Abstract
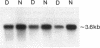
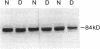
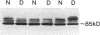
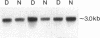
In patients with non-insulin-dependent diabetes mellitus (NIDDM) and matched control subjects we examined the interrelationships between in vivo nonoxidative glucose metabolism and glucose oxidation and the muscle activities, as well as the immunoreactive protein and mRNA levels of the rate-limiting enzymes in glycogen synthesis and glycolysis, glycogen synthase (GS) and phosphofructokinase (PFK), respectively. Analysis of biopsies of quadriceps muscle from 19 NIDDM patients and 19 control subjects showed in the basal state a 30% decrease (P < 0.005) in total GS activity and a 38% decrease (P < 0.001) in GS mRNA/microgram DNA in NIDDM patients, whereas the GS protein level was normal. The enzymatic activity and protein and mRNA levels of PFK were all normal in diabetic patients. In subgroups of NIDDM patients and control subjects an insulin-glucose clamp in combination with indirect calorimetry was performed. The rate of insulin-stimulated nonoxidative glucose metabolism was decreased by 47% (P < 0.005) in NIDDM patients, whereas the glucose oxidation rate was normal. The PFK activity, protein level, and mRNA/microgram DNA remained unchanged. The relative activation of GS by glucose-6-phosphate was 33% lower (P < 0.02), whereas GS mRNA/micrograms DNA was 37% lower (P < 0.05) in the diabetic patients after 4 h of hyperinsulinemia. Total GS immunoreactive mass remained normal. In conclusion, qualitative but not quantitative posttranslational abnormalities of the GS protein in muscle determine the reduced insulin-stimulated nonoxidative glucose metabolism in NIDDM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson K., Galuska D., Thörne A., Sonnenfeld T., Wallberg-Henriksson H. Decreased insulin-stimulated 3-0-methylglucose transport in in vitro incubated muscle strips from type II diabetic subjects. Acta Physiol Scand. 1991 Jun;142(2):255–260. doi: 10.1111/j.1748-1716.1991.tb09154.x. [DOI] [PubMed] [Google Scholar]
- Bak J. F., Pedersen O. Exercise-enhanced activation of glycogen synthase in human skeletal muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):E957–E963. doi: 10.1152/ajpendo.1990.258.6.E957. [DOI] [PubMed] [Google Scholar]
- Browner M. F., Nakano K., Bang A. G., Fletterick R. J. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1443–1447. doi: 10.1073/pnas.86.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Damsbo P., Vaag A., Hother-Nielsen O., Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Apr;34(4):239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Camici M., Lawrence J. C., Jr, Roach P. J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Evidence for interactions among phosphorylation sites and the resolution of electrophoretically distinct forms of the subunit. J Biol Chem. 1983 Sep 10;258(17):10702–10709. [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Falholt K., Jensen I., Lindkaer Jensen S., Mortensen H., Vølund A., Heding L. G., Noerskov Petersen P., Falholt W. Carbohydrate and lipid metabolism of skeletal muscle in type 2 diabetic patients. Diabet Med. 1988 Jan;5(1):27–31. doi: 10.1111/j.1464-5491.1988.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Wallace P., Brechtel G., Olefsky J. M. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metabolism. 1992 Aug;41(8):897–902. doi: 10.1016/0026-0495(92)90174-9. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O., Schmitz O., Bak J., Beck-Nielsen H. Enhanced hepatic insulin sensitivity, but peripheral insulin resistance in patients with type 1 (insulin-dependent) diabetes. Diabetologia. 1987 Nov;30(11):834–840. doi: 10.1007/BF00274790. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Ingebritsen T. S., Stewart A. A., Cohen P. The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. Eur J Biochem. 1983 May 2;132(2):297–307. doi: 10.1111/j.1432-1033.1983.tb07362.x. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Johnson A. B., Argyraki M., Thow J. C., Broughton D., Jones I. R., Taylor R. Effects of intensive dietary treatment on insulin-stimulated skeletal muscle glycogen synthase activation and insulin secretion in newly presenting type 2 diabetic patients. Diabet Med. 1990 Jun;7(5):420–428. doi: 10.1111/j.1464-5491.1990.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. B., Argyraki M., Thow J. C., Jones I. R., Broughton D., Miller M., Taylor R. Impaired activation of skeletal muscle glycogen synthase in non-insulin-dependent diabetes mellitus is unrelated to the degree of obesity. Metabolism. 1991 Mar;40(3):252–260. doi: 10.1016/0026-0495(91)90106-7. [DOI] [PubMed] [Google Scholar]
- Kaslow H. R., Lesikar D. D. Isozymes of glycogen synthase. FEBS Lett. 1984 Jul 9;172(2):294–298. doi: 10.1016/0014-5793(84)81144-8. [DOI] [PubMed] [Google Scholar]
- Katz A., Spencer M. K., Lillioja S., Yan Z., Mott D. M., Haller R. G., Lewis S. F. Basal and insulin-mediated carbohydrate metabolism in human muscle deficient in phosphofructokinase 1. Am J Physiol. 1991 Oct;261(4 Pt 1):E473–E478. doi: 10.1152/ajpendo.1991.261.4.E473. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan R. G., Lamb D. R., Lutz S. A., Perrill C. V., Reimann E. M., Schlender K. K. Glycogen synthase activation in human skeletal muscle: effects of diet and exercise. Am J Physiol. 1979 Jun;236(6):E660–E666. doi: 10.1152/ajpendo.1979.236.6.E660. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Consoli A., Kelley D. E., Reilly J. J., Nurjhan N. Fasting hyperglycemia normalizes oxidative and nonoxidative pathways of insulin-stimulated glucose metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990 Dec;71(6):1544–1551. doi: 10.1210/jcem-71-6-1544. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M. M., Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987 Feb 11;96(2):271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- Mortensen H. B. Quantitative determination of hemoglobin A1c by thin-layer isoelectric focusing. J Chromatogr. 1980 Jun 13;182(3-4):325–333. doi: 10.1016/s0378-4347(00)81481-4. [DOI] [PubMed] [Google Scholar]
- Okubo M., Bogardus C., Lillioja S., Mott D. M. Glucose-6-phosphate stimulation of human muscle glycogen synthase phosphatase. Metabolism. 1988 Dec;37(12):1171–1176. doi: 10.1016/0026-0495(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Roach P. J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990 Sep;4(12):2961–2968. [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Härkonen M., Groop L. C. Impaired activation of glycogen synthase in people at increased risk for developing NIDDM. Diabetes. 1992 May;41(5):598–604. doi: 10.2337/diab.41.5.598. [DOI] [PubMed] [Google Scholar]
- Sharma P. M., Reddy G. R., Babior B. M., McLachlan A. Alternative splicing of the transcript encoding the human muscle isoenzyme of phosphofructokinase. J Biol Chem. 1990 Jun 5;265(16):9006–9010. [PubMed] [Google Scholar]
- Sharma P. M., Reddy G. R., Vora S., Babior B. M., McLachlan A. Cloning and expression of a human muscle phosphofructokinase cDNA. Gene. 1989 Apr 15;77(1):177–183. doi: 10.1016/0378-1119(89)90372-7. [DOI] [PubMed] [Google Scholar]
- Sheorain V. S., Juhl H., Bass M., Soderling T. R. Effects of epinephrine, diabetes, and insulin on rabbit skeletal muscle glycogen synthase. Phosphorylation site occupancies. J Biol Chem. 1984 Jun 10;259(11):7024–7030. [PubMed] [Google Scholar]
- Sheorain V. S., Ramakrishna S., Benjamin W. B., Soderling T. R. Phosphorylation of sites 3 and 2 in rabbit skeletal muscle glycogen synthase by a multifunctional protein kinase (ATP-citrate lyase kinase). J Biol Chem. 1985 Oct 5;260(22):12287–12292. [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Bollen M., Mvumbi L. Control of glycogen synthesis in health and disease. Diabetes Metab Rev. 1987 Jan;3(1):127–161. doi: 10.1002/dmr.5610030107. [DOI] [PubMed] [Google Scholar]
- Strålfors P., Hiraga A., Cohen P. The protein phosphatases involved in cellular regulation. Purification and characterisation of the glycogen-bound form of protein phosphatase-1 from rabbit skeletal muscle. Eur J Biochem. 1985 Jun 3;149(2):295–303. doi: 10.1111/j.1432-1033.1985.tb08926.x. [DOI] [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Vaag A., Damsbo P., Hother-Nielsen O., Beck-Nielsen H. Hyperglycaemia compensates for the defects in insulin-mediated glucose metabolism and in the activation of glycogen synthase in the skeletal muscle of patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992 Jan;35(1):80–88. doi: 10.1007/BF00400856. [DOI] [PubMed] [Google Scholar]
- Vestergaard H., Bjørbaek C., Andersen P. H., Bak J. F., Pedersen O. Impaired expression of glycogen synthase mRNA in skeletal muscle of NIDDM patients. Diabetes. 1991 Dec;40(12):1740–1745. doi: 10.2337/diab.40.12.1740. [DOI] [PubMed] [Google Scholar]
- Vora S. Isozymes of human phosphofructokinase: biochemical and genetic aspects. Isozymes Curr Top Biol Med Res. 1983;11:3–23. [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]