments (yellow), demonstrating their localization within axon-like processes. (c) L. monocytogenes (green) without an actin tail is closely associated with the neurofilament of an axon-like process (red).
FIG 8.
L. monocytogenes bacteria move within axon-like processes. Shown are live-cell images of FBBC-1 cells inoculated with GFP-expressing L. monocytogenes (L146/2007-GFP). One bacterium moves within the axon-like process (arrows). (a) Image from 19 h 33 min p.i. (b) Image from 19 h 35 min p.i. (c) Image from 19 h 37 min p.i. (d) Image from 19 h 39 min p.i. The complete film sequence is available as Video S1 in the supplemental material.
DISCUSSION
There is ample evidence for intra-axonal spread of L. monocytogenes in the peripheral nervous system, whereby the pathogen migrates through cranial nerves to the neuronal bodies of brainstem and midbrain, causing rhombencephalitis (17, 24–26, 43, 44). Such an intra-axonal migration pathway has long been known for neurotropic viruses such as rabies virus, herpesviruses, morbilliviruses, West Nile virus, and poliovirus (45–47), and also, the bacterium Burkholderia pseudomallei is suspected to migrate along cranial nerves (48). Although, once L. monocytogenes has entered the brain, the intrathecal immune response is intense and develops rapidly, L. monocytogenes is able to efficiently replicate and spread within the brain, causing a progressive and frequently fatal neurological disease (17, 29). We hypothesized that this may in part be related to the ability of the agent to spread intracerebrally inside axons, allowing the agent to escape the immune response at least temporarily. As our previously reported neuropathological observations and infection assays of hippocampal brain slices suggested that intra-axonal spread of L. monocytogenes may also occur in the CNS (17, 30), the present study was designed to explore this hypothesis by neuroanatomical mapping of the lesions, precise localization of L. monocytogenes bacteria, and in vitro infections of brain cells.
The observed topography of the microabscesses, which coincide with the presence of bacteria (17), cannot be explained by hematogenous spread of the infection. The latter causes meningitis, choroiditis, and ependymitis or randomly disseminated suppurative lesions within the brain parenchyma that are predominantly associated with small vessels and in some cases associated with septic thromboemboli (49). The rare occurrence of L. monocytogenes bacteria in the Virchow-Robin spaces or in the lumina of blood vessels, the lack of an obvious association of microabscesses with blood vessels, and the lack of extensive involvement of the cerebrospinal fluid (CSF) pathways in the present study argue against hematogenous spread. Rather, the remarkably consistent and specific involvement of certain neuroanatomical areas and the connections between them strongly suggest axonal spread of the agent within the CNS. It is clear from previous work that L. monocytogenes uses the axons of the cranial nerves to enter the CNS and can subsequently be found in the neurons of the cranial nerve nuclei, for example, the sensory trigeminal or the hypoglossal nucleus (17, 18, 24, 25, 50). Further axonal spread to functionally connected structures could explain the high frequency of lesions in the reticular formation and cerebellar peduncles. However, this interpretation is hampered by the severity of the frequently coalescing brainstem lesions, the close proximity of affected structures, and the intense intermingling of gray and white matter in the brainstem.
Consistent with the previously described caudorostral gradient of listeric rhombencephalitis (17), mapping of microabscesses rostral to the medulla revealed a more clear pattern, with disseminated isolated lesions. In addition, such microabscesses were clearly situated more often in the white matter containing the axonal tracts than in the gray matter. Notably, the fasciculus longitudinalis medialis, the tegmental fascicles (Forel), the caudal commissure, and the capsula interna (see Table S1 in the supplemental material) were affected, most of which represent ascending functional connections between the brainstem and forebrain. For example, the tegmental fascicles (Forel) connecting the sensory trigeminal nucleus with the thalamus were involved in >50% of the animals, strongly suggesting that L. monocytogenes could spread along this tract from the medulla to the diencephalon (Fig. 9). Further spread may occur by way of the diencephalic-cortical projections, as indicated by the frequent occurrence of lesions in the internal capsule. The direct demonstration of bacteria in axons confirmed the intra-axonal spread of L. monocytogenes in the CNS, as suggested by the topography of lesions, and the infection model in bovine brain cell cultures demonstrated that L. monocytogenes bacteria are indeed capable of moving inside axon-like processes by employing their actin tail machinery. Live-cell imaging showed that the majority of motile bacteria were moving within filopodium-like cellular protrusions projecting from the cell bodies and axon-like processes. These data, together with our in vivo EM findings, indicate that the actin tail machinery is also involved in the intracerebral cell-to-cell spread of Listeria.
FIG 9.
Schematic illustration of the intracerebral spread of L. monocytogenes exemplified by the sensory trigeminal system in a parasagittally sectioned sheep brain. The functional connection of structures affected by microabscesses indicates the transneuronal spread of intra-axonal L. monocytogenes (represented by black boxes). Arrows indicate the direction of intracerebral spread. V, nervus trigeminus. 1, ganglion trigeminale; 2, nucleus tractus spinalis nervi trigemini; 3, polysynaptic ascending tract through reticular formation; 4, nucleus sensibilis pontinus nervi trigemini; 5, fasciculi tegmenti; 6, thalamus; 7, capsula interna; 8, corpus striatum.
Axonal spread was clearly not random, since we observed a consistent lesion distribution pattern. Anterograde or retrograde axonal transport mechanisms do not appear to be relevant for this pattern, since L. monocytogenes moves inside axons by employing its actin tail, as suggested by our present EM findings reminiscent of previous observations of the peripheral nervous system (25, 43) and our in vitro observations. Rather, in addition to the functional connection between neuronal populations, differential susceptibility of neuronal populations to L. monocytogenes may contribute to the anatomical lesion distribution, as in vitro infection models may suggest (43).
Obviously, migration within axons is a powerful mechanism of spread in light of the extensive interneuronal connections in the brain (31). In neurotropic viral infections, intra- and interneuronal spread is considered to be a mechanism of immune evasion (51, 52). However, our results do not support this assumption for L. monocytogenes. In all cases examined by EM, neutrophils and, to a lesser extent, macrophages were frequently found inside the axonal space still surrounded by an intact myelin sheath, a phenomenon that to our knowledge has not been reported previously. The finding of neutrophils inside splits in otherwise fully intact myelin sheaths suggests that phagocytes are capable of migrating into the axonal space by moving between the myelin lamellae. Intracytoplasmic and intravacuolar L. monocytogenes bacteria were found in phagocytes within the axonal space but not in neutrophils between myelin lamellae, indicating that phagocytes are attracted to the axonal space by the intra-axonal bacteria. It is conceivable that these neutrophils are responding to chemotactic signals, such as formylpeptides released into the axonal space, either by bacteria or by damaged subcellular structures such as mitochondria (53, 54). Formylpeptides are known to be potent chemoattractants for mouse neutrophils, which possess formylpeptide receptors mediating their rapid recruitment during the host defense against L. monocytogenes (53). While it is possible that bacteria by themselves may exert a toxic effect on the axon, it is likely that the intra-axonal invasion of neutrophils associated with the antibacterial immune response is at least partially responsible for the widespread axonal damage observed in the present study.
While macrophages/microglia and neutrophils may eliminate L. monocytogenes from axons by phagocytosis, the formation of adaxonal microabscesses during this process appears to offer a new replication site for the bacteria, since we consistently found the highest L. monocytogenes concentration in such microabscesses. This is in line with previous studies showing that L. monocytogenes replication in ruminant brain slices occurs mainly within microglia cells (30). Also, L. monocytogenes is able to replicate in macrophages and neutrophils by escaping intracellular bactericidal mechanisms (55, 56). Therefore, our results suggest that recognition by the innate immune system paradoxically may favor further spread of the infection by local amplification of L. monocytogenes within microabscesses, which could promote invasion of new axons and further spread of the infection. Studies in ruminants indicate that the support of the adaptive immune response is required to decrease the bacterial load of L. monocytogenes (29). Taken together, intracerebral infection may spread in leaps and bounds, inducing lesions along the axonal trajectory with amplification of the agent in phagocytes before reentering the neuroaxonal pool. This proposed mechanism of spread needs to be further investigated, and it remains to be determined whether L. monocytogenes is also able to spread transneuronally, as suggested by the affection of functionally related structures (Fig. 9). Transneuronal spread is a known pathway of intracerebral spread of rabies virus, measles virus, and herpesviruses and is a pathway suspected for many more viruses (47, 57–59). Proposed mechanisms include trans-synaptic transport, the establishment of microfusions, and non-trans-synaptic neuron-to-neuron transmission but remain to be elucidated for most of these viruses (47).
In summary, the present study provides convincing evidence for the intracerebral spread of L. monocytogenes within axons by employing its actin tail polymerization machinery. Intra-axonal spread leads to the swift invasion of phagocytes into the axonal compartment, possibly creating new bacterial replication sites. We conclude from these results that intra-axonal bacterial migration and possibly also innate immune responses play an important role in the spread of the agent and in the progression of listeric rhombencephalitis. Future studies will need to address how intra-axonal Listeria monocytogenes bacteria become detected by the innate immune system at certain points, leading to encephalitic lesions along axonal trajectories.
Supplementary Material
ACKNOWLEDGMENTS
This work received financial support from the Ernst-Frauchiger Foundation and the Swiss National Foundation (CRSII3_147692). Anna Oevermann's professorship for comparative neuropathology is funded by the Ernst-Frauchiger Foundation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00316-15.
REFERENCES
- 1.Low JC, Donachie W. 1997. A review of Listeria monocytogenes and listeriosis. Vet J 153:9–29. doi: 10.1016/S1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 2.Driehuis F, Oude Elferink SJ. 2000. The impact of the quality of silage on animal health and food safety: a review. Vet Q 22:212–216. doi: 10.1080/01652176.2000.9695061. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi M, Chikindas ML. 2007. Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2013. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009-2011. MMWR Morb Mortal Wkly Rep 62:448–452. [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority. 2013. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J 11:3129. doi: 10.2903/j.efsa.2013.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegman-Igra Y, Levin R, Weinberger M, Golan Y, Schwartz D, Samra Z, Konigsberger H, Yinnon A, Rahav G, Keller N, Bisharat N, Karpuch J, Finkelstein R, Alkan M, Landau Z, Novikov J, Hassin D, Rudnicki C, Kitzes R, Ovadia S, Shimoni Z, Lang R, Shohat T. 2002. Listeria monocytogenes infection in Israel and review of cases worldwide. Emerg Infect Dis 8:305–310. doi: 10.3201/eid0803.010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bula CJ, Bille J, Glauser MP. 1995. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin Infect Dis 20:66–72. doi: 10.1093/clinids/20.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Drevets DA, Bronze MS. 2008. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 53:151–165. doi: 10.1111/j.1574-695X.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Mailles A, Lecuit M, Goulet V, Leclercq A, Stahl JP, National Study on Listeriosis Encephalitis Steering Committee. 2011. Listeria monocytogenes encephalitis in France. Med Mal Infect 41:594–601. doi: 10.1016/j.medmal.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Sigurdardottir B, Bjornsson OM, Jonsdottir KE, Erlendsdottir H, Gudmundsson S. 1997. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 157:425–430. [DOI] [PubMed] [Google Scholar]
- 11.Bartt R. 2000. Listeria and atypical presentations of Listeria in the central nervous system. Semin Neurol 20:361–373. doi: 10.1055/s-2000-9398. [DOI] [PubMed] [Google Scholar]
- 12.Hussein AS, Shafran SD. 2000. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore) 79:360–368. [DOI] [PubMed] [Google Scholar]
- 13.Mylonakis E, Hohmann EL, Calderwood SB. 1998. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 77:313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RW, Fung PC. 1993. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis 16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 15.Heim D, Fatzer R, Hornlimann B, Vandevelde M. 1997. Frequency of neurologic diseases in cattle. Schweiz Arch Tierheilkd 139:354–362 (In German.) [PubMed] [Google Scholar]
- 16.Oevermann A, Botteron C, Seuberlich T, Nicolier A, Friess M, Doherr MG, Heim D, Hilbe M, Zimmer K, Zurbriggen A, Vandevelde M. 2008. Neuropathological survey of fallen stock: active surveillance reveals high prevalence of encephalitic listeriosis in small ruminants. Vet Microbiol 130:320–329. doi: 10.1016/j.vetmic.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Oevermann A, Di Palma S, Doherr MG, Abril C, Zurbriggen A, Vandevelde M. 2010. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol 20:378–390. doi: 10.1111/j.1750-3639.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antal EA, Loberg EM, Dietrichs E, Maehlen J. 2005. Neuropathological findings in 9 cases of Listeria monocytogenes brain stem encephalitis. Brain Pathol 15:187–191. doi: 10.1111/j.1750-3639.2005.tb00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamon M, Bierne H, Cossart P. 2006. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 20.Disson O, Lecuit M. 2013. In vitro and in vivo models to study human listeriosis: mind the gap. Microbes Infect 15:971–980. doi: 10.1016/j.micinf.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Disson O, Lecuit M. 2012. Targeting of the central nervous system by Listeria monocytogenes. Virulence 3:213–221. doi: 10.4161/viru.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oevermann A, Zurbriggen A, Vandevelde M. 2010. Rhombencephalitis caused by Listeria monocytogenes in humans and ruminants: a zoonosis on the rise? Interdiscip Perspect Infect Dis 2010:632513. doi: 10.1155/2010/632513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinverni R, Glauser MP, Bille J, Rocourt J. 1986. Unusual clinical features of an epidemic of listeriosis associated with a particular phage type. Eur J Clin Microbiol 5:169–171. doi: 10.1007/BF02013979. [DOI] [PubMed] [Google Scholar]
- 24.Charlton KM, Garcia MM. 1977. Spontaneous listeric encephalitis and neuritis in sheep. Light microscopic studies. Vet Pathol 14:297–313. [DOI] [PubMed] [Google Scholar]
- 25.Otter A, Blakemore WF. 1989. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol Hung 36:125–131. [PubMed] [Google Scholar]
- 26.Antal EA, Loberg EM, Bracht P, Melby KK, Maehlen J. 2001. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol 11:432–438. doi: 10.1111/j.1750-3639.2001.tb00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madarame H, Seuberlich T, Abril C, Zurbriggen A, Vandevelde M, Oevermann A. 2011. The distribution of E-cadherin expression in listeric rhombencephalitis of ruminants indicates its involvement in Listeria monocytogenes neuroinvasion. Neuropathol Appl Neurobiol 37:753–767. doi: 10.1111/j.1365-2990.2011.01183.x. [DOI] [PubMed] [Google Scholar]
- 28.Olson C Jr, Rollins CL, Bagdonas V, Blore IC, Segre D. 1953. Distribution of Listeria monocytogenes in listeriosis of sheep. J Infect Dis 93:247–256. doi: 10.1093/infdis/93.3.247. [DOI] [PubMed] [Google Scholar]
- 29.Di Palma S, Brunetti B, Doherr MG, Forster U, Hilbe M, Zurbriggen A, Vandevelde M, Oevermann A. 2012. Comparative spatiotemporal analysis of the intrathecal immune response in natural listeric rhombencephalitis of cattle and small ruminants. Comp Immunol Microbiol Infect Dis 35:429–441. doi: 10.1016/j.cimid.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Guldimann C, Lejeune B, Hofer S, Leib SL, Frey J, Zurbriggen A, Seuberlich T, Oevermann A. 2012. Ruminant organotypic brain-slice cultures as a model for the investigation of CNS listeriosis. Int J Exp Pathol 93:259–268. doi: 10.1111/j.1365-2613.2012.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty S, Nazmi A, Dutta K, Basu A. 2010. Neurons under viral attack: victims or warriors? Neurochem Int 56:727–735. doi: 10.1016/j.neuint.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balandyte L, Brodard I, Frey J, Oevermann A, Abril C. 2011. Ruminant rhombencephalitis-associated Listeria monocytogenes alleles linked to a multilocus variable-number tandem-repeat analysis complex. Appl Environ Microbiol 77:8325–8335. doi: 10.1128/AEM.06507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa T. 1967. Atlas of the brains of domestic animals. University of Tokyo Press, Tokyo, Japan. [Google Scholar]
- 34.Balestrino D, Hamon MA, Dortet L, Nahori MA, Pizarro-Cerda J, Alignani D, Dussurget O, Cossart P, Toledo-Arana A. 2010. Single-cell techniques using chromosomally tagged fluorescent bacteria to study Listeria monocytogenes infection processes. Appl Environ Microbiol 76:3625–3636. doi: 10.1128/AEM.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnaud M, Chastanet A, Debarbouille M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol 70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. 1998. A third-generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyss-Fluehmann G, Zurbriggen A, Vandevelde M, Plattet P. 2010. Canine distemper virus persistence in demyelinating encephalitis by swift intracellular cell-to-cell spread in astrocytes is controlled by the viral attachment protein. Acta Neuropathol 119:617–630. doi: 10.1007/s00401-010-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takenouchi T, Iwamaru Y, Sato M, Yokoyama T, Kitani H. 2009. Establishment of an SV40 large T antigen-immortalized bovine brain cell line and its neuronal differentiation by dibutyryl-cyclic AMP. Cell Biol Int 33:187–191. doi: 10.1016/j.cellbi.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Wuilleret A, Despres P, Monteiro L, Bouzakoura C, Wildi E. 1969. Bacteriological and histological diagnosis of listeriosis during an ovine enzootic. Schweiz Arch Tierheilkd 111:622–641 (In French.) [PubMed] [Google Scholar]
- 40.Tilney LG, Connelly PS, Portnoy DA. 1990. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol 111:2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Chastellier C, Berche P. 1994. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun 62:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. 2008. Lifeact: a versatile marker to visualize F-actin. Nat Methods 5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dons L, Weclewicz K, Jin Y, Bindseil E, Olsen JE, Kristensson K. 1999. Rat dorsal root ganglia neurons as a model for Listeria monocytogenes infections in culture. Med Microbiol Immunol 188:15–21. doi: 10.1007/s004300050100. [DOI] [PubMed] [Google Scholar]
- 44.Dons L, Jin Y, Kristensson K, Rottenberg ME. 2007. Axonal transport of Listeria monocytogenes and nerve-cell-induced bacterial killing. J Neurosci Res 85:2529–2537. doi: 10.1002/jnr.21256. [DOI] [PubMed] [Google Scholar]
- 45.Kristensson K. 2011. Microbes' roadmap to neurons. Nat Rev Neurosci 12:345–357. doi: 10.1038/nrn3029. [DOI] [PubMed] [Google Scholar]
- 46.Salinas S, Schiavo G, Kremer EJ. 2010. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 8:645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 47.van Riel D, Verdijk R, Kuiken T. 2015. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 48.St John JA, Ekberg JA, Dando SJ, Meedeniya AC, Horton RE, Batzloff M, Owen SJ, Holt S, Peak IR, Ulett GC, Mackay-Sim A, Beacham IR. 2014. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. mBio 5(2):e00025-14. doi: 10.1128/mBio.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray F, Alonso LM. 2002. Bacterial infections of the central nervous system, p 151–193. In Graham DI, Lantos PL (ed), Greenfield's neuropathology, 7th ed Arnold, London, United Kingdom. [Google Scholar]
- 50.Cordy DR, Osebold JW. 1959. The neuropathogenesis of listeria encephalomyelitis in sheep and mice. J Infect Dis 104:164–173. doi: 10.1093/infdis/104.2.164. [DOI] [PubMed] [Google Scholar]
- 51.Lafon M. 2011. Evasive strategies in rabies virus infection. Adv Virus Res 79:33–53. doi: 10.1016/B978-0-12-387040-7.00003-2. [DOI] [PubMed] [Google Scholar]
- 52.Taylor MP, Enquist LW. 29 January 2015. Axonal spread of neuroinvasive viral infections. Trends Microbiol doi: 10.1016/j.tim.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A, Gao JL, Murphy PM, Wang JM. 2012. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep 2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabiet MJ, Huet E, Boulay F. 2005. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol 35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 55.Hamon MA, Ribet D, Stavru F, Cossart P. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol 20:360–368. doi: 10.1016/j.tim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Schnupf P, Portnoy DA. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Dietzschold B, Schnell M, Koprowski H. 2005. Pathogenesis of rabies. Curr Top Microbiol Immunol 292:45–56. doi: 10.1007/3-540-27485-5_3. [DOI] [PubMed] [Google Scholar]
- 58.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol 70:2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young VA, Rall GF. 2009. Making it to the synapse: measles virus spread in and among neurons. Curr Top Microbiol Immunol 330:3–30. doi: 10.1007/978-3-540-70617-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








