Abstract
Mutations in sunlight-induced melanoma arise from cyclobutane pyrimidine dimers (CPD), DNA photoproducts that are typically created picoseconds after an ultraviolet (UV) photon is absorbed at thymine or cytosine. Here we show that in melanocytes, CPD are generated for >3 hours after exposure to UVA, a major component of the radiation in sunlight and in tanning beds. These “dark CPD” constitute the majority of CPD and include the cytosine-containing CPD that initiate UV-signature C→T mutations. Dark CPD arise when UV-induced reactive oxygen and nitrogen species combine to excite an electron in fragments of the pigment melanin. This creates a quantum triplet state that has the energy of a UV photon but that induces CPD by energy transfer to DNA in a radiation-independent manner. Melanin may thus be carcinogenic as well as protective against cancer. These findings also validate the long-standing suggestion that chemically-generated excited electronic states are relevant to mammalian biology.
The pigment melanin protects against sunlight-induced burns, DNA damage, and skin cancer due to an unusually broad absorption spectrum and perhaps radical scavenging activity (1). Yet paradoxes abound. First, melanin is not solely beneficial: Blondes and redheads, who have a higher ratio of yellow pheomelanin to brown eumelanin in their skin and hair, have a 2-4 times greater risk for melanoma than dark-haired individuals and the pheomelanin/eumelanin ratio accounts for some of this risk (2). UVA-irradiated mice do not develop melanoma if they lack melanin (3) and Braf-mutant mice develop 10 times more spontaneous melanomas if they carry the pheomelanin-associated Mc1re/e allele (4). UV-irradiated melanin, especially pheomelanin, triggers apoptosis and production of reactive oxygen species (ROS) and DNA strand breaks (5, 6). Melanin synthesis itself generates ROS, especially synthesis of pheomelanin (7).
Yet melanin's ROS generating properties do not explain its role in melanoma development because most mutations in human melanomas display the UV signature of C →T substitutions at sites of adjacent pyrimidines (8, 9). Such mutations arise from cyclobutane pyrimidine dimers (CPD), which join adjacent pyrimidines to distort the DNA helix (10, 11); genetic disorders of CPD repair such as xeroderma pigmentosum elevate the risk for childhood melanoma by 10,000-fold. The most energetic component of sunlight, UVB (280-315 nm), creates these DNA photoproducts almost instantaneously so the only obvious effect of melanin is shielding. The lower-energy UVA (315-400 nm), which constitutes ~95% of the ultraviolet energy that penetrates the atmosphere, also induces melanoma in mice and in humans who use tanning beds, but it is inefficient at making the cytosine-containing CPD that cause C→T mutations (12, 13). We provide evidence for an unusual biochemical pathway that resolves these oncological discrepancies by making melanin an active participant in CPD formation.
Delayed Cyclobutane Pyrimidine Dimer Induction in Melanocytes
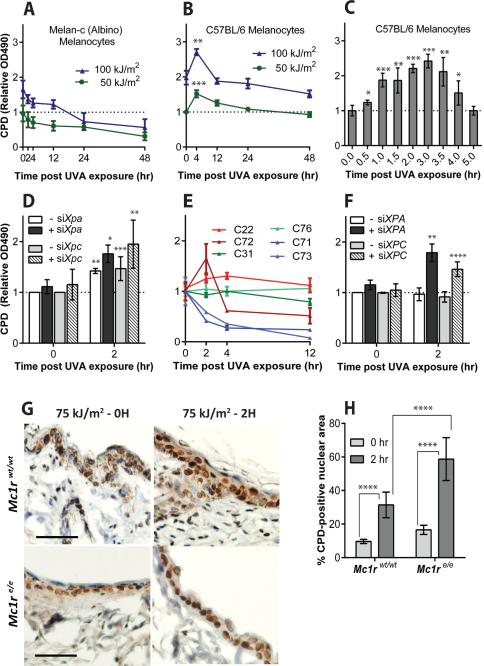
As a positive control for an unrelated experiment, we exposed murine fibroblasts and melanocytes to radiation from a UVA lamp and measured the number of CPD over time using an ELISA assay. Induction of CPD by direct UV absorption is complete in one picosecond (11). Consistent with this, we found that for fibroblasts and for melanocytes derived from albino mice, the peak of CPD induction was reached immediately upon exposure, followed by slow nucleotide excision repair (Fig. 1A) (fig. S1A). (14). Unexpectedly, melanin-containing murine melanocytes instead continued to generate CPD for at least three hours after UVA exposure, at which point generation was offset by DNA repair (Fig. 1B,C). The negative result with albino melanocytes implicated melanin in the process and ruled out the involvement of photosensitizing compounds in the growth medium. We confirmed the delayed production of CPD by using a comet assay after treating lysed cells with a nucleotide excision repair enzyme to induce DNA breaks at CPD sites (fig. S1B). This result also indicated that delayed CPD resided in nuclear DNA. Delayed CPD were also visible by immunofluorescence in normal melanocytes, whereas albino melanocytes showed only repair (fig. S1C, D). We conclude that pigmented melanocytes induce “dark CPD” after UV exposure ends.
Fig. 1. Cyclobutane pyrimidine dimers (CPD) continue to be generated in melanocytes long after UV exposure ends.
(A) In albino murine melanocytes CPD induction at time 0 is followed by repair, but in melanin-containing melanocytes (B, C) CPD continue to increase for 4 h after UVA exposure ends. CPD were assayed by DNA ELISA. (D) Additional dark CPD are revealed in melanocytes when excision repair is suppressed by siXpa or siXpc; dark CPD account for half the total CPD. (E) In melanocytes from humans, the production of dark CPD after UVA varies between individuals but as shown in (F) “non-producers” are revealed as producers after DNA repair is suppressed in the cells by siXPA or siXPC. (G) Dark CPD in mouse melanocytes and keratinocytes in vivo. K14-Kitl transgenic mice, which have epidermal melanocytes containing eumelanin, were crossed to mice carrying the Mc1re/e allele which confers epidermal pheomelanin and yellow fur. Scale bar is 50 μm. (H) Quantitation of CPD in epidermal sections. Error bars are SD from 4, 3, or 2 experiments (A-C, D-F, H, respectively). p values by t-test are for the difference between the asterisked timepoint and 0 h or as indicated. *, p ≤ 0.05; ** 0.005; *** 0.0005; **** 0.00005.
We next examined whether dark CPD induction was masked by concurrent repair and thus might be more extensive than it appeared. Knocking down excision repair of CPD using siRNA against Xpa or Xpc transcripts (corresponding to genes mutated in xeroderma pigmentosum) revealed that dark CPD constituted half of the total CPD (Fig. 1D). This two-fold increase in DNA photoproducts is substantial because cancer-predisposed individuals in xeroderma pigmentosum complementation group D are only 60% defective in CPD repair (15). For the higher-energy UVB, the majority of CPD in melanocytes were created in the dark even without repair knockdown (figs. S1E-H). The UVB process was also dependent on melanin and thus it differs from the delayed production of CPD that has been reported occasionally in other cell types after UVC or UVB exposure (16-18).
Human melanocytes also generated dark CPD after UVA or UVB exposure (Fig. 1E, fig. S1I) although there was inter-individual variation in the response, particularly for UVA. This likely reflects genetic differences between the donors. For human melanocytes in which there was a modest rate of CPD decline after exposure but which showed no obvious dark CPD, the dark CPD were revealed by siRNA knockdown of XPA or XPC transcripts (Fig. 1F). It was not possible to determine whether individual variation was due to variation in melanin type because of privacy restrictions for newborn foreskin tissue.
To examine CPD induction in vivo we used transgenic mice in which melanocytes remain in the epidermis throughout life due to expression of the Kit ligand in keratinocytes (K14-Kitl), thus mimicking human skin. After exposure of these mice to UVA, the level of epidermal CPD at the 2 hour timepoint was 3 times greater than it was immediately after exposure (Fig. 1G,H). Most epidermal cells were keratinocytes, which receive pigment from the melanocytes, suggesting that melanin content rather than synthesis was the crucial requirement for dark CPD induction. Furthermore, in K14-Kitl mice homozygous for the inactive Mc1re/e allele, in which melanocytes synthesize red-yellow pheomelanin, both initial and dark CPD were twice as frequent as in black mice. This suggests that pheomelanin is both a poorer shield against normal CPD formation and a more potent dark CPD generator.
Cytosine-containing Dark CPD
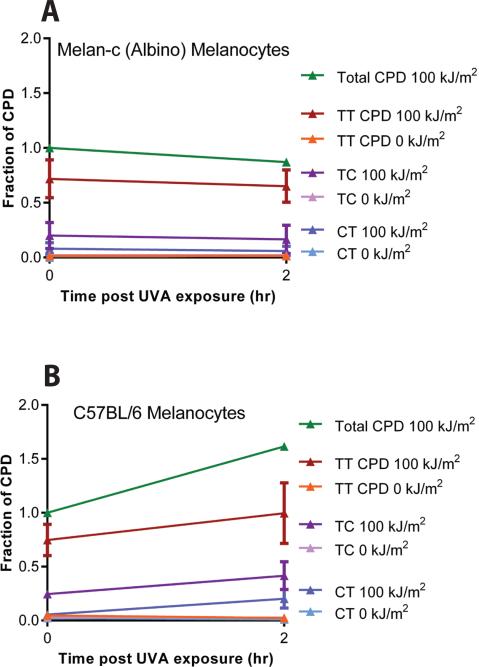
We next used mass spectrometry to identify individual CPD species in murine melanocytes. This analysis revealed that the delayed CPD in melanin-containing melanocytes included the cytosine-containing CPD that generate UV-signature C→T mutations (Fig. 2A,B). Unexpectedly we observed a 4-fold higher ratio of (TC + CT)/TT CPD in the delayed CPD compared to directly-induced CPD, which is suggestive of unusual chemistry. UVA generates primarily TT CPD, with TC and CT CPD together contributing only 10-30% of these three CPD types (13). The (TC + CT)/TT ratio in melanin-containing cells increased from the UVA-like 0.37 at 0 h (directly-induced CPD) to 1.33 in the CPD arising during the following 2 h (p = 0.027), a ratio more typical of cells irradiated with the higher-energy UVB. Mass spectrometry ruled out the possibilities that (i) the ELISA and comet assays cross-reacted with melanin-DNA adducts and (ii) the delayed CPD appearance reflected greater access of the antibody or endonuclease due to a change in DNA conformation after UV exposure.
Fig. 2. Mass spectrometry reveals enrichment of dark CPD for CPD that contain cytosine.
For energetic reasons, direct absorption of UVA generates primarily thymine-containing (TT) cyclobutane pyrimidine dimers. (A) There is no dark CPD formation in albino melanocytes after UVA exposure. The slope of post-UVA CPD induction in albino cells is indistinguishable from the slope in unirradiated cells. Data represent the average of 3 experiments, expressed as a fraction of the total CPD generated at 0 h. Hence, the total CPD line has no error bar. The total number of CPD at 0 h was 169 CPD per megabase of DNA. (B) Dark CPD are induced in melanin-containing melanocytes by UVA and include a greater number of cytosine-containing TC and CT dimers, capable of causing UV signature C→T mutations. Slopes for post-UVA induction of TT, TC, and CT CPD in melanin-containing cells are greater than those in albino, with p = 0.01, 0.05, and 0.03, respectively. The total number of CPD at 0 h was 87 CPD per megabase of DNA, consistent with the shielding function of eumelanin. Data represent the average of 5 experiments.
Photochemical Pathway
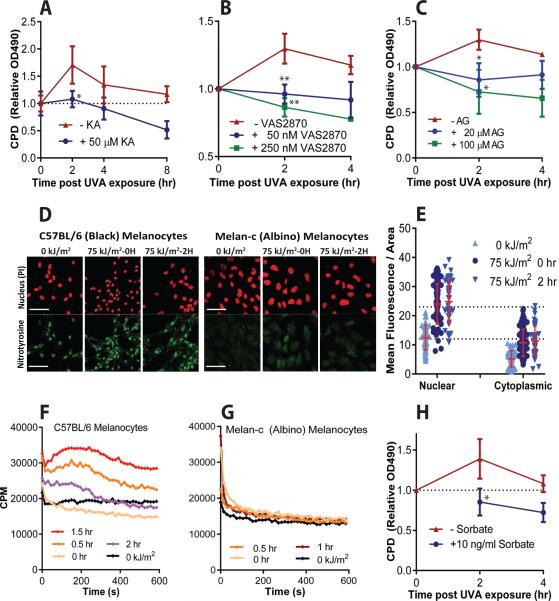
To identify the photochemical pathway that produces dark CPD, we focused on UVA because it reduces background from CPD created by direct UVB absorption, reduces photosensitization from aromatic molecules, and is used in indoor tanning beds. We found that kojic acid, an inhibitor of tyrosinase, the rate-limiting enzyme in melanin synthesis, suppressed production of dark CPD by 85% (Fig. 3A). Because ROS can be produced in cells for long periods after UVA exposure (19), we tested the ROS scavengers N-acetylcysteine and α-tocopherol (vitamin E). The former suppressed production of dark CPD by 64% and the latter abolished it (figs. S2A,B). An effect of antioxidants on CPD detectable immediately after UV has been reported, but was linked to changes in chromatin structure (20). Because melanin generates superoxide radical ion (O2•–) while it is being irradiated, we specifically scavenged it with TEMPOL and found that this also abolished the production of dark CPD after UVA (fig. S2C). A longer-lasting source of O2•– in cells is NADPH oxidase (NOX), which is rapidly induced by UVA (19). The NOX inhibitor VAS2870 also abolished production of dark CPD (Fig. 3B). CPD were inhibited to a level below that at 0 h, indicating that dark CPD were also induced during the irradiation itself.
Fig. 3. In the proposed photochemical pathway for production of dark CPD, high energy triplet-state moieties are created by melanin, superoxide, and nitric oxide.
Dark CPD are blocked by inhibitors of: (A) melanin synthesis (kojic acid, KA), (B) the superoxide generator NADPH oxidase (VAS2870), and (C) the nitric oxide generator iNOS (aminoguanidine). Inhibitors are not expected to reduce CPD levels below those seen at 0 hr, when CPD are generated primarily by direct UV absorption (dotted line). (D) The product of superoxide and nitric oxide, peroxynitrite, generates 3-nitrotyrosine modified proteins in melanocyte nuclei, except in albino melanocytes, and these increase with UVA exposure. (E) Quantitation of 3-nitrotyrosine in melanin-containing melanocytes. The increase at 0 h arises from protein nitration during the 27 min UVA exposure. (F) Ultra-weak chemiluminescence is generated by permeabilized UVA-irradiated melanized melanocytes that had been incubated with a triplet energy acceptor probe, DBAS. Chemiluminescence was quantified by single-photon counting (cpm, counts per minute). (G) Albino melanocytes do not produce chemiluminescence when irradiated. (H) A triplet-state quencher, ethyl sorbate, inhibits production of dark CPD in pigmented mouse melanocytes after they are exposed to UVA. Data are the average of 3 experiments. (F) and (G) show data from one of 3 similar experiments. p values are for the difference between treated and untreated samples.
The O2•– requirement recalled an old observation that CPD can be generated in the dark by dioxetane, a strained 4-member ring peroxide. This moiety undergoes thermolysis to two carbonyls, with one in an electronically excited triplet state having the high energy of a UV photon (> 3 eV, 70 kcal/mol, or 35,000 K) and capable of transferring its energy to DNA by a process that is independent of radiation (21). One of the few biologically-synthesized molecules that can initiate such “photochemistry in the dark” (22, 23) is peroxynitrite (ONOO–) (24), which is generated by the reaction of O2•– with nitric oxide (NO•). Peroxynitrite and NO• are stable enough to diffuse significant distances from the site of generation to the target molecule. NOX and the NO• generator iNOS are UV-inducible within minutes and are expressed in melanocytes, including nuclear NOX4 (25). To determine if NO• is required for production of dark CPD, we inhibited iNOS by treating melanocytes with aminoguanidine. This resulted in complete suppression of dark CPD production (Fig. 3C).
We next investigated whether melanin-containing cells generate peroxynitrite when exposed to UV. Peroxynitrite can be detected by its nitration of tyrosines. Assays for 3-nitrotyrosine revealed that basal nitration activity was present even without UV, that it was located primarily in the nucleus, and that its appearance required melanin (Fig. 3D,E) (fig. S2D). The melanin requirement suggests that the NO• requirement in melanocytes is distinct from that observed in cultured keratinocytes (18). Peroxynitrite's presence in the nucleus without UV exposure is plausible because synthesis of melanin monomers is a redox reaction that releases O2•– (7) and melanosomes are assembled in the perinuclear space. In support of an additional UV role, we found that exposing melanin-containing melanocytes to UVA created as much nuclear and cytoplasmic nitrotyrosine in 30 min as had accumulated from basal induction over ~8 days (Fig. 3D,E) (fig. S2D) (see Methods). This represents a ~400 fold spike in the flux of peroxynitrite per hour.
A diagnostic for excited triplet states is that their energy can also discharge via ultra-weak blue or green luminescence (23). This signal can be enhanced several orders of magnitude by diverting the energy to a triplet energy acceptor, sodium 9,10-dibromoanthracene-2-sulfonate (DBAS), which is a more efficient emitter. To allow rapid DBAS entry, we permeabilized melanocytes within a liquid scintillation counter set to single-photon counting mode. UVA-irradiated melanocytes generated ultra-weak chemiluminescence for several hours post-irradiation and only in cells containing melanin (Fig. 3F,G). The lifetime of triplet carbonyls is ~10 μs (24), so a signal persistent over the course of hours indicates ongoing creation. To test whether this triplet state is required for dark CPD, we added ethyl sorbate, a specific quencher of triplet states (26), to intact melanocytes after UVA exposure. We found that this treatment prevented production of dark CPD (Fig. 3H). Because DBAS diverts triplet energy to luminescence, this compound also blocked dark CPD (fig. S2E). These results provide direct evidence that UV-induced superoxide and nitric oxide lead to a high-energy triplet state moiety, which then creates a dark CPD by energy transfer.
Electronically Excited Melanin as a Molecular Vector
Melanin is located in the cytoplasm yet CPD were generated in the nucleus, raising the question of what molecule acquired the energetic carbonyl. A O2•–-initiated radical chain reaction in lipids was one possible conduit (26), but inducing lipid peroxidation in isolated nuclei using cumene hydroperoxide did not appear to induce CPD in nuclear DNA (fig. S3).
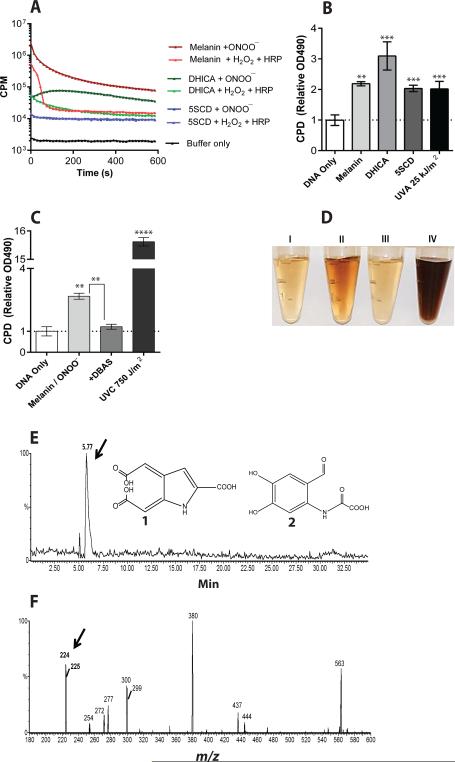
Another candidate for the triplet-state carrier was the set of melanin monomers out of which the final melanin polymers are assembled. These monomers are lipophilic and are therefore potentially able to enter the nucleus, and are concentrated in perinuclear coated vesicles before transfer to melanosomes (27). Melanin polymer rapidly solubilizes when exposed to hydrogen peroxide (H2O2) (28) and its degradation by peroxidation or UV-photoionization has been proposed to involve dioxetane and triplet carbonyls (29, 30). We first investigated whether a triplet state could be created in melanin by a cell-free system. Synthetic melanin oxidized with peroxynitrite and incubated with DBAS generated a chemiluminescence signal 100-fold larger than seen in melanocytes (Fig. 4A)(fig. S4A). We found that chemiluminescence began immediately and proceeded for >10 min. To further test melanin's ability to host a triplet state, we oxidized synthetic melanin with horseradish peroxidase in the presence of hydrogen peroxide, which generates dioxetane intermediates by a mechanism similar to that of peroxynitrite (24). This produced similar levels of chemiluminescence (Fig. 4A, fig. S4B). The eumelanin monomer 5,6-dihydroxyindole-2-carboxylic acid (DHICA) and the pheomelanin monomer 5-S-cysteinyldopa (5SCD) also generated chemiluminescence when oxidized (Fig. 4A). Importantly, we found that CPD were generated in the complete absence of UV when we incubated plasmid DNA and peroxynitrite with melanin, DHICA, or 5SCD (Fig. 4B). We were unable to test whether the triplet quencher, ethyl sorbate blocked these reactions because it was insufficiently soluble in the aqueous buffer. However we found that DBAS, which redirects triplet energy toward luminescence, reduced CPD production by 50-90% (Fig. 4C, fig. S4C). The CPD created by oxidizing melanin or its monomers included the mutagenic cytosine-containing CPD (fig. S4D). The level of CPD induced in the absence of UV was approximately equal to that generated in pure DNA by 25 kJ/m2 of UVA, an exposure about one-quarter of that required to produce a barely-perceptible sunburn (the “minimal erythema dose”). Based on our mass spectrometry data with albino murine melanocytes (Fig. 2), this value is approximately 1 CPD per 24 kb of DNA created solely by oxidized melanin.
Fig. 4. Excited-state melanin may act as a molecular vector.
(A) Synthetic melanin, its eumelanin monomer DHICA, and its pheomelanin monomer 5SCD generate ultra-weak chemiluminescence when oxidized by peroxynitrite or by horseradish peroxidase plus H2O2. DBAS was used as a triplet energy probe, so luminescence indicates the presence of electronically excited triplet states. Data represent one of 6 experiments. (B) CPD are created in the dark when melanin or its monomers are oxidized with peroxynitrite. The level of CPD resulting from direct DNA absorption of UVA is shown for comparison. (C) Diverting triplet energy to luminescence with DBAS blocks production of CPD by oxidized melanin. The UVC dose used for comparison is ~100 fold higher than that lethal to 50% of normal cells. In B and C, data represent the average of 3 experiments. p values are for the difference between treated and “DNA only” samples or as indicated. (D) Synthetic melanin is rapidly solubilized upon exposure to UV or peroxynitrite. Left to right: Supernatants resulting from untreated melanin or from melanin exposed to UVA (200 kJ/m2), 7.8 mM NaOH, or peroxynitrite (1 mM in NaOH stabilizer). (E-F) Identification of a putative triplet-carbonyl carrier. (E) The eumelanin monomer DHICA was oxidized and products were separated by HPLC, monitoring by mass spectrometry in negative ion mode. Monitoring for the 224 m/z ion of 225 dalton compounds 1 and 2, expected for triplet-carbonyl derivatives of dioxetane-adducted DHICA, reveals a single, polar peak. (F) Scanning the polar HPLC peak reveals four principal m/z peaks, one at 224 daltons.
We next explored whether UV exposure overcomes the migration barrier posed by the nuclear membrane. Cell-free experiments revealed that melanin polymer was rapidly solubilized after exposure to UVA or peroxynitrite (Fig. 4D). Because antibodies to melanin monomers do not exist, we were unable to directly monitor melanin migration into the nucleus. We therefore imaged unfixed melanocytes (to avoid artifactually permeabilizing the nuclear membrane), using differential interference contrast microscopy to maintain contrast at high magnification. Unirradiated normal melanocytes but not albino melanocytes showed dark granules in the cytoplasm, especially in the perinuclear area (fig. S5). We presume these are melanin aggregates in melanosomes, coated vesicles, and endoplasmic reticulum because they have the same size and cellular locations as granules seen in melanocytes immunostained for tyrosinase (31). After UVA exposure, 3D images reconstructed from serial planar images revealed granules inside the nucleus (Supplementary Movies S1 and S2). We do not know whether these represent melanin-containing organelles that moved into the nucleus or molecular aggregates formed by spontaneous polymerization of melanin precursors, as occurs during normal melanin synthesis. We conclude that, in cells exposed to UV, melanin or its constituents are able to enter the nucleus.
Finally, we sought to identify carbonyl-containing DHICA reaction products remaining after the triplet excitation energy discharges. The milder reaction of horseradish peroxidase with hydrogen peroxide was used to avoid complete oxidation and so reveal labile reaction intermediates. The DHICA analog of the predicted eumelanin degradation product (29) (Fig. 4E, structure 1) has a molecular weight of 225 daltons and would be highly polar. An alternative reaction is formation of a dioxetane moiety at the pyrrole ring (32), also leading to a polar structure of 225 daltons (structure 2). When we separated reaction products by HPLC and scanned the HPLC eluate by mass spectrometry in negative ion mode for the corresponding 224 m/z product, we observed a single peak eluting in the highly polar region of the buffer gradient (Fig. 4E). Scanning this fraction of the HPLC eluate across the small-molecule region of the mass spectrometer revealed four principal m/z peaks, of which the second largest was 224 daltons (Fig. 4F). Fragmention of these HPLC fractions generated m/z peaks assignable to oxidation products of either structure 1 or 2 created by known oxidation reactions. When we blocked the reactivity of DHICA's 6-membered ring by omitting an OH group (5-hydroxyindole-2-carboxylic acid) (fig. S6A), oxidation still produced chemiluminescence (fig. S6B,C) and CPD (fig. S6D), suggesting that 2 is the predominant product. Together, these results indicate that melanin and melanin fragments are capable of acting as the molecular vectors that acquire an electronically excited triplet state, probably at a carbonyl arising from a dioxetane intermediate.
Proposed Scheme for the Participation of Melanin in Melanoma Development
In summary, we have shown that exposing melanin-containing cells to UV radiation induces superoxide and nitric oxide, causing an orders-of-magnitude peroxynitrite spike that degrades melanin, allows melanin-like granules to appear in the nucleus, and excites melanin derivatives to a triplet state that has the high energy of a UV photon (Fig. 5). These evanescent electronically excited products transfer their triplet energy to DNA hours after the original UV exposure, creating mutagenic cyclobutane pyrimidine dimers in the dark. We speculate that the degradation of melanin polymer into fragments allows these moieties to closely approach the DNA, and that the peroxynitrite–melanin reaction intermediate is a short-lived dioxetane (fig. S6E) whose triplet energy level lies well above that of the DNA bases. The sustained time course of dark CPD generation can be accounted for by the prolonged steps of UV-induction of NOX and iNOS, peroxynitrite-induced solubilization of melanin to fragments or release of pre-melanin monomers from UV-damaged melanosomes and vesicles, and migration to the nucleus. It remains to identify the isozymes generating superoxide and nitric oxide, the cell site(s) at which melanin is degraded and its fragments are excited to a triplet state, the full inventory of eumelanin and pheomelanin fragments that can host triplet states, the chemical intermediate through which ONOO– creates the triplet carbonyl, and the energy transfer process.
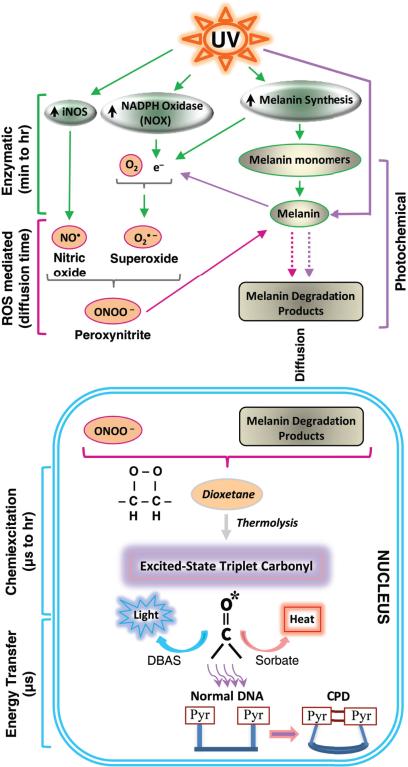
Fig. 5. A mechanistic model for the generation of dark CPD in melanocytes by chemiexcitation, with melanin as an active participant.
Exposing cells to UV radiation is known to upregulate iNOS, NADPH oxidase (NOX), and enzymes of melanin synthesis, presumably causing sustained generation of nitric oxide (NO•) and superoxide (O2•–). Cytoplasmic NOS and NOX are indicated on the figure but some isoforms are located in the nucleus. The present experiments show a UV-induced surge in the product of these two radicals, the powerful oxidant peroxynitrite (ONOO–), and show that peroxynitrite degrades melanin polymer to fragments. Melanin or melanin fragments then appear in the nucleus. Peroxynitrite is also one of the few biologically-synthesized molecules capable of exciting an electron to a triplet state. The present experiments show that, on a faster time scale, peroxynitrite excites an electron in a melanin fragment to a triplet state that has the high energy of a UV photon. The typical triplet-state reaction intermediate, not demonstrated here (hence indicated in italics), is a cycloaddition of –O–O– to create an unstable dioxetane; dioxetanes undergo spontaneousthermolysis to yield two carbonyls, one of which acquires most of the energy and finishes in a high energy triplet state (*). For the melanin-related triplet, the half-life of the reaction intermediate appeared to be minutes and a carbonyl consistent with a dioxetane precursor was identified by mass spectrometry. Triplet energy then discharges on a microsecond timescale to generate visible luminescence, or discharges in a radiation-independent manner to DBAS to be emitted as fluorescence, to sorbate to be dissipated as isomerization and heat, or evidently toDNA bases where it makes CPD. The presence of melanin, activation of iNOS and NOX, and the triplet state were shown to be required for dark CPD formation.
A consequence of these events is that melanin may be carcinogenic as well as protective against cancer. This double nature would explain the apparent cancer-facilitating effects of melanin seen in mice and in human epidemiology (2-4, 6). Melanin is an unusual polymer whose properties set the stage for the events we have described (33). Highly reactive o-quinones created by ROS-generating redox transformations of tyrosine polymerize spontaneously into oligomers. The o-quinones readily accept an electron to become semiquinone radicals, giving melanin a high concentration of free radicals in stable redox equilibrium and stabilized by metal ions. This macromolecule is a photon trap that also acts as an electron-proton photoconductor. These characteristics give melanin its broad light absorption, radical scavenging, and metal reservoir properties, but at a price. First, melanin synthesis generates O2•– and H2O2. UV exposure additionally excites the rings to an energy that, especially for pheomelanin, ejects an electron that is captured by oxygen to yield more O2•- (5, 34). Second, the reactive semiquinones allow melanin to be degraded and these fragments to be adducted to create high-energy unstable moieties such as dioxetanes. Although most of the cell's melanin synthesis is safely isolated inside melanosomes, the early steps occur in close proximity to the nucleus.
It was proposed long ago that chemiexcitation – the creation of chemical reaction products containing excited electrons that underlie bioluminescence in lower organisms – has broad importance in biology (22, 23). Our data suggest that this may be the case in skin. The consequence is that half or more of the CPD in a melanocyte arise after UV exposure ends. In vivo the same appears to be true of keratinocytes, which receive melanosomes donated by melanocytes. If the same holds for human skin, this would mean that past measurements of CPD immediately after UV exposure have underestimated the consequences of UV exposure.
One benefit of dark photochemistry's slow course is that it allows intervention. A blocker of dark CPD, α-tocopherol (vitamin E), is not only an antioxidant but also inactivates dioxetanes by converting them to a pair of diols (35). The triplet quencher, ethyl sorbate is an analog of the widely used food preservative potassium sorbate. Screening for novel triplet quenchers offers the prospect of developing “evening-after” sunscreens that could potentially prevent the carcinogenic processes occurring in skin hours after sun exposure ends.
Supplementary Material
Acknowledgments
We thank M. Bosenberg and V. Muthusamy for UVA-irradiated mouse skin; the Yale Office of Environmental Health and Safety for the single-photon liquid scintillation counter; A. Bommakanti for photography; and D. Mitchell and A. Mennone for helpful discussions. Supported by Department of Defense CDMRP grants CA093473P1 and CA093473 to DEB and RH and NIH grant 2 P50 CA121974 to RH and DEB. Confocal support was provided by NIH grant P30 DK34989 to the Yale Liver Center. SW was supported by a Postgraduate Fellowship from the University of Veterinary Medicine, Vienna. CMM was supported by Doctoral Fellowship 09/02062-8 from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and EJHB was supported by FAPESP grant 06/56530-4 and the INCT - Processos Redox em Biomedicina.
References and Notes
- 1.Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J. Photochem. Photobiol. B. 1991;9:135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- 2.Williams PF, Olsen CM, Hayward NK, Whiteman DC. Melanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burden. Int J Cancer. 2011;129:1730–1740. doi: 10.1002/ijc.25804. [DOI] [PubMed] [Google Scholar]
- 3.Noonan FP, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun. 2012;3:884. doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra D, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chedekel MR, Smith SK, Post PW, Pokora A, Vessell DL. Photodestruction of pheomelanin: role of oxygen. Proc. Natl. Acad. Sci. U.S.A. 1978;75:5395–5399. doi: 10.1073/pnas.75.11.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi S, et al. Melanin acts as a potent UVB sensitizer to cause an atypical mode of cell death in murine skin. Proc. Natl. Acad. Sci. U S A. 2004;101:15076–15081. doi: 10.1073/pnas.0403994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz-Munoz JL, et al. Generation of hydrogen peroxide in the melanin biosynthesis pathway. Biochim Biophys Acta. 2009;1794:1017–1029. doi: 10.1016/j.bbapap.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Brash DE. UV signature mutations. Photochem. Photobiol. 2014 doi: 10.1111/php.12377. doi:10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauthammer M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brash DE, Seetharam S, Kraemer KH, Seidman MM, Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schreier WJ, et al. Thymine dimerization in DNA is an ultrafast photoreaction. Science. 2007;315:625–629. doi: 10.1126/science.1135428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazovich D, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douki T, Reynaud-Angelin A, Cadet J, Sage E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42:9221–9226. doi: 10.1021/bi034593c. [DOI] [PubMed] [Google Scholar]
- 14.Materials and Methods are available as supplementary material on Science Online.
- 15.Kraemer KH, et al. Genetic heterogeneity in xeroderma pigmentosum: complementation groups and their relationship to DNA repair rates. Proc Natl Acad Sci U S A. 1975;72:59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrier W, Snyder R, Regan J. In: The Science of Photomedicine. Regan J, Parrish J, editors. Plenum; New York: 1982. pp. 91–112. [Google Scholar]
- 17.Beecham EJ, Mushinski JF, Shacter E, Potter M, Bohr VA. DNA repair in the cmyc proto-oncogene locus: possible involvement in susceptibility or resistance to plasmacytoma induction in BALB/c mice. Mol Cell Biol. 1991;11:3095–3104. doi: 10.1128/mcb.11.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon-Thomson C, et al. 1alpha,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol Sci. 2012;11:1837–1847. doi: 10.1039/c2pp25202c. [DOI] [PubMed] [Google Scholar]
- 19.Valencia A, Kochevar IE. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J Invest Dermatol. 2008;128:214–222. doi: 10.1038/sj.jid.5700960. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg M, Kohen R, Enk CD. Role of antioxidants in prevention of pyrimidine dimer formation in UVB irradiated human HaCaT keratinocytes. Biomed Pharmacother. 2006;60:233–237. doi: 10.1016/j.biopha.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Lamola AA. Production of pyrimidine dimers in DNA in the dark. Biochem Biophys Res Commun. 1971;43:893–898. doi: 10.1016/0006-291x(71)90701-7. [DOI] [PubMed] [Google Scholar]
- 22.White E, Miano J, Watkins C, Breaux E. Chemically produced excited states. Angw. Chem. Internat. Edit. 1974;13:229–243. [Google Scholar]
- 23.Cilento G. In: Chemical and Biological Generation of Excited States. Adam W, Cilento G, editors. Academic Press; New York: 1982. pp. 277–307. [Google Scholar]
- 24.Knudsen FS, et al. Chemiluminescent aldehyde and beta-diketone reactions promoted by peroxynitrite. Chem Res Toxicol. 2000;13:317–326. doi: 10.1021/tx990176i. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Graillet C, et al. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem. 1996;271:28052–28056. doi: 10.1074/jbc.271.45.28052. [DOI] [PubMed] [Google Scholar]
- 26.Velosa AC, Baader WJ, Stevani CV, Mano CM, Bechara EJ. 1,3-diene probes for detection of triplet carbonyls in biological systems. Chem Res Toxicol. 2007;20:1162–1169. doi: 10.1021/tx700074n. [DOI] [PubMed] [Google Scholar]
- 27.Hatta S, Mishima Y, Ichihashi M, Ito S. Melanin monomers within coated vesicles and premelanosomes in melanin synthesizing cells. J Invest Dermatol. 1988;91:181–184. doi: 10.1111/1523-1747.ep12464454. [DOI] [PubMed] [Google Scholar]
- 28.Kayatz P, et al. Oxidation causes melanin fluorescence. Invest Ophthalmol Vis Sci. 2001;42:241–246. [PubMed] [Google Scholar]
- 29.Slawinska D, Slawinski J. Electronically excited molecules in the formation and degradation of melanins. Physiol Chem Phys. 1982;14:363–374. [PubMed] [Google Scholar]
- 30.Wakamatsu K, Nakanishi Y, Miyazaki N, Kolbe L, Ito S. UVA-induced oxidative degradation of melanins: fission of indole moiety in eumelanin and conversion to benzothiazole moiety in pheomelanin. Pigment Cell Melanoma Res. 2012;25:434–445. doi: 10.1111/j.1755-148X.2012.01011.x. [DOI] [PubMed] [Google Scholar]
- 31.Halaban R, et al. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci U S A. 2000;97:5889–5894. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito I, Matsugo S, Matsuura T. 1,2-Dioxetane formation in an indole system. J. Am. Chem. Soc. 1979;101:4757–4759. [Google Scholar]
- 33.Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006;19:572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 34.Ye T, et al. Photoionization thresholds of melanins obtained from free electron laser-photoelectron emission microscopy, femtosecond transient absorption spectroscopy and electron paramagnetic resonance measurements of oxygen photoconsumption. Photochem. Photobiol. 2006;82:733–737. doi: 10.1562/2006-01-02-RA-762. [DOI] [PubMed] [Google Scholar]
- 35.Adam W, Vargas F, Epe B, Schiffmann D, Wild D. Single-electron-transfer in the reduction of 1,2-dioxetanes by biologically active substrates. Free Radic Res Commun. 1989;5:253–258. doi: 10.3109/10715768909074708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.