Abstract
A cDNA corresponding to a thiol-specific antioxidant enzyme (TSA) was isolated from a rat brain cDNA library with the use of antibodies to bovine TSA. The cDNA clone encoded an open reading frame capable of encoding a 198-residue polypeptide. The rat and yeast TSA proteins show significant sequence homology to the 21-kDa component (AhpC) of Salmonella typhimurium alkyl hydroperoxide reductase, and we have found that AhpC exhibits TSA activity. AhpC and TSA define a family of > 25 different proteins present in organisms from all kingdoms. The similarity among the family members extends over the entire sequence and ranges between 23% and 98% identity. A majority of the members of the AhpC/TSA family contain two conserved cysteines. At least eight of the genes encoding AhpC/TSA-like polypeptides are found in proximity to genes encoding other oxidoreductase activities, and the expression of several of the homologs has been correlated with pathogenicity. We suggest that the AhpC/TSA family represents a widely distributed class of antioxidant enzymes. We also report that a second family of proteins, defined by the 57-kDa component (AhpF) of alkyl hydroperoxide reductase and by thioredoxin reductase, has expanded to include six additional members.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kelley J. M., Gocayne J. D., Dubnick M., Polymeropoulos M. H., Xiao H., Merril C. R., Wu A., Olde B., Moreno R. F. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991 Jun 21;252(5013):1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Harrison P. M., Guest J. R. A molecular analysis of the 53.3 minute region of the Escherichia coli linkage map. J Gen Microbiol. 1991 Feb;137(2):361–367. doi: 10.1099/00221287-137-2-361. [DOI] [PubMed] [Google Scholar]
- Chae H. Z., Kim I. H., Kim K., Rhee S. G. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993 Aug 5;268(22):16815–16821. [PubMed] [Google Scholar]
- Chae H. Z., Uhm T. B., Rhee S. G. Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7022–7026. doi: 10.1073/pnas.91.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive alignment and phylogenetic tree construction of protein sequences. Methods Enzymol. 1990;183:375–387. doi: 10.1016/0076-6879(90)83025-5. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Curry J., Morris C. F., Walker-Simmons M. K. Cloning and expression of an embryo-specific mRNA up-regulated in hydrated dormant seeds. Plant Mol Biol. 1992 Jun;19(3):433–441. doi: 10.1007/BF00023391. [DOI] [PubMed] [Google Scholar]
- Goodman H. J., Woods D. R. Molecular analysis of the Bacteroides fragilis recA gene. Gene. 1990 Sep 28;94(1):77–82. doi: 10.1016/0378-1119(90)90470-c. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988 Aug;7(8):2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Yamada M., Sato H., Matsue M., Taketani S., Nakayama K., Sugita Y., Bannai S. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem. 1993 Sep 5;268(25):18633–18636. [PubMed] [Google Scholar]
- Jacobson F. S., Morgan R. W., Christman M. F., Ames B. N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989 Jan 25;264(3):1488–1496. [PubMed] [Google Scholar]
- Kim I. H., Kim K., Rhee S. G. Induction of an antioxidant protein of Saccharomyces cerevisiae by O2, Fe3+, or 2-mercaptoethanol. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6018–6022. doi: 10.1073/pnas.86.16.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Rhee S. G. Sequence of peptides from Saccharomyces cerevisiae glutamine synthetase. N-terminal peptide and ATP-binding domain. J Biol Chem. 1988 Jan 15;263(2):833–838. [PubMed] [Google Scholar]
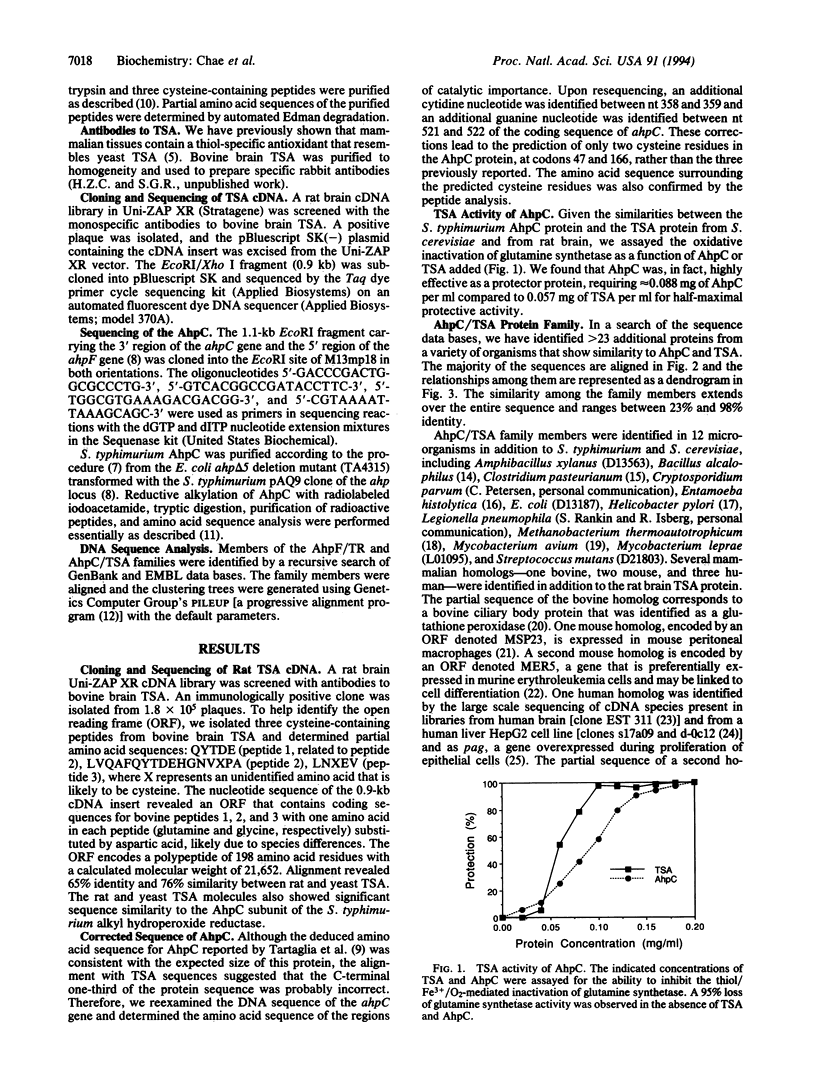
- Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. The isolation and purification of a specific "protector" protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988 Apr 5;263(10):4704–4711. [PubMed] [Google Scholar]
- Lim Y. S., Cha M. K., Kim H. K., Uhm T. B., Park J. W., Kim K., Kim I. H. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993 Apr 15;192(1):273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- Mathieu I., Meyer J., Moulis J. M. Cloning, sequencing and expression in Escherichia coli of the rubredoxin gene from Clostridium pasteurianum. Biochem J. 1992 Jul 1;285(Pt 1):255–262. doi: 10.1042/bj2850255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole P. W., Logan S. M., Kostrzynska M., Wadström T., Trust T. J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991 Jan;173(2):505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo K., Hori N., Matoba R., Niiyama T., Fukushima A., Kojima Y., Matsubara K. Large scale cDNA sequencing for analysis of quantitative and qualitative aspects of gene expression. Nat Genet. 1992 Nov;2(3):173–179. doi: 10.1038/ng1192-173. [DOI] [PubMed] [Google Scholar]
- Poole L. B., Claiborne A. The non-flavin redox center of the streptococcal NADH peroxidase. I. Thiol reactivity and redox behavior in the presence of urea. J Biol Chem. 1989 Jul 25;264(21):12322–12329. [PubMed] [Google Scholar]
- Proenca R., Niu W. W., Cacalano G., Prince A. The Pseudomonas cepacia 249 chromosomal penicillinase is a member of the AmpC family of chromosomal beta-lactamases. Antimicrob Agents Chemother. 1993 Apr;37(4):667–674. doi: 10.1128/aac.37.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospéri M. T., Ferbus D., Karczinski I., Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993 May 25;268(15):11050–11056. [PubMed] [Google Scholar]
- Ross R. P., Claiborne A. Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J Mol Biol. 1992 Oct 5;227(3):658–671. doi: 10.1016/0022-2836(92)90215-6. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- Shau H., Kim A. Identification of natural killer enhancing factor as a major antioxidant in human red blood cells. Biochem Biophys Res Commun. 1994 Feb 28;199(1):83–88. doi: 10.1006/bbrc.1994.1197. [DOI] [PubMed] [Google Scholar]
- Shichi H., Demar J. C. Non-selenium glutathione peroxidase without glutathione S-transferase activity from bovine ciliary body. Exp Eye Res. 1990 May;50(5):513–520. doi: 10.1016/0014-4835(90)90040-2. [DOI] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993 Jul 15;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Storz G., Christman M. F., Sies H., Ames B. N. Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8917–8921. doi: 10.1073/pnas.84.24.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Jacobson F. S., Tartaglia L. A., Morgan R. W., Silveira L. A., Ames B. N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989 Apr;171(4):2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M., Oikawa A., Yasui A. Characterization of a superoxide dismutase gene from the archaebacterium Methanobacterium thermoautotrophicum. Arch Biochem Biophys. 1990 Nov 15;283(1):210–216. doi: 10.1016/0003-9861(90)90633-a. [DOI] [PubMed] [Google Scholar]
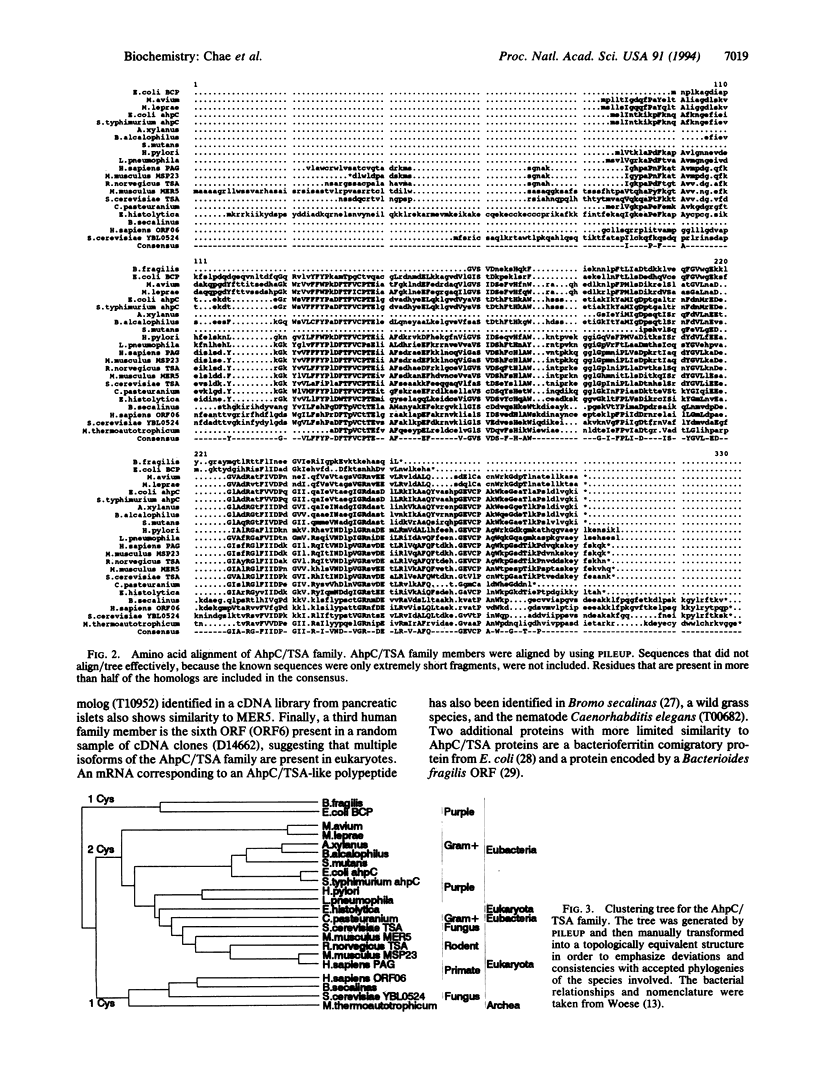
- Tartaglia L. A., Storz G., Brodsky M. H., Lai A., Ames B. N. Alkyl hydroperoxide reductase from Salmonella typhimurium. Sequence and homology to thioredoxin reductase and other flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Jun 25;265(18):10535–10540. [PubMed] [Google Scholar]
- Torian B. E., Flores B. M., Stroeher V. L., Hagen F. S., Stamm W. E. cDNA sequence analysis of a 29-kDa cysteine-rich surface antigen of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6358–6362. doi: 10.1073/pnas.87.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. M., Kanaya S., Koyama N., Sekiguchi T., Nosoh Y., Ohashi S., Tsuda K. Tryptic digestion of NADH dehydrogenase from alkalophilic Bacillus. J Biochem. 1989 Apr;105(4):626–632. doi: 10.1093/oxfordjournals.jbchem.a122715. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Koyama N., Cui M., Yamagishi A., Nosoh Y., Oshima T. Nucleotide sequence of the gene encoding NADH dehydrogenase from an alkalophile, Bacillus sp. strain YN-1. J Biochem. 1991 May;109(5):678–683. doi: 10.1093/oxfordjournals.jbchem.a123440. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Takahashi M., Fukasawa Y., Wada M., Abe C. Cloning and expression of the gene for the Avi-3 antigen of Mycobacterium avium and mapping of its epitopes. Infect Immun. 1992 Mar;60(3):1210–1216. doi: 10.1128/iai.60.3.1210-1216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Matsui Y., Natori S., Obinata M. Cloning of a housekeeping-type gene (MER5) preferentially expressed in murine erythroleukemia cells. Gene. 1989 Aug 15;80(2):337–343. doi: 10.1016/0378-1119(89)90297-7. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chae H. Z., Rhee S. G., Chock P. B., Stadtman E. R. On the protective mechanism of the thiol-specific antioxidant enzyme against the oxidative damage of biomacromolecules. J Biol Chem. 1994 Jan 21;269(3):1621–1626. [PubMed] [Google Scholar]



