Abstract
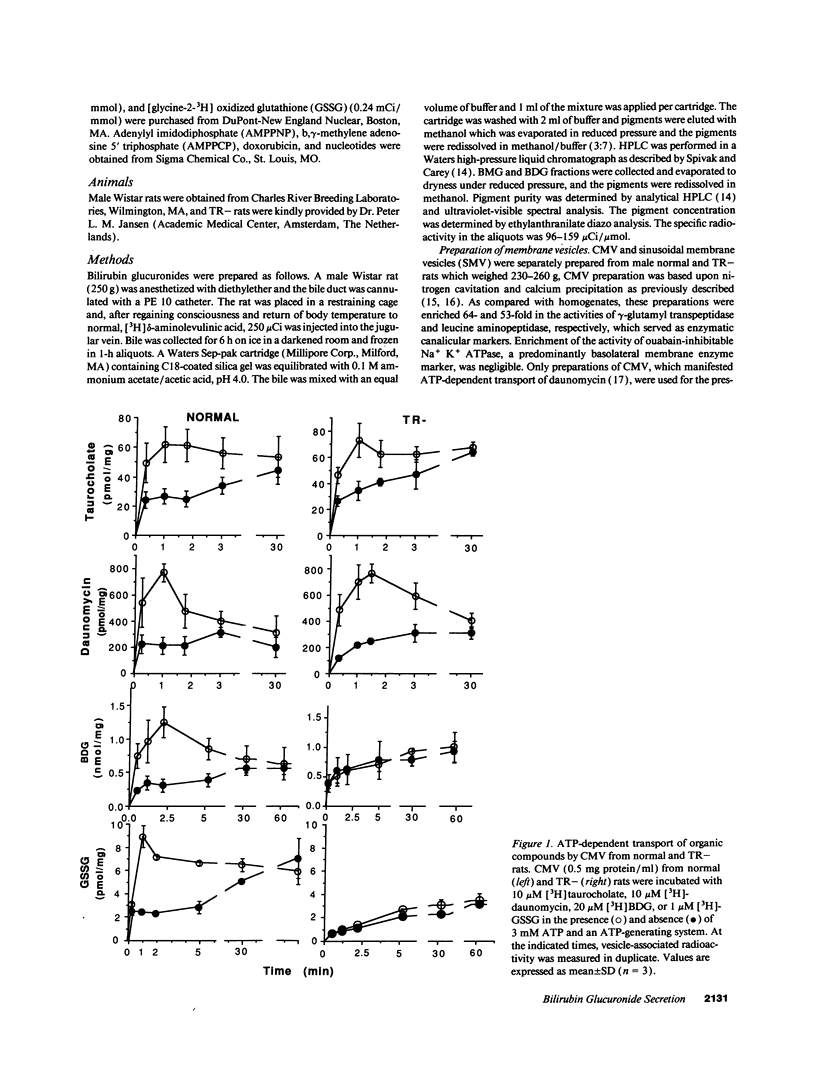
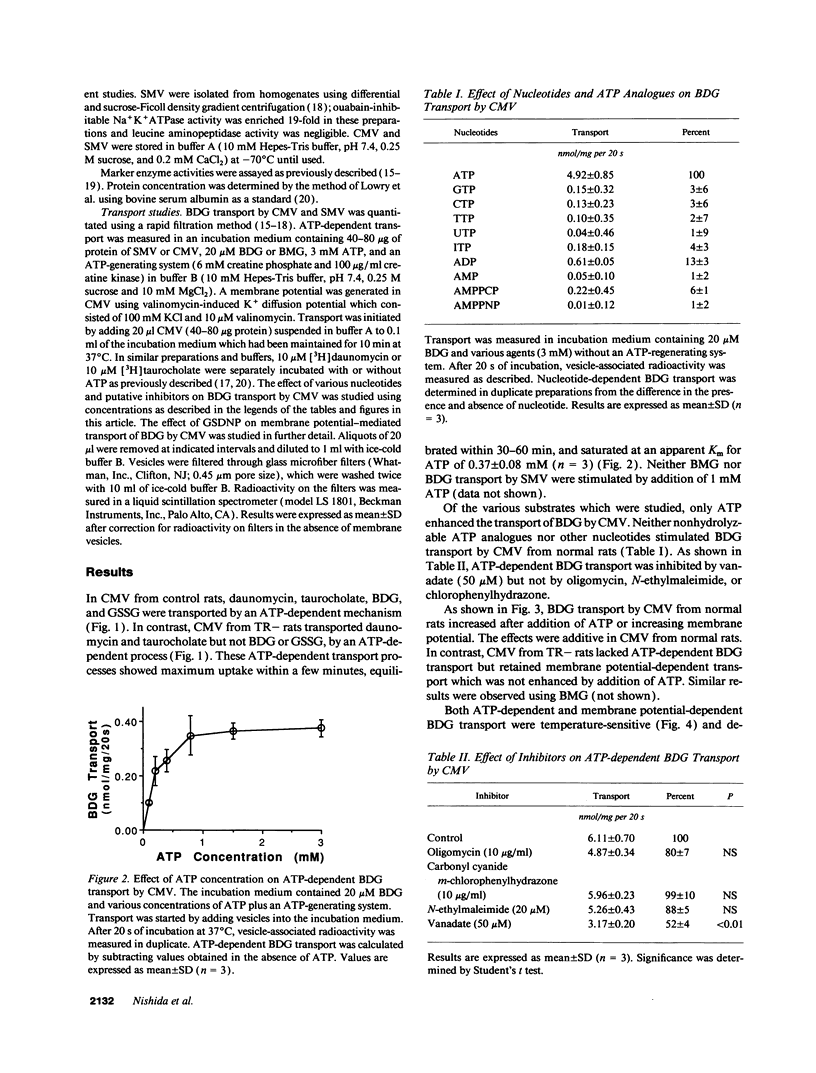
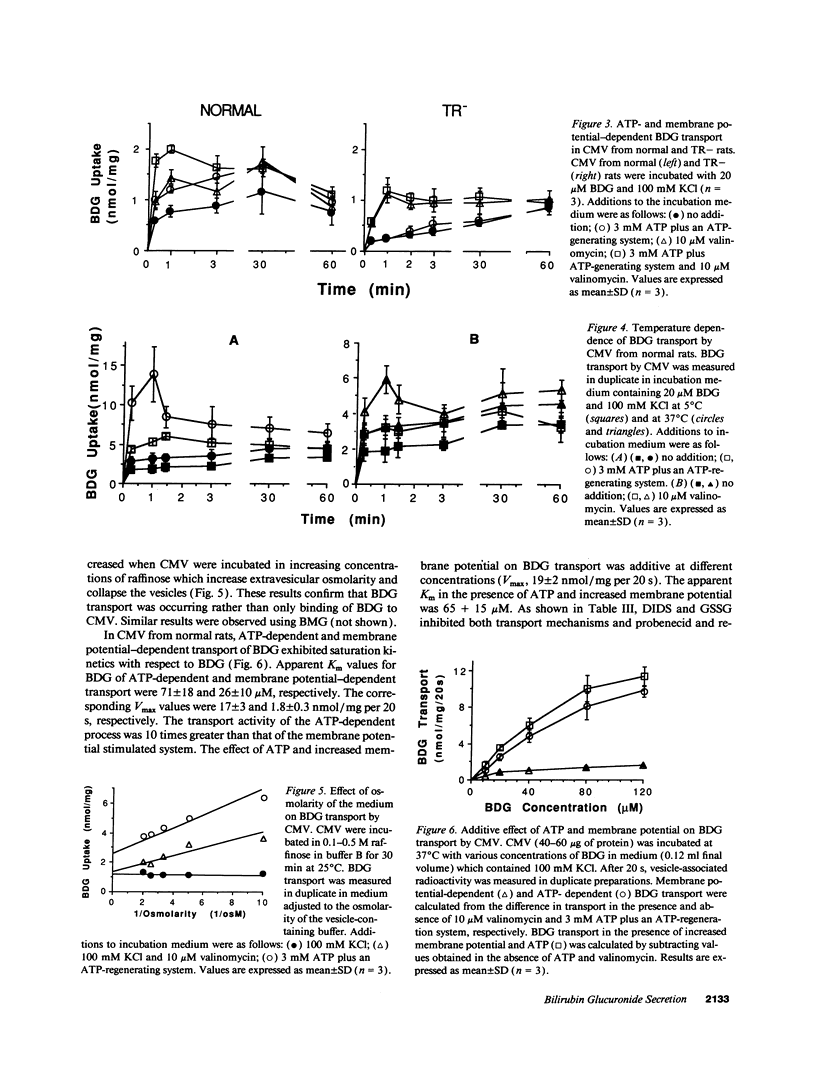
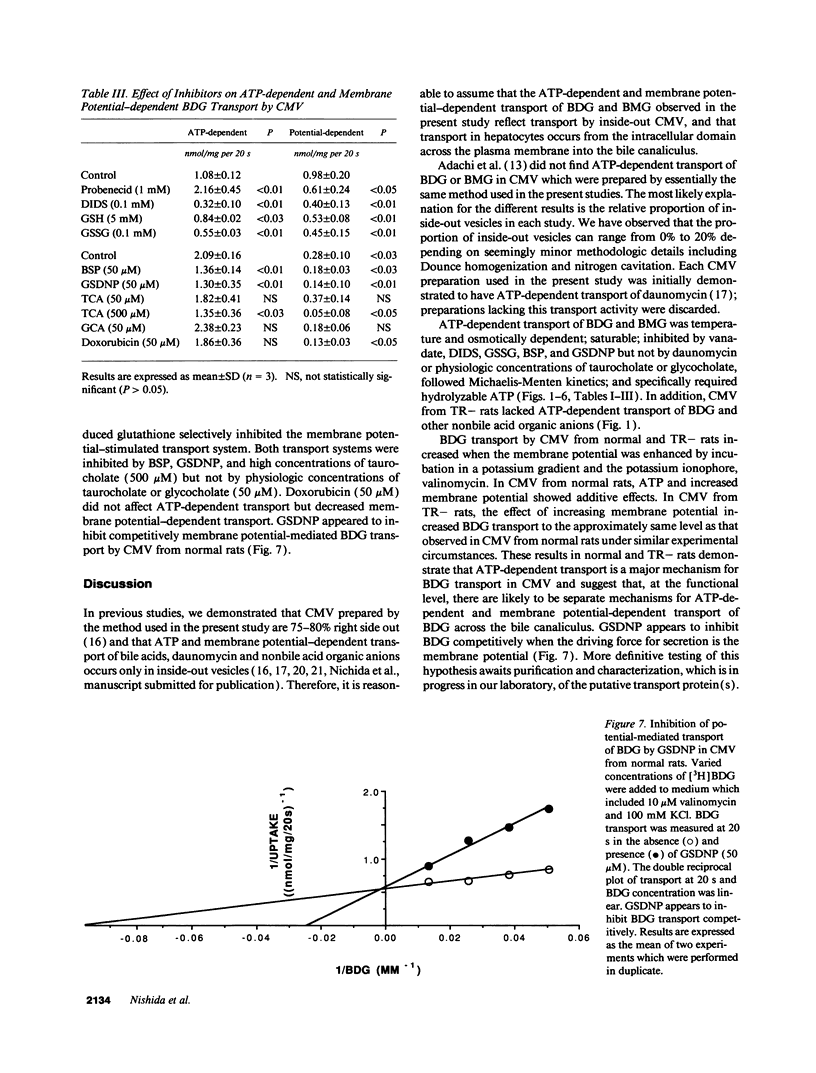
Bilirubin is conjugated with glucuronic acid in hepatocytes and subsequently secreted in bile. The major conjugate is bilirubin diglucuronide. Using sealed vesicles which are primarily derived from the canalicular (CMV) and sinusoidal (SMV) membrane vesicle domains of the plasma membrane of hepatocytes, we demonstrated that bilirubin glucuronides are transported by CMV by both ATP- and membrane potential-dependent transport systems. In CMV from normal rats, these processes are additive. In CMV from TR- rats, which have an autosomal recessively inherited defect in biliary secretion of nonbile acid organic anions, ATP-dependent transport of bilirubin diglucuronide was absent whereas the membrane potential driven system was retained. Other canalicular ATP-dependent transport systems, which were previously described for organic cations and bile acids, are functionally retained in TR- rats. Our study indicates that bilirubin glucuronides are primarily secreted into the bile canaliculus by an ATP-dependent mechanism which is defective in an animal model of the human Dubin-Johnson syndrome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS I. M., JOHNSON L., WOLFSON S. Biliary excretion of injected conjugated and unconjugated bilirubin by normal and Gunn rats. Am J Physiol. 1961 May;200:1091–1094. doi: 10.1152/ajplegacy.1961.200.5.1091. [DOI] [PubMed] [Google Scholar]
- Adachi Y., Kobayashi H., Kurumi Y., Shouji M., Kitano M., Yamamoto T. Bilirubin diglucuronide transport by rat liver canalicular membrane vesicles: stimulation by bicarbonate ion. Hepatology. 1991 Dec;14(6):1251–1258. [PubMed] [Google Scholar]
- Alpert S., Mosher M., Shanske A., Arias I. M. Multiplicity of hepatic excretory mechanisms for organic anions. J Gen Physiol. 1969 Feb;53(2):238–247. doi: 10.1085/jgp.53.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN I. N. Chronic idiopathic jaundice; a review of fifty cases. Am J Med. 1958 Feb;24(2):268–292. doi: 10.1016/0002-9343(58)90315-2. [DOI] [PubMed] [Google Scholar]
- Huber M., Guhlmann A., Jansen P. L., Keppler D. Hereditary defect of hepatobiliary cysteinyl leukotriene elimination in mutant rats with defective hepatic anion excretion. Hepatology. 1987 Mar-Apr;7(2):224–228. doi: 10.1002/hep.1840070204. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver sinusoidal membrane vesicles: evidence of sodium cotransport. Hepatology. 1982 Sep-Oct;2(5):572–579. doi: 10.1002/hep.1840020510. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. The mechanism of biliary secretion of reduced glutathione. Analysis of transport process in isolated rat-liver canalicular membrane vesicles. Eur J Biochem. 1983 Aug 15;134(3):467–471. doi: 10.1111/j.1432-1033.1983.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Lamers W. H. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985 Jul-Aug;5(4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Kitamura T., Jansen P., Hardenbrook C., Kamimoto Y., Gatmaitan Z., Arias I. M. Defective ATP-dependent bile canalicular transport of organic anions in mutant (TR-) rats with conjugated hyperbilirubinemia. Proc Natl Acad Sci U S A. 1990 May;87(9):3557–3561. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klünemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991 Oct 5;266(28):18920–18926. [PubMed] [Google Scholar]
- Nishida T., Gatmaitan Z., Che M., Arias I. M. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Hardenbrook C., Gatmaitan Z., Arias I. M. ATP-dependent organic anion transport system in normal and TR- rat liver canalicular membranes. Am J Physiol. 1992 Apr;262(4 Pt 1):G629–G635. doi: 10.1152/ajpgi.1992.262.4.G629. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Ottenhoff R., Liefting W. G., Schoemaker B., Groen A. K., Jansen P. L. ATP-dependent efflux of GSSG and GS-conjugate from isolated rat hepatocytes. Am J Physiol. 1990 May;258(5 Pt 1):G699–G706. doi: 10.1152/ajpgi.1990.258.5.G699. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., de Haan J., Lambert K. J., Hagey L. R., Hofmann A. F., Jansen P. L. Selective hepatobiliary transport of nordeoxycholate side chain conjugates in mutant rats with a canalicular transport defect. Hepatology. 1989 Jun;9(6):861–865. doi: 10.1002/hep.1840090612. [DOI] [PubMed] [Google Scholar]
- Spivak W., Carey M. C. Reverse-phase h.p.l.c. separation, quantification and preparation of bilirubin and its conjugates from native bile. Quantitative analysis of the intact tetrapyrroles based on h.p.l.c. of their ethyl anthranilate azo derivatives. Biochem J. 1985 Feb 1;225(3):787–805. doi: 10.1042/bj2250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Cohen L. E., Arias I. M. Inheritance of the Dubin-Johnson syndrome. N Engl J Med. 1973 Jan 18;288(3):113–117. doi: 10.1056/NEJM197301182880301. [DOI] [PubMed] [Google Scholar]



