Abstract
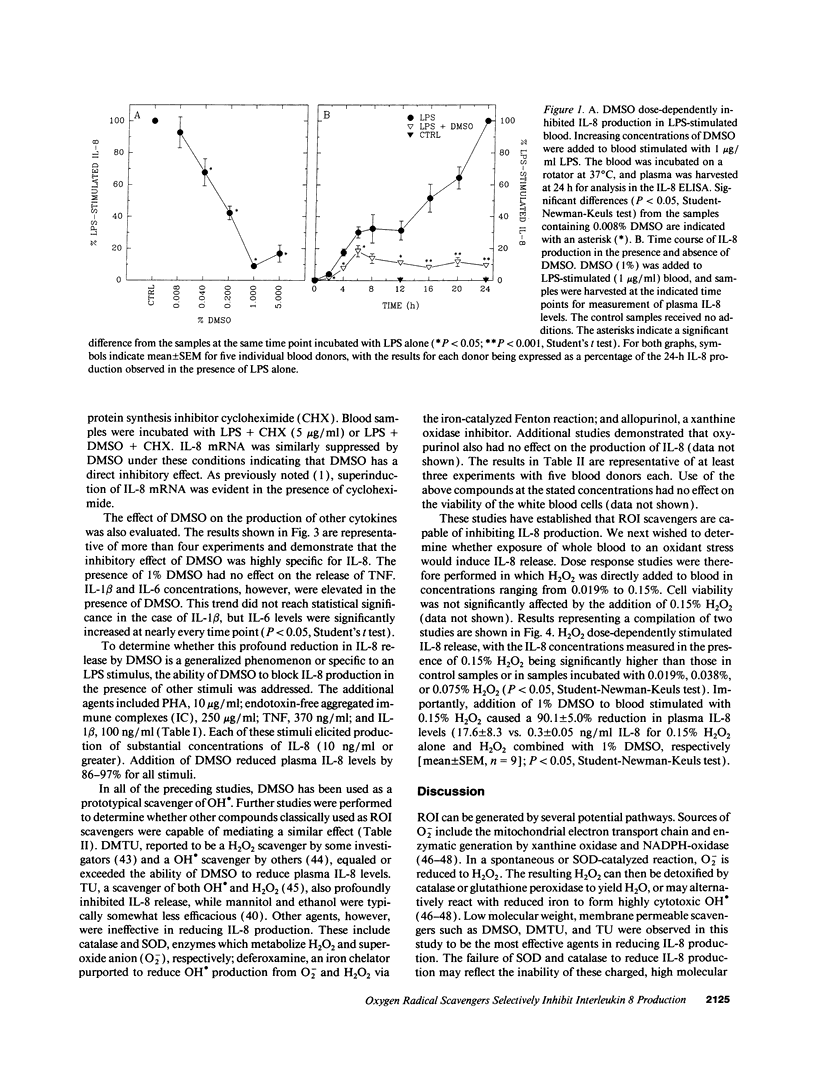
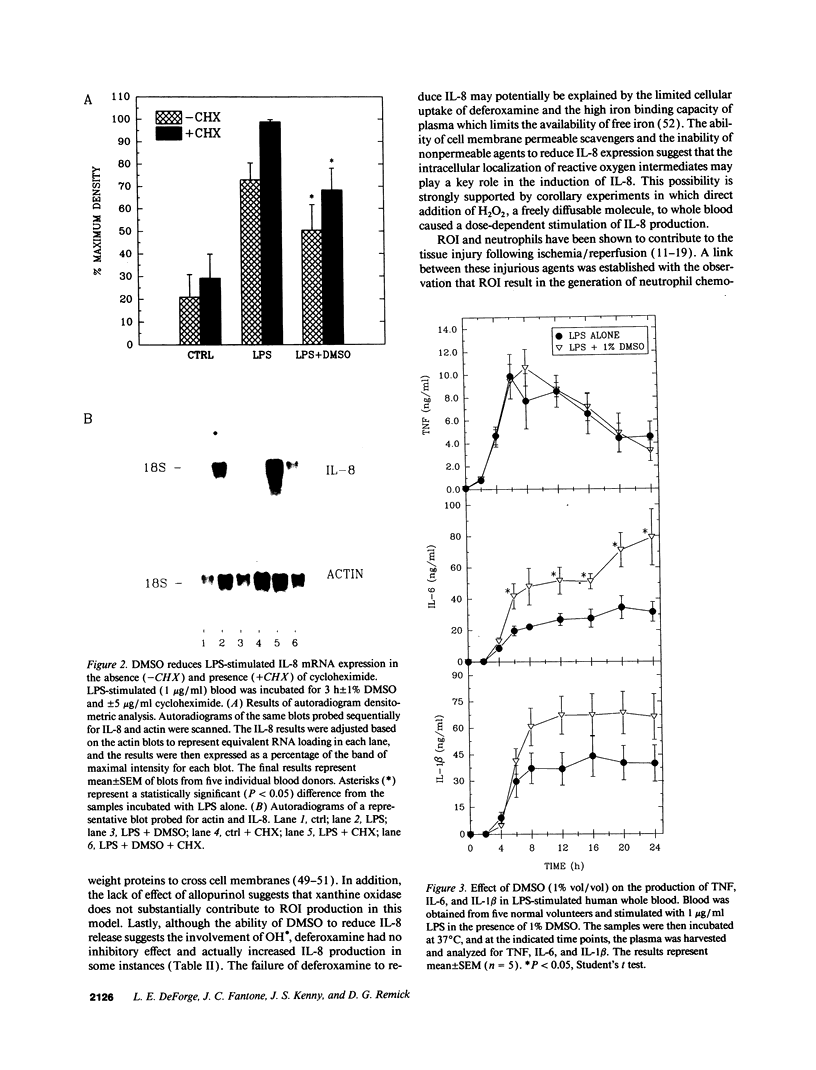
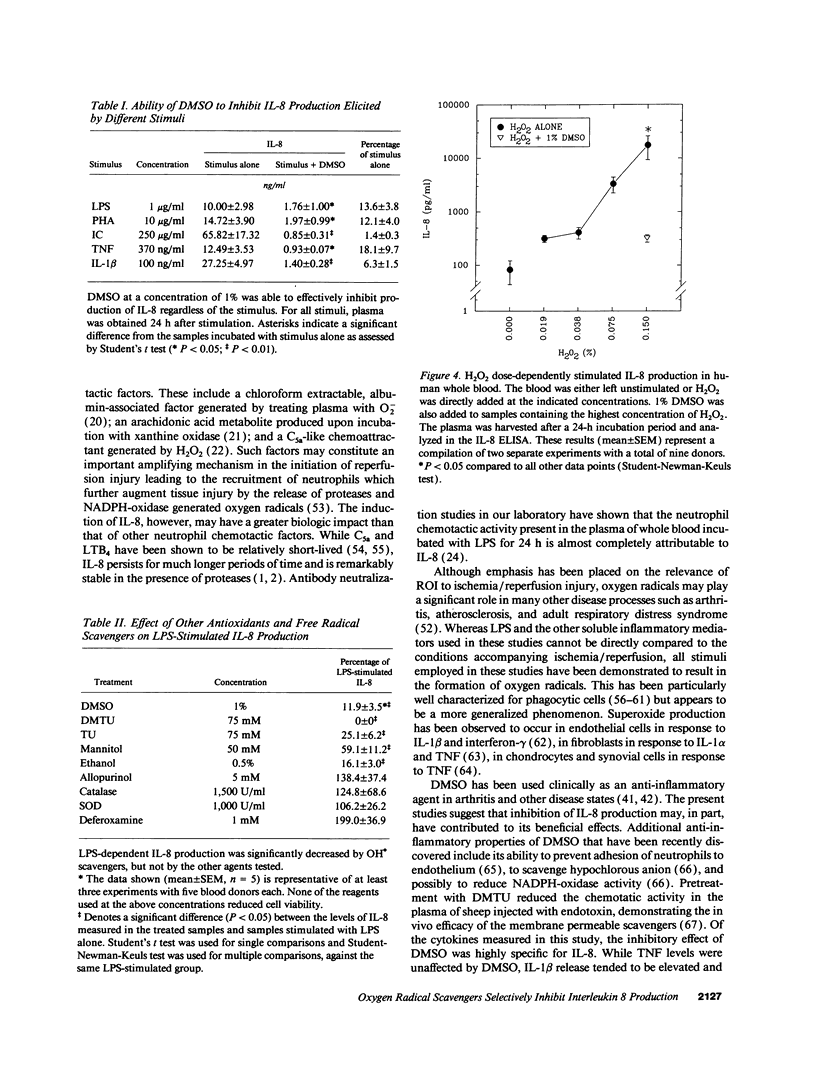
The hydroxyl radical (OH.) scavenger dimethyl sulfoxide (DMSO) was found to dose-dependently inhibit interleukin 8 (IL-8) production in LPS-stimulated human whole blood. At a concentration of 1% (vol/vol), DMSO blocked IL-8 release by approximately 90% in the presence of 1 microgram/ml LPS at a 24-h time point, but did not affect cell viability or reduce the production of tumor necrosis factor (TNF), interleukin 6, or interleukin-1 beta (IL-1 beta). DMSO was found to directly inhibit IL-8 expression at the level of transcription. Furthermore, this effect was not LPS-specific, in that IL-8 production was reduced by DMSO to a similar extent upon stimulation of blood with phytohemagglutinin, aggregated immune complexes, TNF, or IL-1 beta. Other oxygen radical scavengers that have been shown to inhibit OH.-dependent reactions (dimethyl thiourea, thiourea, mannitol, and ethanol) also inhibited IL-8 production. Conversely, addition of H2O2 caused a dose-dependent stimulation of IL-8 release. These results provide evidence that reactive oxygen metabolites play an important role in the regulation of IL-8 production and suggest that reduction of IL-8 release may contribute to the beneficial effects of antioxidants in experimental models of inflammation and ischemia/reperfusion injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh N., Shingu M., Nobunaga M. The effect of recombinant tumor necrosis factor-alpha on superoxide and metalloproteinase production by synovial cells and chondrocytes. Clin Exp Rheumatol. 1990 Jul-Aug;8(4):387–391. [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista A. P., Schuler A., Spolarics Z., Spitzer J. J. Tumor necrosis factor-alpha stimulates superoxide anion generation by perfused rat liver and Kupffer cells. Am J Physiol. 1991 Dec;261(6 Pt 1):G891–G895. doi: 10.1152/ajpgi.1991.261.6.G891. [DOI] [PubMed] [Google Scholar]
- Bautista A. P., Spitzer J. J. Superoxide anion generation by in situ perfused rat liver: effect of in vivo endotoxin. Am J Physiol. 1990 Dec;259(6 Pt 1):G907–G912. doi: 10.1152/ajpgi.1990.259.6.G907. [DOI] [PubMed] [Google Scholar]
- Beilke M. A., Collins-Lech C., Sohnle P. G. Effects of dimethyl sulfoxide on the oxidative function of human neutrophils. J Lab Clin Med. 1987 Jul;110(1):91–96. [PubMed] [Google Scholar]
- Brennan F. M., Zachariae C. O., Chantry D., Larsen C. G., Turner M., Maini R. N., Matsushima K., Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990 Sep;20(9):2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Serody J. S., Hayek M. B., Charniga L. M., Cohen M. S. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J Immunol. 1991 Dec 15;147(12):4271–4277. [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Rubin E., Cohen G. Effect of thiourea on microsomal oxidation of alcohols and associated microsomal functions. Biochemistry. 1979 Apr 3;18(7):1187–1191. doi: 10.1021/bi00574a011. [DOI] [PubMed] [Google Scholar]
- Collins P. D., Jose P. J., Williams T. J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991 Jan 15;146(2):677–684. [PubMed] [Google Scholar]
- DeForge L. E., Kenney J. S., Jones M. L., Warren J. S., Remick D. G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992 Apr 1;148(7):2133–2141. [PubMed] [Google Scholar]
- DeForge L. E., Remick D. G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991 Jan 15;174(1):18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Remick D. G. Sandwich ELISA for detection of picogram quantities of interleukin-8. Immunol Invest. 1991 Feb;20(1):89–97. doi: 10.3109/08820139109054928. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Tracey D. E., Kenney J. S., Remick D. G. Interleukin-1 receptor antagonist protein inhibits interleukin-8 expression in lipopolysaccharide-stimulated human whole blood. Am J Pathol. 1992 May;140(5):1045–1054. [PMC free article] [PubMed] [Google Scholar]
- Desch C. E., Kovach N. L., Present W., Broyles C., Harlan J. M. Production of human tumor necrosis factor from whole blood ex vivo. Lymphokine Res. 1989 Summer;8(2):141–146. [PubMed] [Google Scholar]
- Eskandari M. K., Nguyen D. T., Kunkel S. L., Remick D. G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest. 1990 Feb;19(1):69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Ginsburg I., Ward P. A., Varani J. Lysophosphatides enhance superoxide responses of stimulated human neutrophils. Inflammation. 1989 Apr;13(2):163–174. doi: 10.1007/BF00924787. [DOI] [PubMed] [Google Scholar]
- Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988 Dec;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991 Sep 30;91(3C):14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hofsli E., Lamvik J., Nissen-Meyer J. Evidence that tumour necrosis factor (TNF) is not constitutively present in vivo. The association of TNF with freshly isolated monocytes reflects a rapid in vitro production. Scand J Immunol. 1988 Oct;28(4):435–441. doi: 10.1111/j.1365-3083.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Jacob S. W., Herschler R. Pharmacology of DMSO. Cryobiology. 1986 Feb;23(1):14–27. doi: 10.1016/0011-2240(86)90014-3. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991 Mar;260(3 Pt 1):G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A., Smith C. W. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990 Dec;4(15):3355–3359. [PubMed] [Google Scholar]
- Kenney J. S., Masada M. P., Eugui E. M., Delustro B. M., Mulkins M. A., Allison A. C. Monoclonal antibodies to human recombinant interleukin 1 (IL 1)beta: quantitation of IL 1 beta and inhibition of biological activity. J Immunol. 1987 Jun 15;138(12):4236–4242. [PubMed] [Google Scholar]
- Linas S. L., Whittenburg D., Repine J. E. Role of xanthine oxidase in ischemia/reperfusion injury. Am J Physiol. 1990 Mar;258(3 Pt 2):F711–F716. doi: 10.1152/ajprenal.1990.258.3.F711. [DOI] [PubMed] [Google Scholar]
- Martich G. D., Danner R. L., Ceska M., Suffredini A. F. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991 Apr 1;173(4):1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Puget K. Cell penetration by exogenous superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:67–80. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Karabin G. D., Barker J. N., Griffiths C. E., Sarma V., Mitra R. S., Elder J. T., Kunkel S. L., Dixit V. M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991 Jan;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Palder S. B., Wong C., Hood I., Wenger H., Mannick J. A., Demling R. H. Chemotactic activity of plasma and lung lymph in sheep with endotoxemia: effect of hydroxyl radical scavengers. J Surg Res. 1985 Feb;38(2):162–172. doi: 10.1016/0022-4804(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Parker N. B., Berger E. M., Curtis W. E., Muldrow M. E., Linas S. L., Repine J. E. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes dimethyl sulfoxide consumption in vitro. J Free Radic Biol Med. 1985;1(5-6):415–419. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Weksler B. B., Goldstein I. M. Generation of a chemotactic lipid from a arachidonic acid by exposure to a superoxide-generating system. Inflammation. 1980 Sep;4(3):313–328. doi: 10.1007/BF00915032. [DOI] [PubMed] [Google Scholar]
- Petkau A., Kelly K., Chelack W. S., Barefoot C. Protective effect of superoxide dismustase on erythrocytes of x-irradiated mice. Biochem Biophys Res Commun. 1976 May 17;70(2):452–458. doi: 10.1016/0006-291x(76)91067-6. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J. A., Sylvester I., Smith S., Yoshimura T., Leonard E. J. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J Clin Invest. 1990 Nov;86(5):1556–1564. doi: 10.1172/JCI114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick D. G., DeForge L. E., Sullivan J. F., Showell H. J. Profile of cytokines in synovial fluid specimens from patients with arthritis. Interleukin 8 (IL-8) and IL-6 correlate with inflammatory arthritides. Immunol Invest. 1992 Jul;21(4):321–327. doi: 10.3109/08820139209069371. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. L., Miller C. G., DeForge L. E., Kelty L., Shanley C. J., Bartlett R. H., Remick D. G. Local production of interleukin-8 is associated with nosocomial pneumonia. J Trauma. 1992 Jul;33(1):74–82. doi: 10.1097/00005373-199207000-00015. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Sekizuka E., Benoit J. N., Grisham M. B., Granger D. N. Dimethylsulfoxide prevents chemoattractant-induced leukocyte adherence. Am J Physiol. 1989 Feb;256(2 Pt 2):H594–H597. doi: 10.1152/ajpheart.1989.256.2.H594. [DOI] [PubMed] [Google Scholar]
- Shingu M., Nobunaga M. Chemotactic activity generated in human serum from the fifth component of complement by hydrogen peroxide. Am J Pathol. 1984 Nov;117(2):201–206. [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Lucchesi B. R. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987 Jul;110(1):13–30. [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ham J. M., Colletti L. M., Lynch J. P., 3rd, Kunkel S. L. Tumor necrosis factor-alpha gene expression in human whole blood. J Leukoc Biol. 1990 Apr;47(4):366–370. doi: 10.1002/jlb.47.4.366. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trice J. M., Pinals R. S. Dimethyl sulfoxide: a review of its use in the rheumatic disorders. Semin Arthritis Rheum. 1985 Aug;15(1):45–60. doi: 10.1016/0049-0172(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., DeForge L. E., Fischer E., Marano M. A., Kenney J. S., Remick D. G., Lowry S. F., Moldawer L. L. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991 May 15;146(10):3478–3482. [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Cunningham T. W., Johnson K. J., Ward P. A. Macrophage-derived cytokines amplify immune complex-triggered O2-. responses by rat alveolar macrophages. Am J Pathol. 1988 Mar;130(3):489–495. [PMC free article] [PubMed] [Google Scholar]
- Wharram B. L., Fitting K., Kunkel S. L., Remick D. G., Merritt S. E., Wiggins R. C. Tissue factor expression in endothelial cell/monocyte cocultures stimulated by lipopolysaccharide and/or aggregated IgG. Mechanisms of cell:cell communication. J Immunol. 1991 Mar 1;146(5):1437–1445. [PubMed] [Google Scholar]
- Zimmerman B. J., Grisham M. B., Granger D. N. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am J Physiol. 1990 Feb;258(2 Pt 1):G185–G190. doi: 10.1152/ajpgi.1990.258.2.G185. [DOI] [PubMed] [Google Scholar]