Abstract
Objective
To assess home oxygen use among preterm infants, identify risk factors predicting home oxygen use, and quantify the extent of institutional variation in home oxygen use across NICUs.
Study design retrospective cohort analysis of surviving 23-31 week infants discharged home in 2009, using de-identified electronic medical record information from the Pediatrix Clinical Data Warehouse. Mixed-effects logistic regression quantified clinical risk factors and institutional variation affecting home oxygen use.
Results
8167 infants were identified. Home oxygen use varied by gestational age, from 59% of 23-24 week infants to 7% of 29-31 week infants. Other risk factors included small for gestational age, congenital anomalies, mechanical ventilation in the first 72 hours, fraction of inhaled oxygen >0.4 in the first 72 hours, and patent ductus arteriosus. After adjusting for clinical risk factors, there was still a 4-5 fold difference in institutions’ odds of home oxygen use.
Conclusions
Home oxygen use was common among infants of earlier gestational ages and those with more severe respiratory illness. Institutional variation accounted for 4-5 fold variation in home oxygen use. Families should be counseled regarding the likelihood of home oxygen use, and prospective research must identify optimal management strategies for high-risk infants.
Keywords: bronchopulmonary dysplasia, neonatology, prematurity
Premature infants have high rates of medical service utilization following neonatal intensive care unit (NICU) discharge. (1-2) One population of preterm infants with particularly high need for post-discharge services are patients with bronchopulmonary dysplasia (BPD), which affects 25-35% of infants with birth weights <1500g. (1,3)
Home oxygen therapy is an option to facilitate discharge of infants with BPD as an alternative to prolonged hospitalization. Published guidelines for home oxygen use generally recommend consideration of home oxygen for infants with oxygen saturations <92-95% in room air. (4-6) Despite these consensus statements, however, decision-making regarding use of this therapy is not straight-forward. Observational studies conducted prior to widespread surfactant use found that home oxygen therapy for infants with BPD improved growth, decreased work of breathing, and decreased incidence of sudden infant death syndrome.(7-9) The BOOST and STOP-ROP trials, which compared randomized oxygen saturation targets for infants in the NICU, did not find improved growth at higher oxygen saturations; the STOP-ROP trial additionally found an increased risk of pneumonia and chronic lung disease-related events in infants with higher oxygen saturation targets.(10-11) The SUPPORT trial confirmed an increased risk of retinopathy of prematurity at higher oxygen saturation targets, but an increased mortality risk at lower oxygen saturation targets.(12) No clinical trial to date has specifically assessed the efficacy of home oxygen therapy on growth and neurodevelopment in infants with BPD.
Consistent with the inherent dilemmas in prescribing oxygen therapy, there is a lack of consensus in the U.S. regarding home oxygen use. Ellsbury et al surveyed 181 participants in the Vermont Oxford Network and found pulse oximetry criteria for initiating home oxygen therapy ranging from 84-96%; 51% of respondents additionally reported considering home oxygen for infants with normal saturations but tachypnea or poor growth.(13)
It is important to understand which patients are likely to receive home oxygen upon discharge, in order to prepare families prior to discharge and structure appropriate follow-up.(14) Additionally, as quality improvement in neonatal intensive care strives to optimize outcomes, an assessment of practice variation in the U.S. may spur more comprehensive research on best practices for NICU graduates. Therefore, the goals of this study were: 1) to review the rates of home oxygen use among a large cohort of preterm infants, 2) to identify clinical risk factors predicting home oxygen use in preterm infants, and 3) to determine the extent of institutional variation in home oxygen use in NICUs across the U.S.
Methods
Data were obtained from the Pediatrix Clinical Data Warehouse, which maintains de-identified electronic medical record information including details of admission, clinical course and discharge on all patients cared for in 280 NICUs in 33 states. The Pediatrix Medical Group cares for 15-20% of NICU admissions in the U.S. across a wide range of sizes and types of NICUs. Since 2005, the use of pull-down menus for automated data extraction has enabled more consistent evaluation of detailed information such as home oxygen use.(15)
This study was a retrospective cohort analysis of all surviving infants 23-31 weeks gestation who were discharged home from a Pediatrix center in 2009. Patients who were transferred before discharge were excluded. The primary outcome was home oxygen support; infants were only recorded as discharged home on oxygen if both the home oxygen variable and the respiratory support on the last day of hospitalization were consistent.
Gestational ages were combined into groups: 23-24 weeks, 25-26 weeks, 27-28 weeks, and 29-31 weeks. Birth weight was recorded as a continuous variable; small for gestational age was defined as birth weight less than the 10th percentile for gestational age based on published growth charts.(16) Initial respiratory illness severity was captured by recording the maximum ventilatory support required in the first three days of life (ranging from room air to mechanical ventilation) and the maximum fraction of inhaled oxygen (FiO2) required in the first three days of life (recorded continuously from 0.21 to 1). Length of stay was reported as corrected age at discharge (gestational age at birth + days-hospitalized/7). BPD was defined as supplemental oxygen at 36 weeks’ corrected age (very few infants were discharged on oxygen prior to 36 weeks’ corrected age, or were discharged home on oxygen despite no respiratory support at 36 weeks’ corrected age; these infants were eventually excluded from analysis).(17) The physiologic test for BPD was not used for this analysis.(18) At 36 weeks’ corrected age, infants without an oxygen requirement who still received some degree of non-invasive respiratory support, such as flow via nasal cannula without supplemental oxygen, were not defined as having BPD, but were categorized as requiring non-oxygen respiratory support. Additional variables included maternal demographics, perinatal information and clinical morbidities. Treating hospitals were assigned a random identification number to maintain appropriate de-identification; hospitals’ altitude was recorded as a continuous variable.
Data analysis
Rates of missing demographic and morbidity data were assessed (infants missing data were retained in the data set). Home oxygen use was calculated by gestational age group and respiratory status at 36 weeks corrected age (all infants, those requiring non-oxygen respiratory support, and those with BPD), comparing differences in proportions using Mantel-Haenszel chi-squared tests for trend.
In order to quantify clinical risk factors as well as institutional variation in home oxygen use, two forward-stepwise mixed-effects logistic regression models were developed. In these models, clinical risk factors were treated as fixed effects and each hospital as a random intercept. This approach allowed us to interpret the predictors from the mixed-effects logistic regression comparing infants within a given facility, and also allowed us to quantify the extent of institutional variation after adjusting for infants’ clinical risk factors as well as center altitude. We developed two models. First, we identified early clinical risk factors predicting home oxygen use among all infants; second, we identified predictors of home oxygen use among the subset of infants with BPD.
To assess the relationship between institutional practice variation and home oxygen use, we calculated the proportion of home oxygen use for each institution discharging at least 20 patients. Similarly, we calculated each institution’s proportion of home oxygen use among the subgroup of infants with BPD, including centers discharging at least 10 infants with BPD. In order to adjust institutional variation for differences in patient mix, we used the mixed-effects logistic regression models, converting the standard deviation of the log-odds of random intercepts to the odds scale, in order to interpret this result as the degree to which facility effects increase or decrease the odds of home oxygen use given individual clinical risk factors such as gestational age and initial respiratory illness severity. Finally, we divided centers into groups based upon their rate of home oxygen in the BPD population and used a linear regression model with clustered standard errors to assess the impact of high, medium and low-oxygen-use centers on corrected age at discharge after adjusting for clinical risk factors.
This study was approved by the Medical College of Wisconsin Institutional Review Board; the Clinical Data Warehouse was approved for research use by the Western Institutional Review Board.
Results
Infants (n=8167) were identified who were born between 23-31 weeks’ gestation, survived to NICU discharge, and were discharged home from the admitting institution in 2009; they were cared for in 201 hospitals in 33 states. No infants were missing information regarding the primary outcome of home oxygen use.
A description of the 8167 infants is shown in Table I. Rates of BPD ranged from 71% of surviving 23-24 week infants to 11% of surviving 29-31 week infants. Data were missing in less than 3% of the study population except as indicated in the table. 18 infants were discharged home on oxygen before 36 weeks corrected age; 41 infants were reported as having no respiratory support requirement at 36 weeks corrected age but were discharged home on oxygen; 227 infants had missing or inconsistent information regarding corrected age at discharge. These infants were excluded from further analysis; in total, 7881 infants (97% of the original study sample) were retained for analysis. There were no statistically significant differences between the 7881 infants and the 286 that were excluded in the distribution of gestational age or co-morbidities.
Table 1. Description of surviving neonates discharged home in 2009.
| 23-24 | 25-26 | 27-28 | 29-31 | Total | |
|---|---|---|---|---|---|
| n | 398 | 1032 | 1784 | 4953 | 8167 |
|
| |||||
| BIRTH CHARACTERISTICS AND DEMOGRAPHICS | |||||
|
| |||||
| Birth weight, median (IQR) | 650 (575-710) |
810 (700-900) |
1050 (920-1185) |
1420 (1240-1610) |
1230 (950-1490) |
| Male | 51 | 48 | 54 | 54 | 53 |
| Multiple | 21 | 23 | 26 | 31 | 29 |
| 5-minute Apgar score ≤ 3 | 13 | 6 | 2 | 1 | 3 |
| Antenatal steroids | 77 | 84 | 85 | 83 | 83 |
| Vaginal delivery | 35 | 27 | 23 | 26 | 26 |
| Anomalies | 16 | 12 | 11 | 8 | 9 |
| Race/ethnicitya | |||||
| Non-Hispanic White | 42 | 42 | 47 | 50 | 48 |
| Non-Hispanic Black | 32 | 32 | 27 | 24 | 26 |
| Hispanic | 18 | 17 | 17 | 17 | 17 |
| Other | 5 | 6 | 5 | 5 | 9 |
| Maternal age, median (IQR) | 27 (22-32) |
28 (23-33) |
28 (22-33) |
28 (23-33) |
28 (23-33) |
|
| |||||
| MAXIMUM RESPIRATORY SUPPORT IN THE FIRST THREE DAYS OF LIFE | |||||
|
| |||||
| Maximum early respiratory support | |||||
| Room air | 0 | 0.4 | 2 | 12 | 8 |
| NC, HFNC, or NCPAP | 3 | 11 | 31 | 53 | 40 |
| Mechanical ventilation | 96 | 88 | 67 | 34 | 52 |
| Maximum early FiO2b | |||||
| 21-40% | 68 | 73 | 75 | 71 | 73 |
| 41-60% | 14 | 11 | 9 | 8 | 9 |
| 61-100% | 10 | 7 | 5 | 3 | 4 |
|
| |||||
| CLINICAL CO-MORBIDITIES | |||||
|
| |||||
| IVH grade 3-4c | 14 | 10 | 4 | 1 | 4 |
| NEC | |||||
| All treated | 8 | 9 | 5 | 3 | 4 |
| Treated surgically | 3 | 3 | 0.7 | 0.3 | 0.9 |
| PDA | |||||
| All noted on echo | 78 | 67 | 48 | 21 | 35 |
| Ligated | 35 | 18 | 5 | 0.8 | 6 |
| ROP stage ≥ 3d | 28 | 14 | 2 | 0.8 | 5 |
| BPDe | 71 | 58 | 29 | 11 | 24 |
|
| |||||
| CORRECTED AGE AT DISCHARGE | |||||
|
| |||||
| Corrected age at discharge, weeks, median (IQR) | 40.0 (38.3-42.6) |
38.9 (37.1-41.0) |
37.3 (36.0-39.0) |
36.0 (35-37.3) |
36.7 (35.4-38.4) |
Data are reported as percent unless indicated otherwise. “Early” respiratory support or FiO2 indicates the maximum amount of support required in the first three days of life. NC = nasal cannula. HFNC = high flow nasal cannula. NCPAP = nasal continuous positive airway pressure. All differences between gestational age groups were significant at p < 0.01 by chi-squared analysis or ANOVA as appropriate, except male gender and maternal age. Infants missing data were retained in the analysis, and constituted <3% of each gestational age group unless indicated below.
3-4% missing data on maternal race.
15% missing data on maximum FiO2 in the first three days of life.
HUS information missing on 2-3% of infants 23-26 weeks, 5% of infants 27 weeks, and 12% of infants 29-31 weeks (7% of 29-31 week infants <1500g with initial respiratory requirement).
ROP status missing in <3% of infants 23-28 weeks; 27% of infants 29-31 weeks (11% of infants 29-31 weeks <1500g with initial respiratory requirement).
BPD = bronchopulmonary dysplasia, defined as oxygen requirement at 36 weeks corrected age or discharge, whichever occurred first.
Of infants in the study population, 1305 of 7881 (17%) were discharged home on oxygen. 17% of infants discharged on home oxygen were 23-24 weeks’ gestation; 30% were 25-26 weeks; 26% were 27-28 weeks; 27% were 29-31 weeks. 83% of infants discharged on home oxygen initially required mechanical ventilation; 17% were not intubated in the first 72 hours of life. 75% had low initial oxygen requirements (≤0.4 FiO2). At 36 weeks’ corrected age, 90% had BPD as defined by FiO2 requirement at 36 weeks; the remaining 10% received non-invasive respiratory support but had no oxygen requirement.
Rates of home oxygen use
Infants’ rates of home oxygen use are shown in Table II. Home oxygen use by gestational age ranged from 59% of surviving 23-24 week infants to 7% of surviving 29-31 week infants (Mantel-Haenszel test for trend p <0.0001). Infants born at earlier gestational ages were more likely to have BPD than infants born at later gestational ages (Table I). But infants that did develop BPD were similar in their rate of home oxygen use by gestational age, ranging from 79% of 23-24 week infants to 60% of 29-31 week infants (p=0.0002 overall; p=0.45 between 25 and 31 weeks’ gestation). Infants who were not classified as having BPD but were receiving some form of non-oxygen respiratory support at 36 weeks corrected age had 21-33% home oxygen use on discharge, with no significant differences by gestational age (p=0.39).
Table 2. Home oxygen rates by gestational age and respiratory support needs at 36 weeks corrected age.
| Total | 36 week respiratory support |
|||
|---|---|---|---|---|
| Non-oxygen support | BPD | |||
| ALL INFANTS | n | 7881 | 573 | 1804 |
|
| ||||
| Home oxygen, % | 16.6 | 22.5 | 65.2 | |
|
| ||||
| 23-24 weeks | n | 377 | 46 | 263 |
|
| ||||
| Home oxygen, % | 59.2 | 32.6 | 79.1 | |
|
| ||||
| 25-26 weeks | n | 951 | 116 | 556 |
|
| ||||
| Home oxygen, % | 40.8 | 21.6 | 65.3 | |
|
| ||||
| 27-28 weeks | n | 1687 | 198 | 482 |
|
| ||||
| Home oxygen, % | 20.2 | 20.7 | 62.2 | |
|
| ||||
| 29-31 weeks | n | 4866 | 213 | 503 |
|
| ||||
| Home oxygen, % | 7.3 | 22.5 | 60.1 | |
The first column, labeled “Total,” shows the number of discharged infants, and percentage of that group of infants discharged on home oxygen, for each gestational age group. The two columns to the right show the number of discharged infants and percentage discharged on home oxygen for two subsets of infants requiring respiratory support at 36 weeks corrected age: “non-oxygen support” refers to infants requiring non-invasive respiratory support but without an FiO2 requirement as required for diagnosis of BPD; “BPD” in this table refers specifically to infants requiring supplemental oxygen at 36 weeks corrected age.
Predictors of home oxygen use
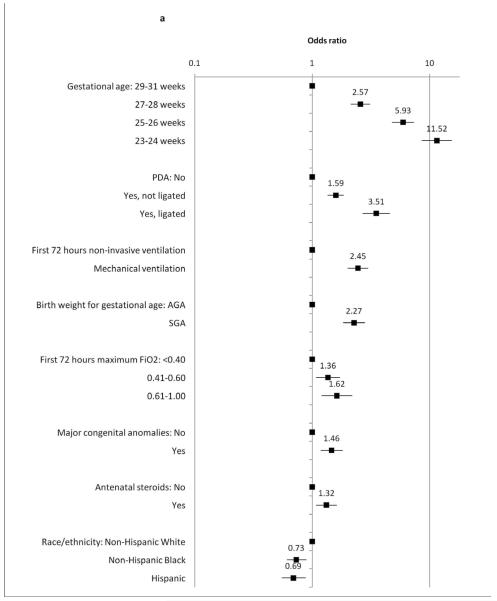
Mixed-effects logistic regression models were used to identify a) early clinical predictors of home oxygen use and b) predictors of home oxygen use among infants with BPD. Results are shown in Figure 1. Earlier gestational age was strongly associated with home oxygen use; other factors increasing odds of home oxygen use included birth weight (small for gestational age), mechanical ventilation in the first three days of life, higher FiO2 requirement in the first three days of life, PDA, antenatal steroids, and presence of congenital anomalies. Decreased odds of home oxygen use were seen among Non-Hispanic Black and Hispanic infants relative to non-Hispanic White infants.
Figure 1. Mixed-effects logistic regression estimating clinical predictors of home oxygen use.
Relative odds of home oxygen use among A, all infants and B,infants with BPD. “Initial respiratory support” and “initial FiO2 requirement” refer to the maximum support in the first three days of life. FiO2= fraction of inhaled oxygen; PDA= patent ductus arteriosus. S.E.= standard error; C.I.= 95% confidence interval.
Clinical Predictors of Home Oxygen Use (n=7881)
Clinical Predictors of Home Oxygen Use: Infants with BPD. (n=1804)
Among the subset of 1804 infants with BPD (Figure 1, B), home oxygen use was still more common among infants of earlier gestational age. Increased odds of home oxygen use were also seen among infants who were small for gestational age, and those who received PDA ligations. Initial respiratory illness severity, presence of congenital anomalies, receipt of antenatal steroids, and race/ethnicity were not statistically significant in predicting home oxygen use among infants with BPD. Decreased odds of home oxygen use were seen with increasing corrected gestational age at discharge.
Institutional variation in home oxygen use
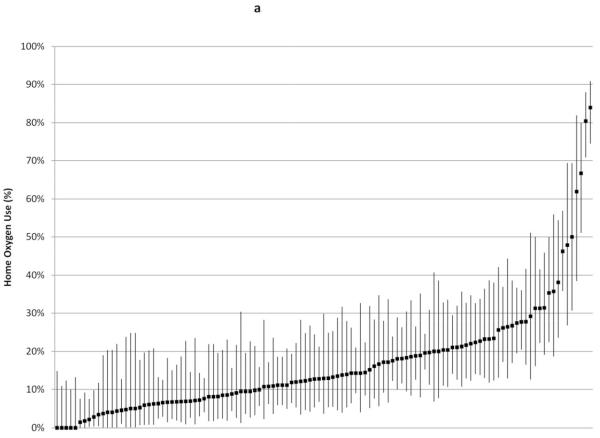
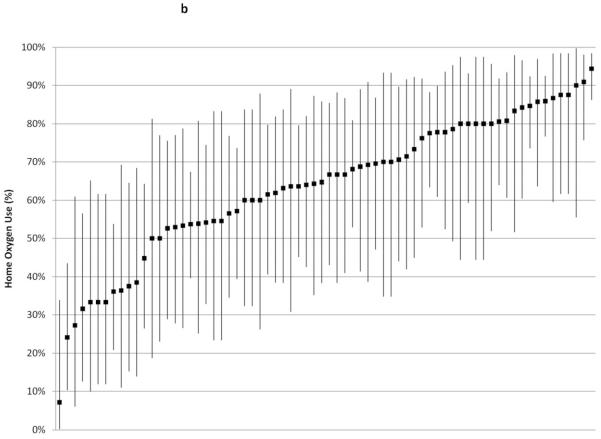
Institutional rates of home oxygen use are shown in Figure 2. Snalysis was restricted to the 117 centers discharging at least 20 infants, which included 7242 infants (1738 with BPD, or 96% of the total infants with BPD in the study cohort); analysis was restricted to the 70 centers discharging at least 10 infants with BPD (1524 infants with BPD out of 5434 infants total in those centers; 88% of the total study cohort with BPD) (Figure 2, A and B, respectively). Without adjusting for institutions’ patient mix, home oxygen use across institutions ranged from 0-80%; among infants with BPD, institutions’ home oxygen use ranged from 7-95%. Although centers’ BPD rate correlated strongly with centers’ home oxygen use rate (r2 = 0.8418), among infants with BPD there was much less correlation between centers’ BPD rate and home oxygen use rate (r2 = 0.1018).
Figure 2.
Rates of home oxygen use were calculated for A, 117 centers that discharged at least 20 infants and B, 70 centers that discharged at least 10 infants with BPD in 2009. Bars indicate exact 95% confidence intervals except for centers reporting 0% home oxygen use, in which case one-sided 97.5% confidence intervals were reported.
Home Oxygen Use: 117 institutions Discharging ≥ 20 Infants
Home Oxygen Use Among Infants with BPD: 70 Institutions Discharging ≥ 10 Infants with BPD
In order to adjust for institutional differences in patient mix, we used the institutional random effects from the mixed-effects logistic regression models shown in Figure 2. After adjusting for individual clinical risk factors as well as center altitude, institutional variation accounted for a 4.8-fold change (95% confidence interval 3.6-6.7) in odds of home oxygen use among all infants – a greater magnitude than all other odds ratios except gestational age. Even when considering only infants with BPD, institutional variation accounted for a 4.2-fold change (95% confidence interval 3.0-6.6) in odds of home oxygen use – a greater magnitude than any identified individual clinical risk factor except the difference between 23-24 weeks’ gestation and 29-31 weeks’ gestation.
Overall, infants discharged with home oxygen had a significantly later corrected gestational age at discharge (39.8 weeks) versus those discharged without home oxygen (36.9 weeks; t-test p <0.001). Among infants with BPD, however, those discharged home on oxygen had a significantly earlier corrected gestational age at discharge (39.9 weeks) than those discharged without oxygen (40.3 weeks; t-test p = 0.0283). In infants with BPD, individual center variation in home oxygen use had a statistically significant effect on corrected age at discharge. After dividing centers into groups based upon their rate of home oxygen use for infants with BPD (<50%, 50-75%, and >75% home oxygen use), we assessed the corrected age at discharge for infants in these centers via a forward-stepwise linear regression model with clustered errors adjusting for additional clinical risk factors impacting corrected age at discharge (gestational age, small for gestational age, PDA, race, initial mechanical ventilation and FiO2 requirements, ROP stage, medical and surgical NEC). Infants with BPD treated in a high-oxygen-use center versus a low-oxygen-use center had an earlier corrected age at discharge by 1.2 weeks (95% confidence interval of 0.5 to 2 weeks earlier; p = 0.002). Infants with BPD treated in a medium-oxygen-use center had a similar mean corrected age at discharge as infants treated in a low-oxygen-use center (p = 0.847).
Discussion
This study was designed to report the rate of home oxygen use among preterm infants, identify clinical risk factors for home oxygen use, and quantify the extent of institutional variation in home oxygen use across a large variety of U.S. NICUs. Risk factors increasing odds of home oxygen use include early gestational age, small for gestational age, mechanical ventilation in the first three days of life, higher FiO2 requirement in the first three days of life, PDA, antenatal steroids, and presence of congenital anomalies. The majority of preterm infants with BPD are discharged home on oxygen, including those of later gestational ages. Institutional variation is one of the strongest determinants of home oxygen use, both overall and among infants with BPD.
It is not surprising that earlier gestational age increases risk for home oxygen therapy. The NICHD Neonatal Research Network has reported higher rates of home oxygen use in infants of lower birth weight, with 15% home oxygen use among VLBW infants from 1995-96 and 11% oxygen use among VLBW infants from 1997-2002 (19-20). In reporting by gestational age groups, a single-center study of 508 infants reported 48% home oxygen use among surviving infants 23-24 weeks and 23% among infants 25-27 weeks, and 81-85% among those infants with BPD.(21) This is the first study reporting on home oxygen use across hundreds of centers with detailed information by gestational age and diagnosis of BPD. Generalizability of these gestational-age-specific rates of home oxygen use is enhanced by the similarity of the Pediatrix group’s reported rates of BPD and home oxygen use to other major reports in the literature over the past fifteen years, including the most recent Neonatal Research Network report as well as the Vermont Oxford Network (19-20, 22-23).
Why study home oxygen use? One important reason is to prepare parents for their infants’ discharge needs. Research on parents of preterm infants has shown that having medical equipment in the home is a major stressor; these studies suggest that specialized discharge needs such as home oxygen use should be described early in the discharge process.(24-25) Recent research by Laughon et al shows an increasing ability to predict BPD over time in the NICU; conversations with families over days-weeks in the NICU may present an opportunity to prepare parents earlier for potentially stressful medical treatments such as home oxygen therapy.(26) In particular, parents of sicker moderate preterm infants such as those 29-31 weeks could be updated as their infant’s anticipated home needs change over time, as expectations may change significantly from the time of birth (7% home oxygen use) to the diagnosis of BPD at 36 weeks corrected age (60% home oxygen use).
A second reason to study home oxygen use is to attempt to gain insight into the factors that drive its use. We found that, controlling for clinical risk factors, institutional variation still accounted for 4-5 fold differences in use of home oxygen. The fact that some institutional variation exists in the use of home oxygen therapy is not surprising, given the varying recommendations and practitioners’ reported decision-making strategies regarding home oxygen use.(4-6,13) Rates of home oxygen use have been shown to vary depending on the oxygen saturation target chosen; the BOOST trial reported 17-30% home oxygen use depending upon the oxygen saturation target chosen, and the Neonatal Research Network’s birth-weight-specific rates of home oxygen use have been reported to vary widely between centers as well.(10,19-20) BPD rates are known to be affected by use of the physiologic test to define BPD; however, even when standardizing centers to using a physiologic test for BPD, rates of this illness vary substantially within centers.(18-20,22,27) But the fact that such wide variability in home oxygen use is due to institutional variation rather than gestational age, illness severity or BPD highlights the fact that this clinical dilemma remains unanswered. Is home oxygen use a good thing or a bad thing? Future research prospectively evaluating growth patterns, rates of re-hospitalization, and markers of which infants derive the most benefit from home oxygen therapy could optimize outcomes for these infants at highest risk for significant medical and developmental needs post-discharge.
This study found that high-oxygen-use centers had a 1.2 week earlier mean corrected age at discharge than low-oxygen-use centers after adjusting for gestational age and illness severity. Although the clinical significance of this finding is uncertain, it suggests that at least some degree of institutional variability in home oxygen use may be determined not by competing concerns regarding optimal physiologic management, but by competing concerns regarding resource utilization. BPD is one of the major predictors of length of hospital stay and increased costs among extremely preterm infants.(28) Decisions regarding home oxygen use may be affected by health insurance status; a retrospective study of preterm births <37 weeks found twice the rate of home oxygen and apnea monitor use among infants with private insurance versus those with Medicaid managed care, though they did not address specific institutional variation.(29) Decisions regarding home oxygen use may also be affected by regional access to home care after discharge, which has been shown to affect timing of discharge for VLBW infants.(30)
Strengths of this study include the large number of infants available for analysis, the recent single-year data collection minimizing changes in treatment patterns over time, the similar rates of BPD and other common preterm morbidities compared with reported literature, and the broad variety of types and sizes of NICUs represented in the data set.
As with any retrospective review, despite internal data consistency checks and low rates of missing data, these findings may be subject to data entry error. Also, the level of detail available in the clinical data warehouse was not sufficient for analysis of some factors that might guide decision-making regarding individual babies’ home oxygen needs, such as how active was the attempt to wean each infant from oxygen over how many days/weeks, what clinical factors led to decisions regarding home oxygen use (such as physiologic testing or oxygen saturation targets), or what family preferences or insurance company stipulations were regarding length of stay. Future prospective research could address some of these important issues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Wade KC, Lorch SA, Bakewell-Sachs S, Medoff-Cooper B, Silber JH, Escobar GJ. Pediatric care for preterm infants after NICU discharge: high number of office visits and prescription medications. J Perinatol. 2008;28:696–701. doi: 10.1038/jp.2008.74. [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely-low-birth-weight in the 1990s. JAMA. 2005;294:318–25. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 4.Panitch H. Bronchopulmonary dysplasia. In: McConnell MS, Imaizumi SO, editors. Guidelines for Pediatric Home Health Care. American Academy of Pediatrics; 2002. pp. 323–42. [Google Scholar]
- 5.American Thoracic Society Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:456–96. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Poets CF, Stebbens VA, Alexander JR, Arrowsmith WA, Salfield SA, Southall DP. Arterial oxygen saturation in preterm infants at discharge from the hospital and six weeks later. J Pediatr. 1992;120:447–54. doi: 10.1016/s0022-3476(05)80919-9. [DOI] [PubMed] [Google Scholar]
- 7.Groothuis JR, Rosenberg AA. Home oxygen promotes weight gain in infants with bronchopulmonary dysplasia. Am J Dis Child. 1987;141:992–5. doi: 10.1001/archpedi.1987.04460090069028. [DOI] [PubMed] [Google Scholar]
- 8.Hudak BB, Allen MC, Hudak ML, Loughlin GM. Home oxygen therapy for chronic lung disease in extremely-low-birth-weight infants. Am J Dis Child. 1989;143:357–60. doi: 10.1001/archpedi.1989.02150150115028. [DOI] [PubMed] [Google Scholar]
- 9.Tay-Uyboco JS, Kwiatkowski J, Cates DB, Kavanagh L, Rigatto H. Hypoxic airway constriction in infants of very low birth weight recovering from moderate to severe bronchopulmonary dysplasia. J Pediatr. 1989;115:456–9. doi: 10.1016/s0022-3476(89)80855-8. [DOI] [PubMed] [Google Scholar]
- 10.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. NEJM. 2003;349:959–967. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 11.The STOP-ROP Multicenter Study Group Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 12.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Target ranges of oxygen saturations in extremely preterm infants. NEJM. 2010;362:1959–69. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellsbury DL, Acarregui MJ, McGuinness GA, Eastman DL, Klein JM. Controversy surrounding the use of home oxygen for premature infants with bronchopulmonary dysplasia. J Perinatol. 2004;24:36–40. doi: 10.1038/sj.jp.7211012. [DOI] [PubMed] [Google Scholar]
- 14.AAP Committee on Fetus and Newborn Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119–26. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps Improvement Project System – tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatrics. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. NICHD/NHLBI/ORD workshop summary: bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 19.Lemons JA, Bauer CR, Oh W, Korones SB, Papile L, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107:e1–8. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 20.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196:147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Stephens BE, Tucker R, Vohr BR. Special health care needs of infants born at the limits of viability. Pediatrics. 2010;125:1152–8. doi: 10.1542/peds.2009-1922. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horbar JD, Carpenter JH, editors. Vermont Oxford Network 2005 Very Low Birth Weight Database Summary. Vermont Oxford Network; Burlington, Vermont: 2006. [Google Scholar]
- 24.Greenough A, Alexander J, Burgess S, Chetcuti PAJ, Cox S, Lenney W, et al. Home oxygen status and rehospitalisation and primary care requirements of infants with chronic lung disease. Arch Dis Child. 2002;86:40–3. doi: 10.1136/adc.86.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanardo V, Freato F. Home oxygen therapy in infants with bronchopulmonary dysplasia: assessment of parental anxiety. Early Human Dev. 2001;65:39–46. doi: 10.1016/s0378-3782(01)00190-6. [DOI] [PubMed] [Google Scholar]
- 26.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Resp Crit Care Med. 2011 doi: 10.1164/rccm.201101-0055OC. doi:10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambalavanan N, Walsh M, Bobashev G, Das A, Levine B, Carlo WA, et al. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011;127:e106–16. doi: 10.1542/peds.2010-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hintz SR, Bann CM, Ambalavanan N, Cotton CM, Das A, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network Predicting time to hospital discharge for extremely preterm infants. Pediatrics. 2010;125:e146–54. doi: 10.1542/peds.2009-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandon GD, Adeniyi-Jones S, Kirkby S, Webb D, Culhane JF, Greenspan JS. Are outcomes and care processes for preterm neonates influenced by health insurance status? Pediatrics. 2009;124:122–7. doi: 10.1542/peds.2008-1318. [DOI] [PubMed] [Google Scholar]
- 30.Spinner SS, Girifalco RB, Gibson E, Stavis RL, Greenspan JS, Spitzer AR. Earlier discharge of infants from neonatal intensive care units: a pilot program of specialized case management and home care. Clin Pediatr. 1998;37:353–8. doi: 10.1177/000992289803700604. [DOI] [PubMed] [Google Scholar]