Abstract
The mechanisms that underlie valvular inflammation in streptococcus-induced infective endocarditis (IE) remain unclear. We previously demonstrated that streptococcal glucosyltransferases (GTFs) can activate human heart valvular interstitial cells (VIC) to secrete interleukin-6 (IL-6), a cytokine involved in T helper 17 (Th17) cell differentiation. Here, we tested the hypothesis that activated VIC can enhance neutrophil infiltration through sustained IL-17 production, leading to valvular damage. To monitor cytokine and chemokine production, leukocyte recruitment, and the induction or expansion of CD4+ CD45RA− CD25− CCR6+ Th17 cells, primary human VIC were cultured in vitro and activated by GTFs. Serum cytokine levels were measured using an enzyme-linked immunosorbent assay (ELISA), and neutrophils and Th17 cells were detected by immunohistochemistry in infected valves from patients with IE. The expression of IL-21, IL-23, IL-17, and retinoic acid receptor-related orphan receptor C (Rorc) was upregulated in GTF-activated VIC, which may enhance the proliferation of memory Th17 cells in an IL-6-dependent manner. Many chemokines, including chemokine (C-X-C motif) ligand 1 (CXCL1), were upregulated in GTF-activated VIC, which might recruit neutrophils and CD4+ T cells. Moreover, CXCL1 production in VIC was induced in a dose-dependent manner by IL-17 to enhance neutrophil chemotaxis. CXCL1-expressing VIC and infiltrating neutrophils could be detected in infected valves, and serum concentrations of IL-17, IL-21, and IL-23 were increased in patients with IE compared to healthy donors. Furthermore, elevated serum IL-21 levels have been significantly associated with severe valvular damage, including rupture of chordae tendineae, in IE patients. Our findings suggest that VIC are activated by bacterial modulins to recruit neutrophils and that such activities might be further enhanced by the production of Th17-associated cytokines. Together, these factors can amplify the release of neutrophilic contents in situ, which might lead to severe valvular damage.
INTRODUCTION
Subacute infective endocarditis (IE) is a chronic infectious disease of heart valves that is primarily caused by viridans streptococci (1), which are oral commensals capable of efficiently evading immune surveillance upon entering the bloodstream (2). Transient bacteremia following dental surgery facilitates the colonization of valve tissues by oral streptococci, particularly in patients with preexisting valvular damage (3, 4). Using a model of experimental endocarditis in rats, we found that endocarditis-inducing Streptococcus mutans or Streptococcus gordonii could induce platelet aggregation through distinct mechanisms on the injured heart valves to form biofilms which were refractory to antibiotic prophylaxis (5). Furthermore, we demonstrated that S. mutans is embedded inside bacterium-platelet biofilms and actively secretes pathogen-associated molecular pattern (PAMP) molecules to trigger valvular inflammatory responses through the induction of cytokines such as interleukin-6 (IL-6) (6–8).
One class of PAMPs shared by these endocarditis-inducing streptococci is composed of glucosyltransferases (GTFs), a group of cell wall-associated or extracellular proteins with molecular masses of ∼150 kDa that convert sucrose into exopolysaccharides (glucans). The GTFs share conserved C-terminal carbohydrate binding motifs, such as those found in clostridial toxins (9, 10). GTFs, or C-terminal carbohydrate binding motifs, are potent PAMPs that can directly activate and upregulate the expression and release of cytokines by endothelial or human (h) heart valve interstitial cells (hVIC) through mitogen-activated protein kinase (MAPK) or nuclear factor (NF)-κB signaling pathways (7, 8). Heart VIC represent the major stromal population in valve leaflets and exhibit diverse and dynamic phenotypes that range from fibroblast-like to myofibroblast-like (11). In response to PAMP stimulation, VIC can secrete specific cytokines and growth factors that are involved in tissue remodeling and inflammation to combat infection (12–15). Using immunohistochemical analysis of diseased valves, we found that VIC are activated to secrete inflammatory cytokines, such as IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) (8). This finding suggests that VIC can play an important role in mediating the inflammatory responses triggered by PAMPs that are released by bacterial biofilms on infected valves.
The pathogenesis of IE is characterized histopathologically by a chronic inflammation with leukocyte infiltration. In infected heart valves, infiltrating leukocytes are predominantly localized at the base of the vegetation or around neocapillaries, and they largely consist of neutrophils, macrophages, and CD4+ or CD8+ T cells (8, 16). Therefore, leukocyte recruitment plays a crucial role in both coagulation and inflammation in IE, although the mediators and mechanisms responsible for neutrophil recruitment during IE remain unclear. In chronic inflammatory microenvironments, Th17 cells and IL-17 play essential roles in recruiting and sustaining neutrophil migration and recruitment, thereby helping the host to resist persistent infections (17, 18). We hypothesized that Th17 cells and cytokines are involved in the persistent inflammation of IE and that activated VIC induce or expand the recruited Th17 cells to sustain neutrophil migration. Here, we provide data to support these hypotheses and delineate the underlying mechanisms. We also investigate the levels of the Th17-related cytokines, IL-17, IL-21, and IL-23, and characterize their association with valvular destruction in IE.
MATERIALS AND METHODS
Streptococcus-induced IE/bacteremia and HD specimens.
This study was approved by the National Taiwan University Hospital (NTUH) Institutional Review Board (NTUH no. 201203082RIC). Informed consent was obtained from the patients and healthy donors (HD). A total of 24 patients with cases of streptococcus-induced IE/bacteremia were enrolled at the NTUH between July 2012 and June 2014, including 12 patients who were diagnosed as harboring IE with either echocardiographic or definite pathological findings of valvular vegetation. Peripheral blood was collected from IE patients and healthy donors for leukocyte separation and serum harvest.
Additionally, IE heart valves were obtained from a 12-year-old male with congenital heart disease who received surgical intervention for recurrent endocarditis caused by S. gordonii and a patient with a history of perimembranous ventricular septal defect with S. mutans bacteremia. Normal heart valves were obtained from forensic biopsy specimens. To culture hVIC, valve tissues were obtained from patients receiving heart transplants for either ischemic heart disease or cardiomyopathy. The investigation was performed in accordance with the Declaration of Helsinki.
Tissue collection and histology.
All tissues were fixed by submersion in 10% neutral buffered formalin for 18 to 24 h and were then transferred to 70% ethyl alcohol prior to processing. Standard histological methods for dehydration in ascending grades of ethyl alcohol, clearing in xylene, and paraffin infiltration were used. Paraffin blocks were processed using a rotary microtome to cut 8-μm-thick sections. Tissue sections were stained with hematoxylin and eosin (H&E).
Cell detection and immunohistochemistry.
Leukocytes were initially identified by H&E staining. T cells, neutrophils, and VIC in tissue sections were confirmed by the use of antibodies (Abs) against CD4 (H370) (sc-7219; Santa Cruz Biotechnology), myoperoxidase (MPO) (PAB7992; Abnova), and anti-smooth-muscle actin (α-SMA) (clone 1A4; R&D Systems), respectively. Cells producing IL-17, chemokine (C-X-C motif) ligand 1 (CXCL1), and elastase were detected by antibodies directed against IL-17A (13082-1-AP; ProteinTech), CXCL1 (NBP1-51188; Novus), and elastase (H-57) (sc-25621; Santa Cruz Biotechnology). Immunostaining was performed using avidin/biotin complex kits (Vector Laboratories Inc.) followed by color-based detection using diaminobenzidine (Dako) or fluorescein isothiocyanate (FITC)- and tetramethylrhodamine isothiocyanate (TRITC)-labeled secondary antibodies. Cell nuclei were stained with Hoechst 33258.
Cell isolation and culture.
Primary VIC were prepared from trimmed valve leaflets and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (hi-FCS) as described previously (8). VIC stained positively with antibodies against α-SMA, prolyl 4-hydroxylase, and vimentin but negatively for von Willebrand factor and desmin, indicating that there was no endothelial or smooth-muscle cell contamination. All experiments were performed on subcultures of confluent cells between the second and seventh passages. Mononuclear cells (MNCs) and polymorphonuclear (PMN) cells were isolated by layering on Histopaque (Sigma). CD4+ T cells were obtained by negative selection with a human CD4+ T cell enrichment cocktail (RosetteSep; Stem Cell Technology) and were cultured in DMEM supplemented with 10% hi-FCS.
Activation of hVIC with GTF and IL-17.
His-tagged recombinant GTFC, an isoform of glucosyltransferase, was purified using nickel-chelating nitrilotriacetic acid (Ni-NTA) agarose resin (Qiagen) as described previously (7). Any possible endotoxin content in the purified GTFC was removed using polymyxin B-agarose beads (Sigma). For cell activation studies, GTFC (25 μg/ml) or IL-17 (PeproTech) (20 ng or 50 ng/ml) was added to confluent VIC in DMEM containing 1% hi-FCS. Culture supernatants were collected 24 h after treatment with medium or GTFC and were designated cultured medium (CM) or conditioned medium-GTF (CM-GTF), respectively. Cells were then harvested, and expression of the genes encoding IL-1β, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), RANTES, CXCL10, matrix metalloprotease 2 (MMP2), MMP9, and actin was determined by reverse transcription-PCR (RT-PCR) (see Table S1 in the supplemental material). To confirm that the observed effects were not caused by lipopolysaccharide (LPS) contamination, all experiments (except those involving an LPS-treated group) were performed in the presence of polymyxin B (Sigma) (40 μg/ml).
PCR array analysis of the human chemokines.
Cells were seeded in 6-well plates and cultured for 1 day to allow for attachment. Complete medium was removed, and cells were treated with DMEM containing 1% hi-FCS for 24 h. Cells received GTF stimulation or media for another 24 h. Finally, total RNA was extracted. Complementary (c)DNA was generated from 2 μg total RNA. Then, a single 96-well human chemokine and receptor RT2 Profiler PCR array that included 84 genes was initially adopted to identify expression of candidate chemokines and the release of these chemokines was subsequently confirmed by the antibody array (SuperArray; Bioscience Corp.). Data are represented as fold changes compared to unstimulated cells.
Antibody array analysis for cytokines.
A commercially available antibody array (RayBio Human Antibody Array 5; RayBiotech Inc.) for the analysis of 80 human cytokines (see Table S2 in the supplemental material) was used for screening. The name and location of each cytokine in the array are listed in Table S2. Each membrane was placed into the 8-well tray that was provided, blocking buffer was added, and the trays were incubated at room temperature for 30 min to block the membranes. After incubating the membranes with 1 ml supernatant collected from resting or GTF-stimulated VIC cultured for 24 h at 4°C overnight, membranes were washed three times and incubated with biotin-conjugated antibodies that were diluted in blocking buffer at room temperature for 1 h. Membranes were washed again and incubated with horseradish peroxidase (HRP)-conjugated streptavidin at room temperature for 1 h and detection buffer for 2 min. Finally, membranes were exposed to X-ray film and signals were detected. Relative expression levels of cytokines were evaluated by comparing the signal intensities, which were quantified by densitometry. A positive control was used to normalize the results from the different membranes being compared, which allowed the normalized intensity value corresponding to each cytokine in each group to be converted into a relative n-fold change value. A 2-fold change in spot intensity compared to the control was considered to be significant.
CD4+ T cell cytokine response and proliferation.
CD4+ T cells (5 × 105) were stimulated with media, GTFC, CM, or CM-GTF for 5 days in the presence of soluble anti-human CD3 (clone OKT3; eBioscience) and anti-CD28 (clone CD28.2; eBioscience) antibodies (1 μg/ml). For the inhibition assay, anti-IL-6 (clone MQ2-13A5; eBioscience) and control rat IgG1 (eBioscience) were pretreated with CM for 30 min. After coculture for 120 h, 5 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin were added for another 4 h. After reactivation, the supernatant was collected for cytokine analysis.
Purified CD4+ T cells were sorted with peridinin chlorophyll protein complex (PerCP)-labeled anti-CD4 (clone SK3; BD Biosciences), phycoerythrin (PE)-labeled anti-CD45RO (clone UCHL1; BD Bioscience), FITC-labeled anti-CD25 (clone M-A251; BD Bioscience), and Alexa Fluor 647-labeled anti-CCR6 (clone TG7/CCR6; Biolegend) antibodies using a FACSAria sorter (BD Biosciences) through the service provided by the Cell Sorting Core Facility (the First Core Laboratory) of the National Taiwan University College of Medicine. The acquisition and analysis gates were restricted to the lymphocyte gate as determined by characteristic forward- and side-scatter properties of lymphocytes. For analysis, 1 × 105 lymphocytes were acquired. The sorting strategy produced results as follows: the CD4+CD45RO− subset (naive CD4+ T cells); the CD4+CD45RO+CCR6− subset (non-IL-17-producing CD4+ T cells); and the CD4+CD45RO+CCR6+CD25− subset (IL-17-producing CD4+ T cells). The purity and potential to secrete IL-17 were determined by PMA-plus-ionomycin reactivation for another 4 h, and the supernatant was collected for IL-17 measurement. Sorted IL-17-producing CD4+ cells (5 × 104) were treated with media or CM from hVIC, with or without GTFC stimulation, in the presence of plate-bound anti-human CD3 and soluble anti-CD28. Cell proliferation was analyzed by flow cytometry by carboxyfluorescein succinimidyl ester (CFSE) labeling. Cells were collected, and the expression levels of IL-6, IL-17, IL-21, IL-23, and retinoic acid receptor-related orphan receptor C (Rorc) were determined by real-time PCR (see Table S3 in the supplemental material). For neutralization experiments, related neutralizing antibodies were preincubated with CM-GTF for 30 min prior to stimulation. All experiments were conducted in triplicate, and data are shown as mean percentages ± standard errors of the means (SEM).
Chemotaxis assay.
A chemotaxis assay was performed using a 24-well two-chamber apparatus. PMN cells and MNCs (2 × 106) were suspended in a total volume of 150 μl DMEM supplemented with 1% hi-FCS. MNCs or PMN cells were added to 6.5-mm-diameter, 5.0-μm-pore-size polycarbonate Transwell cell culture inserts (Millipore), and 600 μl CM was added to the lower chamber. The migration assay was performed at 37°C for 2 h. Migrated cells were counted by trypan blue staining and stained by monoclonal antibodies (MAb) specific for CD14 and CD4 to enable fluorescence-activated cell sorting (FACS) analysis. For the neutralization assay, anti-CXCL1 antibodies (Peprotech Inc.) were preincubated with CM for 30 min before the migration assay was performed. All experiments were performed in triplicate.
Detection of cytokines, chemokines, and PMN activity by ELISA.
Concentrations of IL-6, IL-17, IL-21, IL-23, gamma interferon (IFN-γ), and CXCL1 in cell culture supernatants from treated or untreated controls were quantified by sandwich immunoassays using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol. Experiments were conducted in triplicate, and results are presented as mean concentrations (pg/ml) ± SEM.
PMN cells were cultured with CM, 1 μg/ml LPS, or 25 μg/ml GTFC for 2 h. Cells were harvested, and the expression of IL-1β, IL-6, IL-8, CXCL1, CXCL10, MMP9, and actin genes was determined by RT-PCR. Supernatants were collected for measurements of MPO and elastase activity by ELISA using anti-MPO (Abnova) and anti-elastase (sc-25621; Santa Cruz Biotechnology) antibodies. MMP9 in supernatant from GTFC-activated neutrophils was concentrated 50-fold and detected by anti-MMP9 antibodies (GTX100458; GeneTex Inc.). The gelatinase activity in cultured supernatants from activated neutrophils or plasma from IE patients or HD was monitored by gelatin zymography.
Statistical analyses.
Data were analyzed statistically using an unpaired (two-tailed) t test to compare the mean levels of cytokine secretion following a particular treatment. Differences with P values of <0.05 were considered to be statistically significant and are indicated with a single asterisk (*), while differences with P values of <0.01 are indicated with two asterisks (**) and differences with P values of <0.001 are indicated with three asterisks (***).
RESULTS
Detection of Th17 cells and IL-17 in infected heart valves and patient sera.
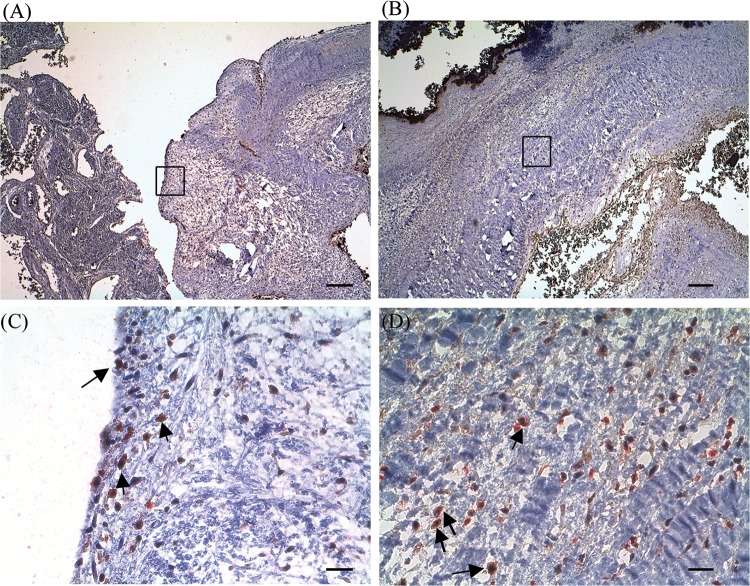
To confirm whether Th17 cells are recruited in the streptococcus-induced IE, infiltrating leukocytes in infected valves were examined by immunostaining. Infiltrating CD4+ T cells were identified in infected heart valve sections (Fig. 1A and B) but not in normal valves obtained from an autopsy or in the infected heart valve sections stained with isotype control antibodies (data not shown). Double immunostaining for CD4 and IL-17 confirmed the identity and presence of Th17 cells and the production of IL-17 in the infected heart valves (Fig. 1C and D). IL-17-producing cells could also be detected in the surrounding neocapillaries (data not shown).
FIG 1.
IL-17-producing CD4+ T cells in infected valves from patients with infective endocarditis (IE). (A and B) Sections of valvular endothelial linings or interstitial stroma of formalin-fixed specimens from a patient with IE induced by Streptococcus gordonii were stained with CD4 (red) and IL-17 (brown). (C and D) Doubly positive cells are indicated by arrows, and panels C and D show the enlarged insets from panels A and B, respectively. Slides were counterstained with Mayer's hematoxylin; original magnification, ×40 (scale bar, 250 μm) (A and B) or ×400 (scale bar, 25 μm) (C and D).
The demographics and clinical characteristics of patients with infective endocarditis (IE) or streptococcus bacteremia without IE are shown in Table 1. Most of the affected sites were mitral valves (n = 8), whereas involvement of the aortic valves was less common (n = 2). The most frequent cardiac complication was rupture of the chordae tendineae (RCT) leading to pericardial effusion. In accordance with the valve inflammatory responses, levels of IL-17- and Th17-associated cytokines, including IL-21 and IL-23, were significantly elevated in the sera of endocarditis patients compared to sera of patients with streptococcus bacteremia without IE (Table 1). The patients with IE complicated with rupture of the chordae tendineae exhibited significantly higher levels of IL-21 (P = 0.004).
TABLE 1.
Demographics and clinical characteristics of patients with IE or streptococcus bacteremia and correlations of serum cytokine levels with valvular destruction in streptococcus-induced IE patientsa
| Parameter | Value(s)± SEM for: |
|
|---|---|---|
| Streptococcus-induced IE patients (n = 12) | Non-IE patients with streptococcus bacteremia (n = 12) | |
| No. of males/no. of females | 7/5 | 8/4 |
| Patient age (yrs) (range) | 44.0 ± 22.68 (5–77) | 57.5 ± 21.47 (4–82) |
| No. (%) of patients with aortic valve involvement | 2 (16.67) | NA |
| No. (%) of patients with mitral valve involvement | 8 (16.67) | NA |
| No. (%) of patients with indicated complication of infectious endocarditis or bacteremia with (n = 5)/without (n = 7) rupture of chordae tendineae | ||
| Glomerunephritis | 1 (20.00)/1 (14.28) | 0 |
| Pericardial effusion | 2 (40.00)/2 (28.57) | 0 |
| Thromoembolic event(s) | 1 (20.00)/3 (42.86) | 1 |
| Acute decompensated heart failure | 1 (20.00)/1 (14.28) | 0 |
| Serum cytokine levels (pg/ml) | ||
| IL-17 | 53.54 ± 65.49/104.29 ± 103.55 | 47.40 ± 29.36 |
| IL-21 | 1,392.77 ± 808.86**/260.19 ± 209.26** | 534.03 ± 623.87 |
| IL-23 | 119.09 ± 213.95/19.85 ± 31.63 | 76.52 ± 169.71 |
| IL-1β | 60.85 ± 35.02/66.40 ± 46.41 | 55.94 ± 51.32 |
| IL-6 | 177.51 ± 175.16/515.40 ± 686.43 | 668.02 ± 803.48 |
Valvular or cardiac interstitial destruction included the presence of ruptured chordae tendineae and pericardial effusion. P values were calculated using Student's t test. **, P = 0.004. IE, infective endocarditis; NA, not applicable.
Chemokine expression and leukocyte migration induced by activated VIC.
To test whether activated VIC could induce the migration and chemotaxis of CD4+ T cells and neutrophils, in vitro culture assays for VIC and leukocyte migration were performed as described previously (8). GTF-activated VIC showed upregulated expression and release of a panel of chemokines. Compared to unstimulated VIC results, significant (greater than 4-fold) increases were detected for the mRNA expression of the genes encoding CCL4 (11.3-fold), CCL8 (7.57-fold), CCL20 (22.32-fold), CCL3 (8.82-fold), CXCL10 (22.78-fold), CCL7 (4.44-fold), CXCL1 (4.56-fold), CXCL2 (4.47-fold), CXCL8 (4.92-fold), and CXCL11 (5.86-fold) as determined by PCR array (see Fig. S1A in the supplemental material). Accordingly, GTF strongly influenced the release of chemokines, including CCL7 (9.52-fold increase), CXCL1 (16.16-fold increase), and CXCL6 (7.51-fold increase), into culture supernatants (see Fig. S1B). These data suggest that hVIC, upon activation by PAMPs such as GTF, release multiple chemokines that recruit neutrophils and CD4+ T cells.
The chemokine-containing culture supernatants from GTF-activated VIC induced the chemotaxis and migration of PMN cells and CD4+ T cells to a significantly greater level than did unstimulated supernatants (see Fig. S1C and D in the supplemental material). Furthermore, both naive (CD45RA+) and memory (CD45RO+) CD4+ T cells were induced to migrate toward media containing GTF-activated VIC (see Fig. S1E). The expression levels of the genes encoding IL-6, IL-8, RANTES, CXCL10, and MMP9 were upregulated in GTF-activated VIC (see Fig. S1F).
Enhancement of Th17-related genes in memory T cells by GTF-stimulated VIC is dependent on IL-6.
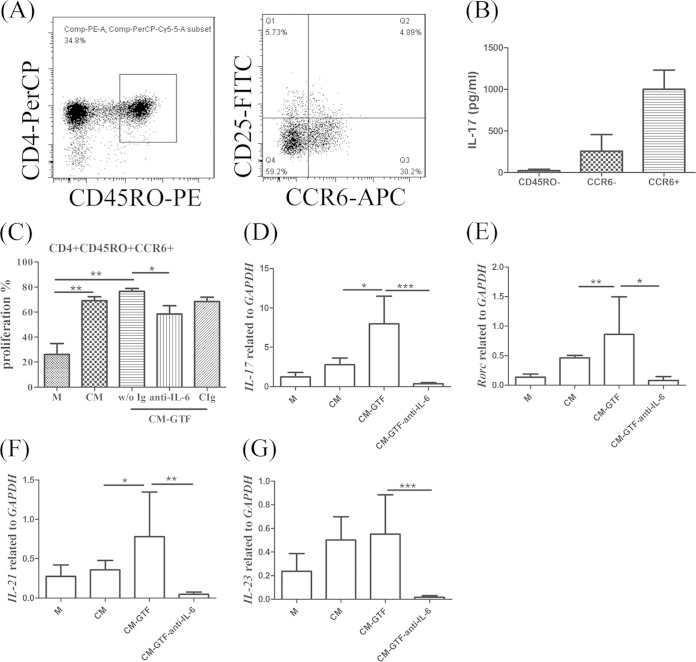
In human peripheral mononuclear cells (PBMCs), Th17 cells could be identified based on their surface markers as the CD4+ CD45RO+ CD25− CCR6+ subset (19, 20). To test whether the cytokine milieu from activated valvular interstitial cells (VIC) could induce Th17 cell differentiation or expansion, IL-17A-producing cells in the cell sorter-enriched CD4+ CD45RA+ (naive) or CD4+ CD45RO+ CD25− CCR6+ Th17 cell subsets were examined by intracellular staining and flow cytometry analysis after coculture with supernatants from activated or nonactivated VIC for 5 days in the presence of anti-CD3 and anti-CD28 antibodies, as described previously (21). The FACS-sorted CD4+ CD45RO+ CD25− CCR6+ T cell subset was capable of secreting IL-17 at a significantly greater level than the CD4+ CD45RO+ CD25− CCR6− T cell subset; therefore, it contained enriched Th17 cells. In contrast, the CD4+ CD45RA+ naive T cell subset failed to secrete detectable levels of IL-17 (Fig. 2A and B). Under the in vitro culture conditions, the hVIC exhibited activated status (8) and the culture supernatants from these activated cells could efficiently induce the proliferation of the CCR6+ Th17 cells (Fig. 2C). However, we demonstrated that stimulation by bacterial modulins such as GTFC could further enhance the expression of genes encoding retinoic acid receptor-related orphan receptor gamma (ROR-γ) and IL-17 in the Th17 cells (Fig. 2D to G) but failed to enhance further the proliferation of Th17 cells. These effects on proliferation and production of cytokines were attenuated in the presence of IL-6 neutralizing antibody. Coculture of CD4+ CD45RA+ naive T cells with supernatants from activated VIC also failed to induce detectable levels of IL-17-producing cells (data not shown). Therefore, these results suggest that the supernatants from GTFC-activated VIC may exert modulatory effects on the functionality rather than the proliferative capacity of the Th17 cells.
FIG 2.
Enhancement of Th17-related genes in memory T cells by GTF-stimulated valvular interstitial cells (VIC) is dependent on IL-6. (A) Sorting of IL-17-producing cells. CD4+ T cells were purified by a CD4+ enrichment cocktail and stained with anti-CD4, anti-CD45RO, anti-CD25, and anti-CCR6 antibodies. The CD4+ CD45RO− population was gated as a CD45RO− population. The CD4+ CD45RO+ CD25− population was gated and separated into CCR6− and CCR6+ populations. (B) After reactivation using PMA plus ionomycin for 4 h, IL-17 production was measured by ELISA. (C) Proliferation of CD4+ CD45RO+ CD25− CCR6+ cells (labeled with carboxyfluorescein succinimidyl ester) was examined with or without IL-6 neutralizing or control antibody. (D to G) The expression of the genes encoding IL-17 (D), Rorc (E), IL-21 (F), and IL-23 (G) was determined by RT-PCR. Data from six independent experiments are represented as fold change compared to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression. Significant differences between groups, as determined by Student's t test, are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001). M, medium control; GTF, GTF stimulation; CM, cultured medium from resting VIC; CM-GTF, conditioned medium from GTF-activated VIC; CIg, control Ig.
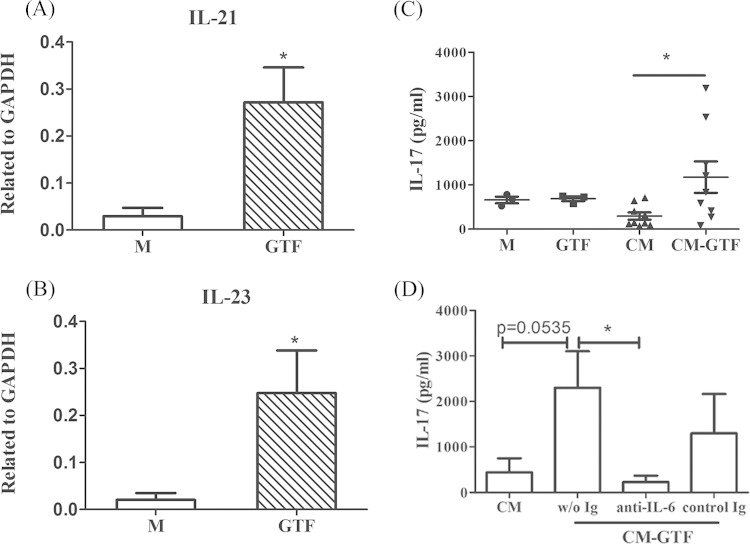
To exclude the possibility that streptococcal GTF could exert a direct stimulatory effect to induce Th17 cell differentiation or expansion, enriched CD4+ T cells were cocultured with or without GTF alone, GTF-activated supernatants, or nonactivated supernatants from VIC for cytokine analysis. In streptococcal GTF-activated VIC, the expression levels of the Th17 cell differentiation- or expansion-related IL-21 or IL-23 cytokines were significantly increased (Fig. 3A and B). However, GTF alone did not induce IL-17 production or Th17 expansion in the in vitro coculture assay. Only supernatants from GTF-activated VIC could stimulate the production and secretion of IL-17, which was analogous to the response of the enriched CCR6+ Th17 cell subset. The induction of IL-17 secretion was inhibited by IL-6 neutralizing antibody treatment (Fig. 3C and D). Together, these data suggest that GTF-activated VIC induce Th17 cell proliferation and IL-17 secretion in an IL-6-dependent manner.
FIG 3.
Th17-associated cytokine production is induced by glucosyltransferase (GTF)-stimulated valvular interstitial cells (VIC). (A and B) The expression of the genes encoding IL-21 (A) and IL-23 (B) in VIC stimulated with control media or GTF for 24 h was examined by RT-PCR. Data from three independent experiments are presented as fold changes compared to GAPDH expression. (C and D) Secretion of IL-17 was measured in CD4+ T cells cocultured with cultured medium (n = 3) or GTF-conditioned medium (n = 3) from resting (CM; n = 8) or GTF-stimulated (CM-GTF; n = 8) VIC in the presence of 1 μg/ml anti-CD3 and anti-CD28 antibody for 120 h with or without IL-6 neutralizing or control antibody (control Ig). After reactivation using phorbol myristate acetate plus ionomycin, the production of IL-17 was measured in supernatants using an ELISA kit. Data are presented as mean concentrations (pg/ml) ± SEM. Significant differences among groups, as determined by Student's t test, are indicated by asterisks (*, P < 0.05).
Neutrophil migration could be enhanced by GTF- or IL-17-activated VIC.
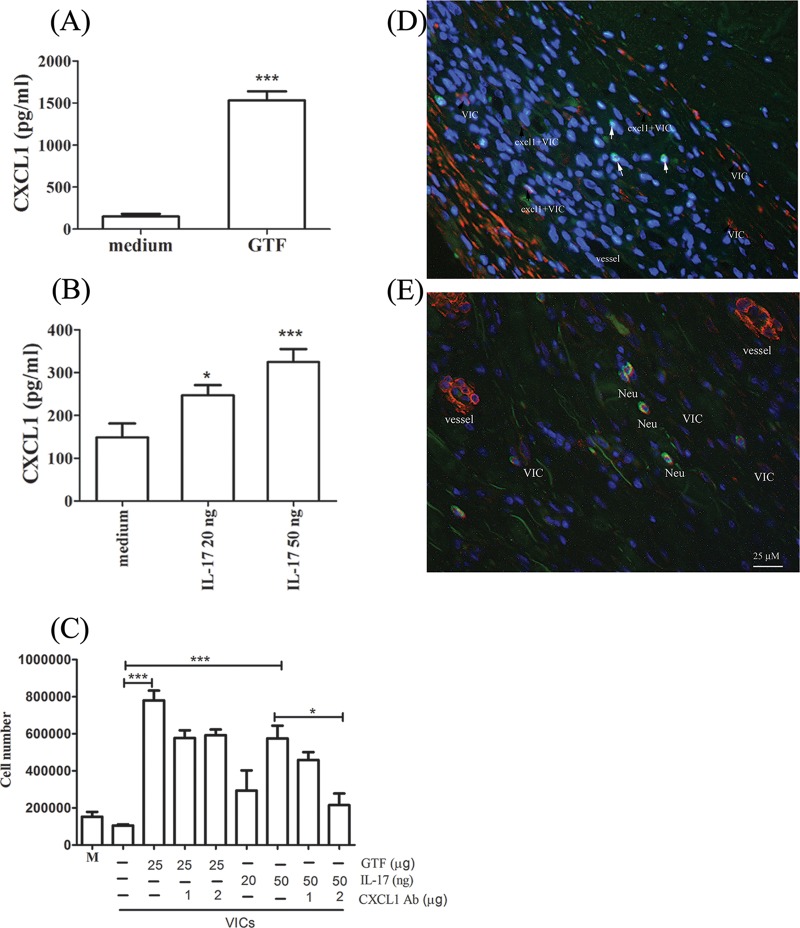
In addition to GTF, IL-17 can also directly activate the expression and secretion of the CXCL1 chemokine (Fig. 4A and B). Interestingly, CXCL1-mediated neutrophil chemotaxis and migration were found to play a major role in neutrophil recruitment in IL-17-activated VIC but not in GTF-activated VIC. CXCL1 neutralizing antibody inhibited neutrophil migration induced by IL-17 activation completely but inhibited CM-GTF-induced migration only partially (Fig. 4C). Notably, double staining of α-SMA and CXCL1 confirmed that human VIC can secrete CXCL1 in infected valves (Fig. 4D). Neutrophils (positive for elastase antibody) were present surrounding the capillaries or VIC (positive for anti-α-SMA staining), suggesting that activated VIC might play an important role in recruiting or sustaining these inflammatory infiltrates (Fig. 4E).
FIG 4.
CXCL1 expression in activated valvular interstitial cells (VIC) and the induction of neutrophil migration. (A and B) The production of CXCL1 was examined in VIC stimulated with glucosyltransferase (GTF; n = 14) (A) and IL-17 (n = 12) (B). (C) Neutrophil (Neu) migration experiments were performed using resting or activated conditioned media (CM), with or without CXCL1 neutralizing antibody (n = 6). Statistically significant differences between columns, as determined by Student's t test, are indicated by asterisks (*, P < 0.05; ***, P < 0.001). (D and E) Immunostaining of α-SMA and CXCL1 (D) or of SMA and elastase (E) in an infected valve. VIC are indicated by α-SMA-positive cells (red); CXCL1- and elastase-expressing cells are indicated in green (original magnification, ×400; scale bar, 25 μm).
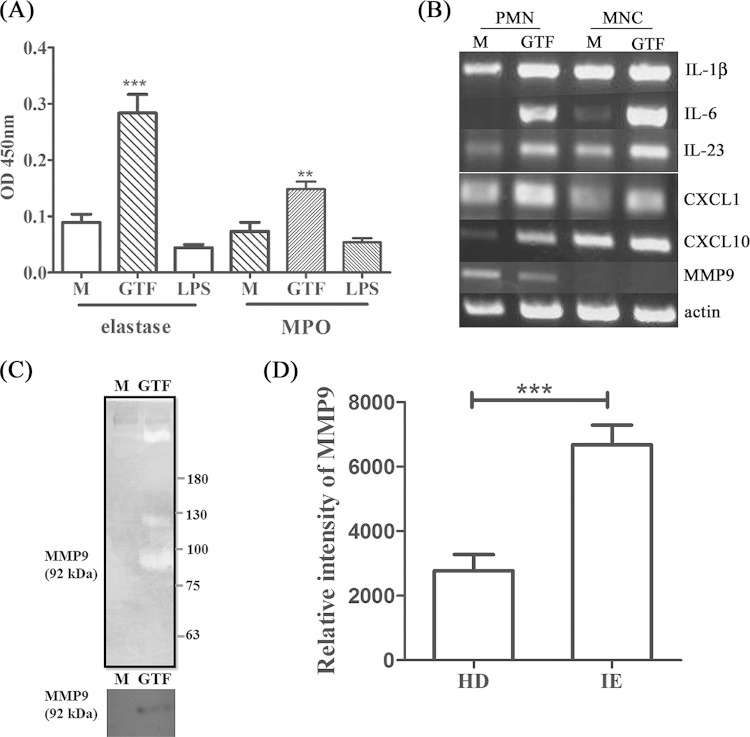
Elastase, MPO, and MMP9 (matrix metalloprotease 9) activity has been reported to be involved in the destruction of heart valves (22, 23). The level of circulating MMP9 was increased in embolic infective endocarditis, but the source of MMP9 secretion was not identified. Similarly to previous reports, we detected elastase in infected valves (Fig. 4E). Interestingly, streptococcal GTF-activated but not IL-17- or IL-17-activated VIC stimulated the release of MPO and elastase (Fig. 5A). Notably, LPS, a potent activator of neutrophils, had no effect. The expression of Th17-related cytokines, including IL-1β, IL-6, and IL-23, and chemokines, including CXCL1 and CXCL10, was upregulated in GTF-activated PMN cells and MNCs. MMP9 was expressed only in PMN cells but showed no difference in expression after GTF activation (Fig. 5B). Active MMP9 secreted from GTF-activated PMN cells exerted gelatinase activity, which we confirmed by Western blotting (Fig. 5C). The gelatinase activity of MMP was also higher in plasma from IE patients than in plasma from HD (Fig. 5D). Therefore, streptococcal GTF released from bacterium-platelet biofilms could activate VIC to sustain IL-17 production, enhance neutrophil recruitment and activation, and induce tissue damage in infected heart valves.
FIG 5.
Proinflammatory cytokines and tissue remodeling-related gene expression in glucosyltransferase (GTF)-stimulated neutrophils and mononuclear cells. Purified polymorphonuclear (PMN) cells or mononuclear cells were stimulated by GTF or lipopolysaccharide (LPS) for 2 h. (A) MPO activity and elastase activity in culture supernatants were measured by ELISA. Data from three independent experiments are shown with a mean absorbance value of 450 nm. (B) The expression of cytokines and matrix metalloprotease in PMN cells or MNCs with or without GTF stimulation was determined by RT-PCR. (C) Gelatinase activity (top panel) and MMP9 expression (bottom panel) in culture supernatants from GTF-activated PMN cells were detected by gelatin zymography and Western blotting, respectively. (D) Gelatinase activity of MMP9 in plasma from patients with infective endocarditis (IE) or from healthy donors (HD) (IE, n = 42; HD, n = 21). Significant differences between groups, as determined by Student's t test, are indicated by asterisks (**, P < 0.01; ***, P < 0.001).
DISCUSSION
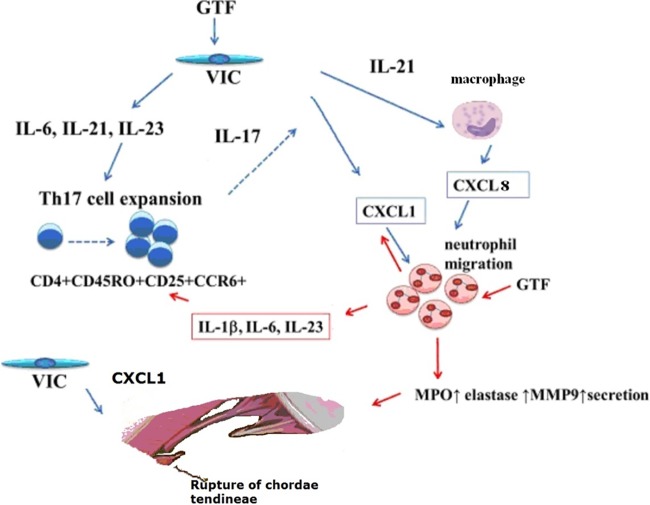
Based on the findings of our study, we propose a model for the underlying mechanisms involved in chronic inflammatory responses of heart valves during streptococcus-induced endocarditis. The streptococcus-platelet biofilm on the infected valves can release PAMPs, such as GTFs, which might directly activate the VIC to produce various chemokines, including CXCL1, to recruit both T cells and neutrophils. Interestingly, both Th1 cell-associated cytokine CXCL10 (gamma interferon-induced protein 10 [IP-10]) and Th17 cell-associated CCL20 were significantly upregulated in GTF-activated VIC, whereas Th2 cell-associated cytokine CCL17 was slightly downregulated by GTF-activated VIC. Proliferation and expression of the genes encoding Rorc, IL-17, and IL-21 in CD4+ CD45RO+ CD25− CCR6+ Th17 cells were induced by GTF-activated VIC. Reciprocally, IL-17 promoted CXCL1 expression in VIC, which amplified neutrophil recruitment and the release of tissue-damaging MPO, elastase, and MMP9, resulting in valvular damage. Furthermore, expression of Th17 cell-related cytokines, including IL-1, IL-6, and IL-23, was upregulated in GTF-activated neutrophils. This paracrine loop may sustain the persistent inflammatory response in IE (Fig. 6).
FIG 6.
A hypothetical model for heart valve inflammation during streptococcus-induced endocarditis. GTFs were released from streptococcal biofilm in the infected valves, which may directly activate the VIC to produce IL-6, IL-21, and IL-23. These secreted factors promote the expansion of CD4+ CD45RO+ CD25− CCR6+ Th17 cells. Reciprocally, IL-17 promoted CXCL1 expression in VIC to amplify neutrophil recruitment and the release of tissue-damaging MPO, elastase, and MMP9. IL-21 could also induce the recruitment of neutrophils indirectly by priming macrophages to increase CXCL8 secretion. Additionally, IL-1β, IL-6, and IL-23 expression was elevated in GTF-activated neutrophils and monocytes, which sustained Th17 cells in infected valves. Both CXCL8 and CXCL1 could elicit angiogenic responses, which could in turn lead to ruptures of the chordae tendineae.
Th17 cell differentiation and expansion were induced by cytokine transforming growth factor (TGF)-β and IL-6, while IL-23 may have a critical role in stabilizing the Th17 phenotype (24–26). In humans, however, TGF-β and IL-6 are not capable of differentiating human Th17 cells (27, 28). In contrast, TGF-β and IL-21 uniquely promote the differentiation of human naive CD4+ T cells into Th17 cells. Additionally, IL-1β and IL-6 can induce IL-17A secretion from human central memory CD4+ T cells (29). These results are consistent with our data showing that CCR6+ Th17 cell expansion induced by GTF-activated VIC was mediated by IL-6. IL-1β in its active form was undetectable in supernatants from GTF-activated VIC and, therefore, exhibited negligible capacity to trigger Th17 cell differentiation from naive CD4+ T cells. Furthermore, the production and secretion of IFN-γ, a Th1 cytokine, could also be induced by GTF-activated VIC (data not shown), suggesting that the cytokine milieu generated by activated VIC can exert multipotent immunostimulatory effects. Recently, it was found that neutrophils do not express IL-21R (30) and, therefore, that IL-21 had no direct effect on neutrophil function. Interestingly, IL-21 could induce the recruitment of neutrophils indirectly through monocyte-derived macrophages by upregulating the secretion of CXCL8, a potent neutrophil chemoattractant (30), suggesting that the enhanced levels of IL-21 detected in the IE patients may play a role as important as that of IL-17 in recruiting neutrophils to the infected valves.
Notably, the concentrations of IL-21 and IL-23 were also upregulated in supernatants of GTF-activated VIC. Expression of the genes encoding IL-21 and IL-23 in CCR6+ cells cocultured with CM-GTF was inhibited by either IL-6 or IL-21 neutralizing antibody (data not shown). IL-23 alone and IL-23 synergistically combined with prostaglandin E2 (PGE2) were essential for the maintenance and survival of Th17 cells in vitro in cultures in the presence of IL-1β, TGF-β, and IL-6 (31, 32). When CD4+ T cells were cocultured with bronchial fibroblasts, the expression levels of the genes encoding IL-1β, TGF-β, and IL-23 were upregulated, resulting in enhanced IL-17 secretion from CCR6+ T cells (33). In our coculture system, IL-21 and IL-23 were also involved in the Th17 cell expansion, and IL-21R and IL-23R were upregulated by IL-6 (34). Therefore, the loop of IL-6–IL-21–IL-23 secretion by both VIC and CCR6+ CCD4+ T cells in response to GTF stimulation may synergistically trigger the expansion of Th17 cells in vitro.
It has been reported that IL-21 might enhance the production of chemokines and tissue-degrading MMPs by epithelial cells and fibroblasts (35, 36). IL-21R was also present in cases of human tendinopathy, where it was expressed by tenocytes and macrophages (37). IL-21-induced MMP elevation might account for the correlation of serum IL-21 in our IE patients with rupture of the chordae tendineae (Table 1). Although ruptures of chordae tendineae could be attributed to primary tears, these conditions often occur in elderly people. The IE patients in this study were much younger than those in a previous report (38). We also checked the serum levels of several types of MMPs among our patients, but none of them were associated with IL-21 or valvular destruction (data not shown). This might be a consequence of consumption of MMPs in affected tissues. Furthermore, a negative trend for an association with IL-17 in patients with rupture of chordae tendineae was also observed. The local expression of IL-17 in affected sites might not be as persistent as expression of IL-21 in the context of chronic bacterial endocarditis.
Rupture of the chordae tendineae has been associated with the local expression of vascular endothelial growth factor A and of several types of MMPs, along with an increased level of inflammatory cell infiltrate. In contrast, tenomodulin, an antiangiogenic factor, is less abundant in these physiologically avascular areas. Inflammation and angiogenesis may both contribute to degeneration and, subsequently, to ruptures of the chordae tendineae (39). CXCL8 has been found to elicit angiogenic responses in microvascular endothelial cells from the human intestine (40). Therefore, IL-21-driven CXCL8 secretion by macrophages could also account for the neocapillary formation observed in infected valves. Additionally, the significant elevation of CXCL1 production by hVIC upon activation by GTF might also contribute to enhanced angiogenesis (41). Sterile Libman-Sacks endocarditis in systemic lupus erythematosus (SLE) is also well known to be associated with valvular destruction. Patients with SLE have higher serum levels of IL-17, IL-21, and IL-23 than healthy controls (42, 43). Moreover, increased levels of IL-21 might synergize with Toll-like receptor 9 (TLR-9) signaling to generate pathogenic plasma cells that contribute to SLE activity. Notably, antiphospholipid antibodies are associated with both Libman-Sacks endocarditis and severe valvular regurgitation in SLE (44). Our findings suggest that IL-21 and IL-17 in infective endocarditis or Libman-Sacks endocarditis might similarly mediate valvular destruction.
In chronic infective endocarditis, protease activity associated with MPO, elastase, or MMP9 has been related to tissue damage and inflammation (23, 45). In vitro, GTF, but not LPS, could induce the release of MPO and elastase. These findings are consistent with a previous report that a high concentration of LPS failed to induce the release of elastase. Only PMA exhibited a stimulatory effect on neutrophils that included elastase release (46). In contrast, PMA is also a potent inducer of the in vitro formation of human neutrophil extracellular traps (NETs) (47). NETs were detected in vegetation, and cell-free DNA was elevated in serum from rats administered Enterococcus faecalis bacteria in an infective endocarditis rat model (45). The MPO and elastase detected in or at the base of the vegetation could also have contributed to the NET formation. However, our study demonstrated an alternative route for the release of these tissue-damaging proteases from recruited neutrophils through the direct activation of neutrophils by streptococcal GTFs.
Supplementary Material
ACKNOWLEDGMENTS
We declare that we have no conflicts of interest.
This study was supported by the National Science Council (NSC 101-2321-B-002-055, NSC102-2321-B-002-010, and 103-2320-B-002-037-MY3), Ministry of Science and Technology (MOST 103-2321-B-002-024), and Academia Sinica and Ministry of Science and Technology (104-0210-01-09-02).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02965-14.
REFERENCES
- 1.Mylonakis E, Calderwood SB. 2001. Infective endocarditis in adults. N Engl J Med 345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 2.Jung CJ, Zheng QH, Shieh YH, Lin CS, Chia JS. 2009. Streptococcus mutans autolysin AtlA is a fibronectin-binding protein and contributes to bacterial survival in the bloodstream and virulence for infective endocarditis. Mol Microbiol 74:888–902. doi: 10.1111/j.1365-2958.2009.06903.x. [DOI] [PubMed] [Google Scholar]
- 3.Fekete T. 1990. Controversies in the prevention of infective endocarditis related to dental procedures. Dent Clin North Am 34:79–90. [PubMed] [Google Scholar]
- 4.Robert RB. 1995. Streptococcal endocarditis, p 1318–1330. Raven Press, New York, NY. [Google Scholar]
- 5.Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, Chia JS. 2012. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis 205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 6.Shun CT, Lu SY, Yeh CY, Chiang CP, Chia JS, Chen JY. 2005. Glucosyltransferases of viridans streptococci are modulins of interleukin-6 induction in infective endocarditis. Infect Immun 73:3261–3270. doi: 10.1128/IAI.73.6.3261-3270.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh CY, Chen JY, Chia JS. 2006. Glucosyltransferases of viridans group streptococci modulate interleukin-6 and adhesion molecule expression in endothelial cells and augment monocytic cell adherence. Infect Immun 74:1273–1283. doi: 10.1128/IAI.74.2.1273-1283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shun CT, Yeh CY, Chang CJ, Wu SH, Lien HT, Chen JY, Wang SS, Chia JS. 2009. Activation of human valve interstitial cells by a viridians streptococci modulin induces chemotaxis of mononuclear cells. J Infect Dis 199:1488–1496. doi: 10.1086/598485. [DOI] [PubMed] [Google Scholar]
- 9.Yeh CY, Lin CN, Chang CF, Lin CH, Lien HT, Chen JY, Chia JS. 2008. C-terminal repeats of Clostridium difficile toxin A induce production of chemokine and adhesion molecules in endothelial cells and promote migration of leukocytes. Infect Immun 76:1170–1178. doi: 10.1128/IAI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL, ESC Committee for Practice Guidelines. 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 11.Roy A, Brand NJ, Yacoub MH. 2000. Molecular characterization of interstitial cells isolated from human heart valves. J Heart Valve Dis 9:459–464. [PubMed] [Google Scholar]
- 12.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC Jr, Fullerton DA. 2008. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol 294:C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 13.Heo SK, Yun HJ, Noh EK, Park WH, Park SD. 2008. LPS induces inflammatory responses in human aortic vascular smooth muscle cells via Toll-like receptor 4 expression and nitric oxide production. Immunol Lett 120:57–64. doi: 10.1016/j.imlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Babu AN, Meng X, Zou N, Yang X, Wang M, Song Y, Cleveland JC, Weyant M, Banerjee A, Fullerton DA. 2008. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg 86:71–76. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 15.López J, Fernández-Pisonero I, Dueñas AI, Maeso P, San Román JA, Crespo MS, García-Rodríguez C. 2012. Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol 158:18–25. doi: 10.1016/j.ijcard.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi R, Tanaka M, Kume N, Minami M, Kawamoto T, Togi K, Shimaoka T, Takahashi S, Yamaguchi J, Nishina T, Kitaichi M, Komeda M, Manabe T, Yonehara S, Kita T. 2004. Upregulation of SR-PSOX/CXCL16 and recruitment of CD8+ T cells in cardiac valves during inflammatory valvular heart disease. Arterioscler Thromb Vasc Biol 24:282–287. doi: 10.1161/01.ATV.0000114565.42679.c6. [DOI] [PubMed] [Google Scholar]
- 17.Maione F, Paschalidis N, Mascolo N, Dufton N, Perretti M, D'Acquisto F. 2009. Interleukin 17 sustains rather than induces inflammation. Biochem Pharmacol 77:878–887. doi: 10.1016/j.bcp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Bian Z, Guo Y, Ha B, Zen K, Liu Y. 2012. Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol 188:844–853. doi: 10.4049/jimmunol.1101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brucklacher-Waldert V, Steinbach K, Lioznov M, Kolster M, Holscher C, Tolosa E. 2009. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL-17A expression. J Immunol 183:5494–5501. doi: 10.4049/jimmunol.0901000. [DOI] [PubMed] [Google Scholar]
- 21.Lee JJ, Chang YL, Lai WL, Ko JY, Kuo MY, Chiang CP, Azuma M, Chen CW, Chia JS. 2011. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck 33:1301–1308. doi: 10.1002/hed.21607. [DOI] [PubMed] [Google Scholar]
- 22.Soini Y, Satta J, Määttä M, Autio-Harmainen H. 2001. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol 194:225–231. doi: 10.1002/path.850. [DOI] [PubMed] [Google Scholar]
- 23.Al-Salih G, Al-Attar N, Delbosc S, Louedec L, Corvazier E, Loyau S, Michel JB, Pidard D, Duval X, Meilhac O. 2012. Role of vegetation-associated protease activity in valve destruction in human infective endocarditis. PLoS One 7:e45695. doi: 10.1371/journal.pone.0045695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 26.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal MR. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier M, Bouchard A, Girard D. 2004. In vivo and in vitro roles of IL-21 in inflammation. J Immunol 173:7521–7530. doi: 10.4049/jimmunol.173.12.7521. [DOI] [PubMed] [Google Scholar]
- 31.Stritesky GL, Yeh N, Kaplan MH. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol 181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. 2008. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loubaki L, Hadj-Salem I, Fakhfakh R, Jacques E, Plante S, Boisvert M, Aoudjit F, Chakir J. 2013. Co-culture of human bronchial fibroblasts and CD4+ T cells increases Th17 cytokine signature. PLoS One 8:e81983. doi: 10.1371/journal.pone.0081983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 35.Yuan FL, Hu W, Lu WG, Li X, Li JP, Xu RS, Li CW, Chen FH, Jin C. 2011. Targeting interleukin-21 in rheumatoid arthritis. Mol Biol Rep 38:1717–1721. doi: 10.1007/s11033-010-0285-x. [DOI] [PubMed] [Google Scholar]
- 36.Fina D, Sarra M, Caruso R, Del Vecchio BG, Pallone F, MacDonald TT, Monteleone G. 2008. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57:887–892. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 37.Campbell AL, Smith NC, Reilly JH, Kerr SC, Leach WJ, Fazzi UG, Rooney BP, Murrell GA, Millar NL. 2014. IL-21 receptor expression in human tendinopathy. Mediators Inflamm 2014:481206. doi: 10.1155/2014/481206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portugese S, Amital H, Tenenbaum A, Bar-Dayan Y, Levy Y, Afek A, Shemesh J, Shoenfeld Y. 1998. Clinical characteristics of ruptured chordae tendineae in hospitalized patients: primary tear versus infective endocarditis. Clin Cardiol 21:813–816. doi: 10.1002/clc.4960211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura N, Shukunami C, Hakuno D, Yoshioka M, Miura S, Docheva D, Kimura T, Okada Y, Matsumura G, Shin'oka T, Yozu R, Kobayashi J, Ishibashi-Ueda H, Hiraki Y, Fukuda K. 2008. Local tenomodulin absence, angiogenesis, and matrix metalloproteinase activation are associated with the rupture of the chordae tendineae cordis. Circulation 118:1737–1747. doi: 10.1161/CIRCULATIONAHA.108.780031. [DOI] [PubMed] [Google Scholar]
- 40.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. 2003. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 41.Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, Kunkel SL, Burdick MD, Strieter RM. 1999. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol 154:1503–1512. doi: 10.1016/S0002-9440(10)65404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. 2008. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol 127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Nakou M, Papadimitraki ED, Fanouriakis A, Bertsias GK, Choulaki C, Goulidaki N, Sidiropoulos P, Boumpas DT. 2013. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin Exp Rheumatol 31:172–179. http://www.clinexprheumatol.org/pubmed/find-pii.asp?pii=23137515. [PubMed] [Google Scholar]
- 44.Perez-Villa F, Font J, Azqueta M, Espinosa G, Pare C, Cervera R, Reverter JC, Ingelmo M, Sanz G. 2005. Severe valvular regurgitation and antiphospholipid antibodies in systemic lupus erythematosus: a prospective, long-term, follow up study. Arthritis Rheum 53:460–467. doi: 10.1002/art.21162. [DOI] [PubMed] [Google Scholar]
- 45.Augustin P, Alsalih G, Launey Y, Delbosc S, Louedec L, Ollivier V, Chau F, Montravers P, Duval X, Michel JB, Meilhac O. 2013. Predominant role of host proteases in myocardial damage associated with infectious endocarditis induced by Enterococcus faecalis in a rat model. Infect Immun 81:1721–1729. doi: 10.1128/IAI.00775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceusters JD, Serteyn DA, Minguet G, de la Rebière de Pouyade G, Romainville J, Deby-Dupont GP, Mouithys-Mickalad AA, Franck TJ. 2012. An in vitro whole blood model to test the effects of different stimuli conditions on the release of myeloperoxidase and elastase by equine neutrophils. Vet Immunol Immunopathol 150:221–227. doi: 10.1016/j.vetimm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.