Abstract
Purpose
The pathophysiology of interstitial cystitis/painful bladder syndrome (IC/PBS) remains incompletely understood, but is thought to involve a central disturbance in the processing of pain and viscerosensory signals. We aimed to identify differences in brain activity and connectivity between female IC/PBS patients and healthy controls in order to advance clinical phenotyping and treatment efforts for IC/PBS.
Materials and Methods
We examined oscillation dynamics of intrinsic brain activity in a large sample of well-phenotyped female IC/PBS patients and female healthy controls collected during a 10-minute resting fMRI scan as part of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network* project. The BOLD signal was transformed to the frequency domain and relative power was computed for multiple frequency bands.
Results
The results demonstrated altered frequency distributions in a viscerosensory region (post Insula) and sensorimotor cortices (postcentral gyrus, paracentral lobule, supplementary motor area (SMA), including a region likely involved in control of pelvic floor muscles (PelMotor). Additionally, the SMA, paracentral lobule and PelMotor all demonstrated increased functional connectivity to the midbrain (red nucleus) and cerebellum. This increased functional connectivity was greatest in patients reporting pain during bladder filling.
Conclusions
These findings suggest that women with IC/PBS have a neuromotor component to their pathology involving an alteration in the intrinsic oscillations and connectivity within a cortico-cerebellar network previously associated with urinary bladder function.
Keywords: Interstitial cystitis, painful bladder syndrome, resting state, chronic pain, urologic chronic pelvic pain syndrome
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic pelvic pain condition associated with urinary urgency and frequency that may affect as many as 7.9 million women in the United States.1 The specific pathophysiology remains incompletely understood, but several lines of evidence suggest a role for central amplification of viscerosensory signals.2,3 The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network* is a multi-site endeavor to identify epidemiological and neuroimaging parameters to advance clinical phenotyping and treatment efforts for urological chronic pelvic pain, including IC/PBS. Standardized acquisition parameters for structural as well as resting scan were applied at 5 MAPP sites across the USA as part of the trans-MAPP neuroimaging study.
Resting scan fMRI capitalizes on the fact that a wealth of information can be extracted from the intrinsic fluctuations in the blood oxygen-level dependent (BOLD) signal without the need of an external stimulus. Advantages of resting scan fMRI over task-based fMRI include the ability for a greater degree of standardization across research centers. In addition, brain responses to acute, experimental pain in the laboratory may have limited utility for understanding chronic pain. Monitoring how the brain processes ongoing signals from the body and environment may provide novel insights into altered neurocircuitry in chronic pain patients.
In the current study, we examined intrinsic brain oscillations (resting state fMRI) from female IC/PBS patients and healthy controls for alterations in frequency power distribution and functional connectivity patterns. Based on previous reports in other chronic pain conditions3–5, we hypothesized that altered oscillation frequency and functional connectivity seen in IC/PBS would include viscerosensory and sensorimotor regions.
METHODS
Subjects
The present analysis included 85 female healthy controls (HC) and 82 IC/PBS patients collected from 5 different MAPP discovery sites (University of California at Los Angeles (UCLA), Northwestern University (NWU), University of Michigan (UM), University of Alabama at Birmingham (UAB), and Stanford University (SU)). This cohort represents all female HC and IC/PBS subjects from the trans-MAPP neuroimaging study for which completed resting scans existed with the exception of one subject that failed normalization during preprocessing of the resting scan data. Approval was received from each site’s respective ethics committee and consent was obtained from all participants. The number of subjects from each site is given inTable 1.
Table 1.
Participant characteristics: mean (standard error)
| Overall | UCLA | NWU | UM | UAB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | IC/PBS | HC | IC/PBS | HC | IC/PBS | HC | IC/PBS | HC | IC/PBS | |
| N | 85 | 82 | 14 | 9 | 9 | 29 | 18 | 14 | 9 | 9 |
| Age | 35.3(1.2) | 38.8(1.4) | 36.7(3.6) | 32.3(2.6) | 36.8(4.8) | 35.1(2.7) | 32.8(2.2) | 36.7(2.2) | 32.3(2.7) | 36.8(4.3) |
| Duration (yrs) | N/A | 10.4(1.3) | N/A | 6.9(2.7) | N/A | 8.1(4.5) | N/A | 6.6(1.5) | N/A | 23.1(3.4) |
| GUPI total | 1.5(0.3) | 26.2(1.0) | 1.3(0.4) | 24.8(2.8) | 0.9(0.4) | 26.0(2.7) | 0.9(0.2) | 25.9(1.7) | 3.1(2.5) | 27.2(3.4) |
| GUPI pain | 0.4(0.2) | 12.8(0.5) | 0.1(0.1) | 11.8(1.4) | 0.4(0.4) | 13.6(1.2) | 0.1(0.1) | 12.5(0.9) | 1.4(1.4) | 13.6(1.8) |
Procedures
Participants completed the female version of the Genitourinary Pain Index (GUPI) prior to completing the resting scan fMRI. The GUPI is used to quantify symptoms in men and women with urological pain conditions.6 The GUPI consists of pain, urinary and quality of life subscales. Thus, the GUPI total score incorporates responses from these 3 symptom domains.
fMRI Acquistion and Analysis
fMRI Acquisition
MRI scanning was performed at multiple sites using different scanner technology (3T Siemens Trio (NWU and UCLA), 3T Phillips Ingenia (UM), 3T Philips Achieva (UAB), and 3T GE Discovery (SU)). Trans-MAPP neuroimaging data was collected, quality controlled and archived according to multi-site imaging procedures developed collaboratively between the MAPP Research Network, the UCLA PAIN repository and the UCLA Laboratory of Neuroimaging. Detailed procedures and description of the repository are available at PAINrepository.org. Scanner compatible acquisition parameters were developed and all sites were required to complete and pass a site qualification including a set of pilot scans of a human volunteer; the initial scans were reviewed for quality control by the UCLA site, and recommendations and adjustments were made as necessary prior to commencement of study scans. A high resolution structural image was acquired from each subject with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2200 ms, echo time (TE) = 3.26 ms, slice thickness = 1 mm, 176 slices, 256×256 voxel matrices, and 13 mm voxel size. Resting state scans were acquired while subjects rested with eyes closed for 10 minutes in 40-slice whole brain volumes, slice thickness = 4 mm, TR = 2000 ms, TE = 28 ms, flip angle = 77°.
fMRI Preprocessing
Using Data Processing Assistant for Resting-State fMRI (DPARSF)7 (http://www.restfmri.net/forum/DPARSF) which is based on SPM8 (Welcome Department of Cognitive Neurology, London, UK) and Resting-State fMRI Data Analysis Toolkit8, data were slice-time and motion corrected. Nuisance covariate regression was then performed to minimize physiological noise using six head motion parameters, white matter signal and corticospinal fluid signal. Data were spatially normalized to the Montreal Neurological Institute (MNI) template using structural scans. Spatial smoothing with a 3 mm3 Gaussian kernel occurred after calculation of frequency and connectivity maps (see below).
Statistical Analyses
Frequency Analysis
Using the framework developed by Buzsaki and colleagues9,10 the BOLD signal timecourse data of each voxel was transformed to the frequency domain and subdivided into 3 frequency bands, referred to in the literature as slow-5 (0.01–0.027 Hz), slow-4 (0.027–0.073 Hz), and slow-3 (0.073–0.198 Hz). For convenience, we will refer to these 3 frequency bands as low frequency (LF), medium frequency (MF) and high frequency (HF) respectively. A mask created from the first image of each subject intersected with a grey matter mask was applied to restrict analyses to grey matter regions without dropout of signal. The fractional amplitude of frequency fluctuation for each of the bands was computed for each voxel and normalized to the mean.11 A Group (HC; IC)×Band (LF; MF; HF)×Site (NWU; UCLA; UAB; UM; SU) flexible factorial analysis was performed in SPM8 with age of subject entered as a covariate. Contrasts were performed for each band to identify regions with altered frequency power distribution in IC/PBS compared to HC. Regions with a cluster-level familywise error (FWE) corrected p < 0.05 were considered significant. Small volume corrections were performed for the right and left insula as well as for a 6mm sphere centered at −2 −18 70 (PelMotor) as this region has previously been shown to be maximally activated with pelvic floor contraction, and which has been shown to have altered resting state functional connectivity in men with urologic chronic pelvic pain syndrome5,12. The insula was selected as a region of interest (ROI) as it has been shown to have structural and functional alterations in patients with IC/PBS and chronic pain disorders that are co-morbid with IC/PBS.3,4,13 Finally, given the small size of nuclei within the brainstem, it is particularly difficult to detect alterations within the brainstem using whole brain statistics, thus small volume correction was performed for the brainstem.
Seed-based Functional Connectivity Analyses
The marsbar toolkit for SPM was used to create five seed ROIs of significant clusters from the above frequency analysis.14 The preprocessed functional data was bandpass filtered using LF, MF, and HF bands and band-specific fisher transformed maps of the bivariate correlation between seed ROI time-course and all other voxels were created using DPARSF. Group differences in band-specific functional connectivity for each of the identified seeds were examined using a flexible factorial model in SPM8 with Site and Group factors and age of subject as a covariate.
RESULTS
Subject characteristics
Average age, symptom duration, total GUPI scores and GUPI pain subscores are given inTable 1. Age showed a trend to be greater in IC/PBS patients compared to the healthy controls (t(165)=−1.875, p=.062) with UAB demonstrating older patients with significantly longer symptom duration (23.1 years) compared to other sites (7.6 years).
Group differences in oscillation frequency power
Female IC/PBS patients and healthy controls were examined for regional brain differences in frequency power within 3 frequency bands (LF, MF, HF) thought to represent different neuronal oscillation classes.10 Alterations were found exclusively in the lowest frequency band (LF; 0.01–0.027 HZ). IC/PBS patients demonstrated significantly greater LF power in postcentral gyrus (primary sensory cortex), paracentral lobule (within primary motor cortex), supplementary motor area (SMA) and in PelMotor (Figure 1A;Table 2). In addition, the right posterior insula (pINS) demonstrated significantly less LF power in patients compared to HCs (Figure 1B;Table 2).
Figure 1.
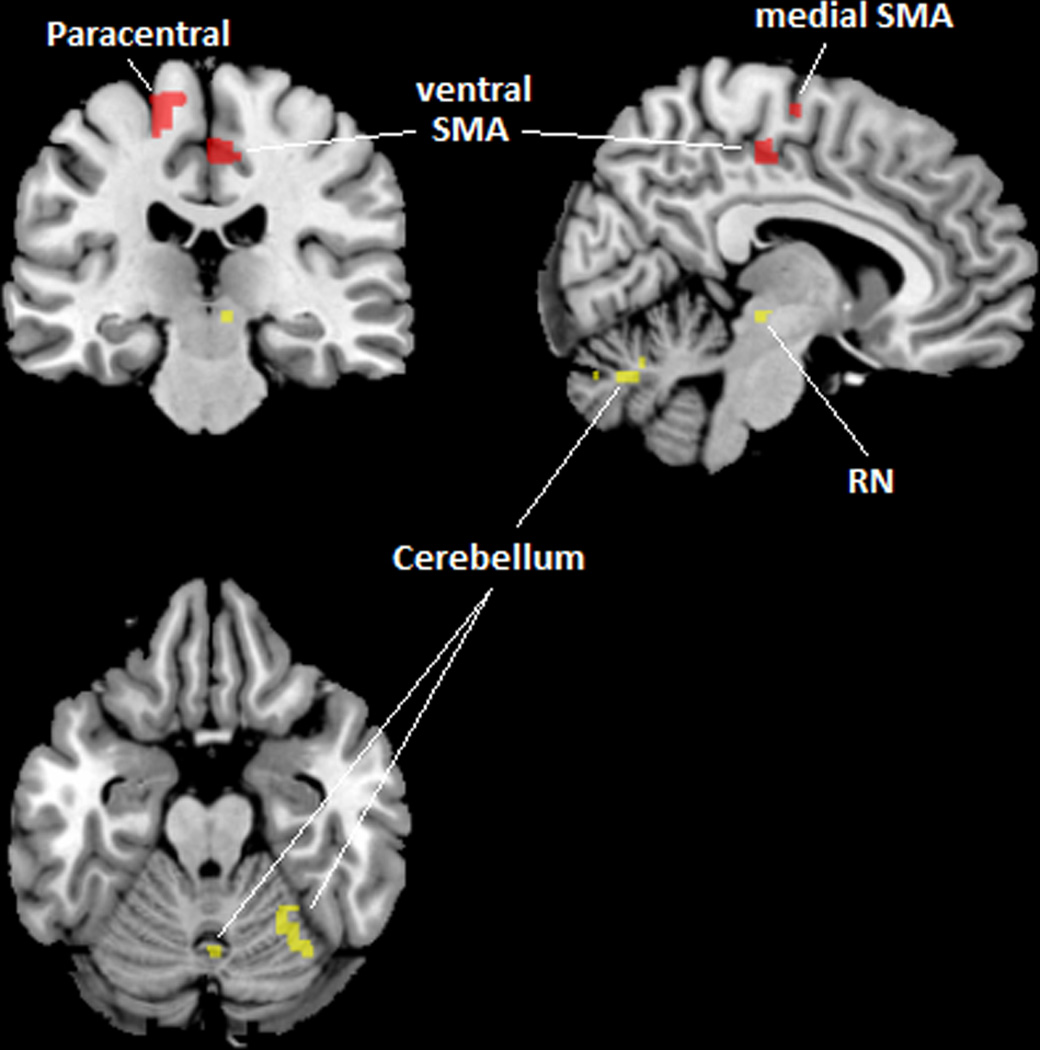
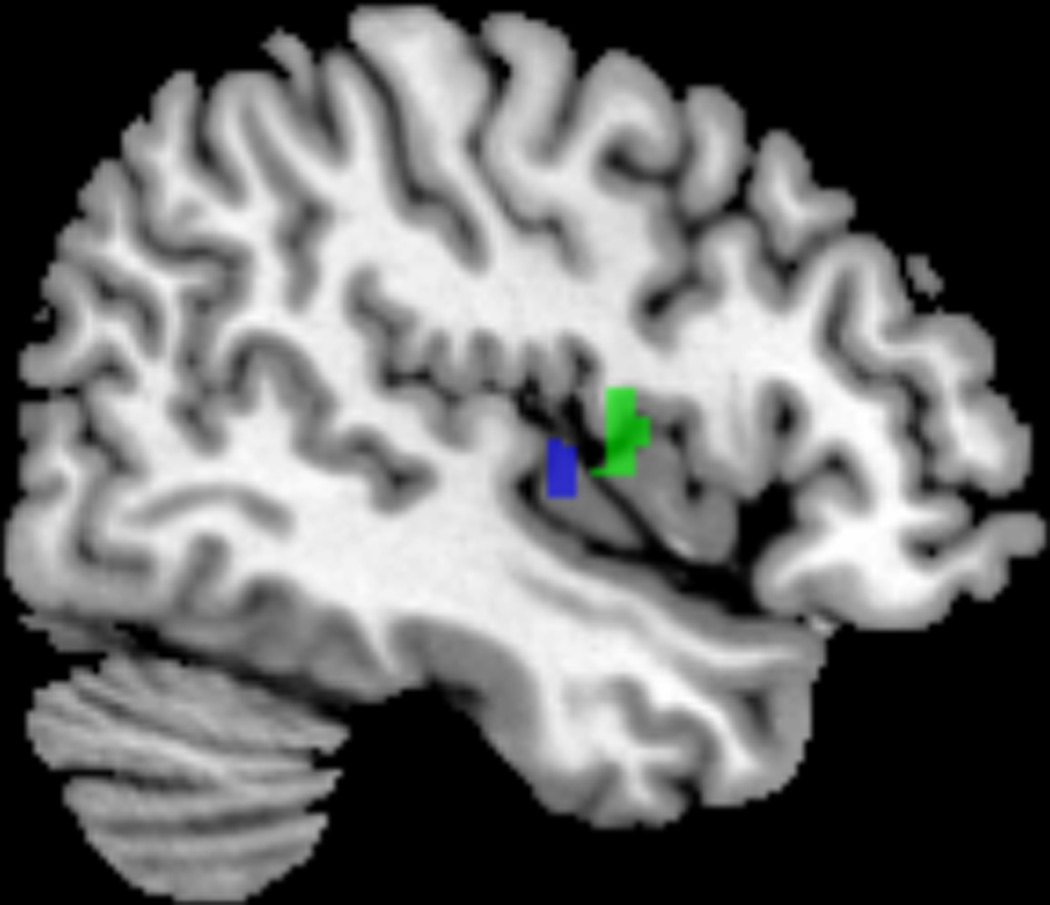
(A) PelMotor, SMA, and paracentral lobule (shown in red) demonstrated increased LF power and increased LF connectivity with a region in the right midbrain (red nucleus) and regions within the cerebellular vermis VI/VII and right lobule VI (shown in yellow) in patients compared to controls. (B) pINS (shown in blue) demonstrated decreased LF power and decreased LF connectivity with mINS (shown in green) in patients compared to controls.
Table 2.
| Region | Hemi- sphere |
X | Y | Z | Size | Z | PFWE |
|---|---|---|---|---|---|---|---|
| IC/PBS greater LF power compared to HC | |||||||
| PelMotor | Bilateral | −2 | −16 | 68 | 10 | 3.95 | .007 (SVC) |
| SMA | Right | 4 | −22 | 48 | 95 | 4.98 | .025 |
| Paracentral | Left | −16 | −24 | 58 | 80 | 5.13 | .025 |
| Postcentral | Right | 44 | −14 | 30 | 295 | 5.43 | <.001 |
| IC/PBS less LF power compared to HC | |||||||
| pINS | Right | 46 | −10 | 4 | 16 | 3.80 | .049 (SVC) |
| IC/PBS greater LF functional connectivity compared to HC (seed is listed first) | |||||||
| PelMotor-midbrain | Right | 8 | −24 | −8 | 66 | 4.30 | .007 (SVC) |
| SMA-midbrain | Right | 6 | −24 | −6 | 22 | 3.96 | .048 (SVC) |
| Left | −6 | −24 | −4 | 28 | 3.92 | .035 (SVC) | |
| SMA-cerebellum | Bilateral | 2 | −70 | −30 | 240 | 3.98 | .002 |
| Right | 20 | −66 | −18 | 173 | 4.16 | .011 | |
| Paracentral-midbrain | Right | 8 | −26 | −10 | 24 | 3.69 | .042 (SVC) |
| Bilateral | 0 | −26 | −20 | 41 | 3.52 | .018 (SVC) | |
| Paracentral-cerebellum | Left | −34 | −82 | −18 | 536 | 4.27 | <.001 |
| Right | 36 | −64 | −20 | 163 | 4.38 | .012 | |
| Bilateral | 0 | −66 | −24 | 260 | 4.08 | .001 | |
| Paracentral-superior parietal | Left | −8 | −70 | 54 | 153 | 3.82 | .017 |
| Left | −30 | −62 | 44 | 230 | 4.49 | .002 | |
| Paracentral-precuneus | Bilateral | 0 | −48 | 58 | 373 | 4.08 | <.001 |
| IC/PBS less LF functional connectivity compared to HC (seed is listed first) | |||||||
| pINS-mINS | Right | 46 | 0 | 6 | 32 | 3.93 | .027 (SVC |
Group differences in functional connectivity
The functional connectivity within the LF band of the 5 regions displaying altered LF power was examined in patients and healthy controls. Increased connectivity in patients was found between: 1) PelMotor and right midbrain; 2) SMA and cerebellum; 3) SMA and bilateral midbrain; 4) paracentral lobule and superior parietal cortex; 5) paracentral lobule and cerebellum; and 6) paracentral lobule and right midbrain (Figure 1;Table 2). Reduced connectivity in patients compared to controls was found between right pINS and right mid INS (mINS;Table 2). The altered functional connectivity was selective to the LF range and was not seen in MF or HF range. Notably, three of the regions (PelMotor, SMA, paracentral lobule) demonstrated altered connectivity with the same region of the right midbrain (in the area of the red nucleus, centered at 6 −24 −7 with a volume of 64 mm). Similarly, two of the regions (SMA and paracentral lobule) demonstrated increased LF functional connectivity with the same regions in the cerebellum located in vermis VII (centered at 5 −69 −27 with a volume of 712 mm) and in right lobule VI (centered at 28 −58 −20 with a volume of 432 mm). PelMotor also demonstrated increased LF functional connectivity with these cerebellular regions once a small volume correction was applied.
Clinical Correlates
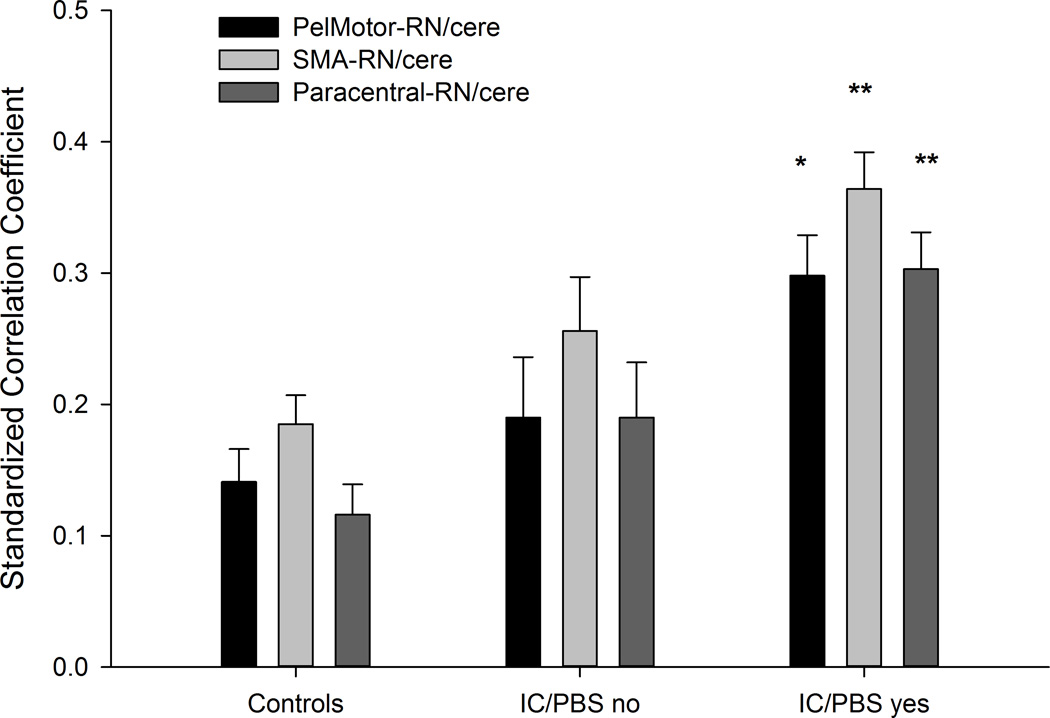
The cortico-cerebellar and insular functional connectivity identified as altered in patients was evaluated for associations with symptom duration and GUPI scores in IC/PBS patients (controlling for age and site). None of the functional connectivity measures correlated with self-reported duration of urological symptoms or with composite GUPI scores (p’s > 0.05). To further evaluate if the observed brain changes were correlated with symptoms, we tested for differences among the patients (using a general linear model controlling for age and site) based on the yes/no responses to individual items on the GUPI regarding pain during urination and pain during bladder filling. 48% of the patients indicated pain during urination and 64% of the patients indicated pain during bladder filling. LF functional connectivity measures did not differ between IC/PBS patients endorsing the presence of pain during urination from those that did not (p’s > 0.05). However, those endorsing presence of pain during bladder filling compared to those who did not showed increased functional connectivity between: 1) PelMotor and red nucleus/cerebellum (F(1,74) = 4.91, p = 0.03), 2) SMA and red nucleus/cerebellum (F(1,74) = 4.24, p = 0.043), and 3) paracentral lobule and red nucleus/cerebellum (F(1,74) = 6.41, p = 0.013) (Figure 2).
Figure 2.
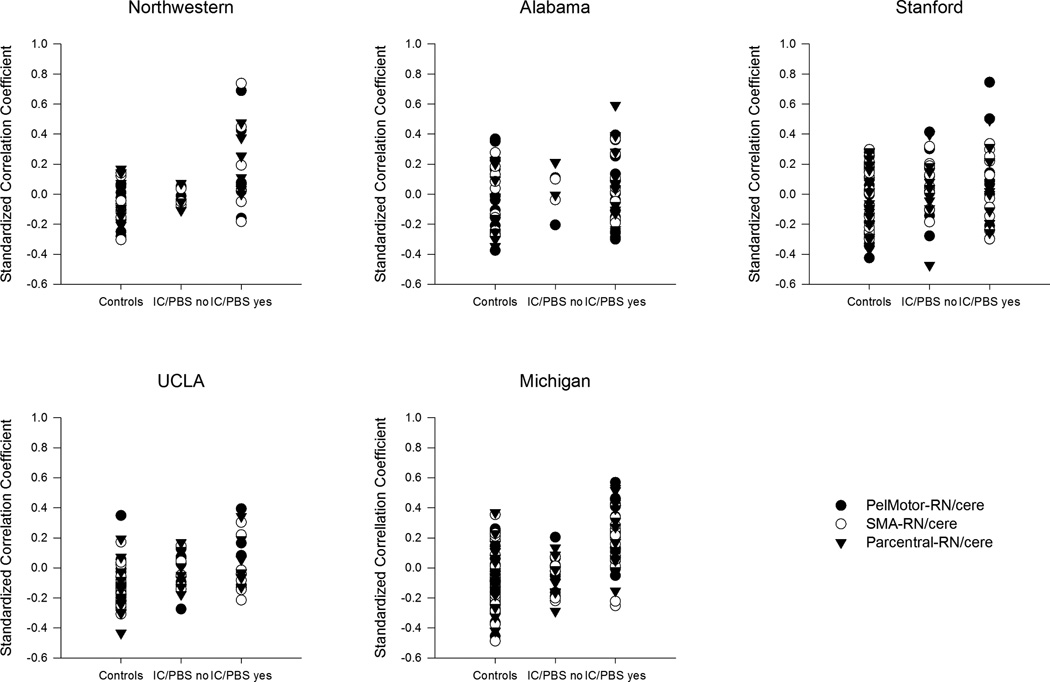
(A) The estimated mean functional connectivity (controlling for age ) between PelMotor, SMA, paracentral lobule and red nucleus/cerebellar regions (RN/cere) is shown for healthy controls, IC/PBS with pain during bladder filling (IC/PBS yes) and IC/PBS without pain during bladder filling (IC/PBS no). (B) Functional connectivity values are plotted for each individual within each of the 5 scanning sites.
*p<0.05 compared to controls
**p<0.05 compared to controls and IC/PBS no
Discussion
To our knowledge, this is the first demonstration of abnormalities in the intrinsic oscillation of the resting brain in a large sample of well phenotyped women with IC/PBS. The results demonstrated disease related alterations in frequency distributions in 1) viscerosensory regions (pINS) which showed reduced LF power in patients, and in 2) sensorimotor-related cortices (postcentral gyrus, SMA, paracentral lobule, and a motor region likely involved in pelvic muscle control (PelMotor))12 which showed increased LF power in patients. In addition, most of the clusters demonstrating altered frequency distribution also demonstrated frequency-specific alterations in functional connectivity. Strikingly, 3 of the increased LF frequency clusters (SMA, paracentral lobule, and PelMotor) demonstrated increased LF functional connectivity with the same areas of the midbrain (right red nucleus) and cerebellum (vermis VI/VII, right lobule VI) in the patient group. The increased functional connectivity between motor cortices and red nucleus/cerebellum was greatest in patients endorsing the presence of pain during bladder filling. These results suggest an alteration in the ongoing activity in known cortico-cerebellar pathways in women with IC/PBS that may relate to one particular aspect of IC/PBS symptomology, namely the experience of pain during bladder filling.
Functional neuroanatomy of micturition
Preclinical and human imaging studies have identified a number of cortical (insula, prefrontal, anterior cingulate, motor cortex, cerebellum), midbrain (hypothalamus, red nucleus) and medullary pontine brain regions (periaqueductal gray, Barrington’s nucleus, locus coeruleus complex, inferior olive) directly involved in the control of micturition.15 The voiding reflex is mediated by spinal and medullary pontine relay nuclei, but in humans receives strong input from cortical regions, including the anterior cingulate, prefrontal and motor regions. Conscious perception of urinary sensations (including fullness urgency and pain) involves activation of the insular cortex. In the current study, alterations in the frequency power distribution of intrinsic oscillations were observed in several of these micturitioin related brain regions.
1. Alterations in viscerosensory regions
The insula has a posterior-to-mid-to-anterior integration of interoceptive information with primary interoceptive representations located in the pINS and subjective awareness of interoceptive information in anterior insula.16 Activation of the posterior and mid insula have been shown to be involved in sensorimotor tasks while the anterior insula is an association cortex, integrating cognitive, affective and interoceptive inputs.17 The lower LF power in the pINS of women with IC/PBS compared to healthy controls may suggest increased neural activity in pINS (however, see Limitations for other interpretations), consistent with tonically increased viscerosensory input to the brain. Such input may come from increased afferent input from the urinary bladder, or from dorsal horn neurons, sensitized by descending facilitatory input from the brain. In addition, the reduced connectivity between pINS and mINS suggests altered integration of interoceptive information into subjective awareness. However, no significant correlations between any of the insula subregions and clinical symptoms were observed.
2. Alterations in pelvic motor related cortical and subcortical regions and pathways
Several regions with known involvement in micturition and pelvic motor control (postcentral gyrus, SMA, paracentral lobule, and a motor region likely involved in pelvic muscle control (PelMotor)) showed an increase in LF power in the patient group. One possible interpretation of this finding may be related to these regions being less active in women with IC/PBS, although other interpretations are possible (see Limitations below).
PelMotor is a region within the primary motor cortex that has previously been shown to be maximally activated with pelvic floor contraction.5,12 Many IC/PBS patients demonstrate pelvic floor dysfunction;18 thus, PelMotor was a region of interest in the current study. The red nucleus is a relay center, whose functions include the integration of information from motor cortex and cerebellum. Information is sent back to the cerebellum via the inferior olive and to the spinal cord via the rubrospinal tract.19 Several observations implicate a role of the red nucleus in bladder motility. For example, injection of virus tracers into the bladder has demonstrated that the red nucleus is a supraspinal center connected to the bladder20. Electrical stimulation of the red nucleus results in inhibition of spontaneous contractions of the urinary bladder during instillation of fluid into the bladder.21 In addition, elevated levels of nerve growth factor in the red nucleus has been associated with development of neuropathic pain following peripheral nerve injury in rats.22 Thus, an alteration in the coordination between the cerebellum and motor cortex involving the red nucleus may have consequences for bladder function and pain. Even though cerebellar activation has often been observed in evoked brain responses to experimental pain stimuli, its specific role in pain processing/modulation remains unclear.23 On one hand, the cerebellum plays an important role in central autonomic control, and receives strong noradrenergic input from the pontine locus coeruleus complex (Barrington’s nucleus and locus coeruleus), a region that is often referred to as the pontine micturition center.15 On the other hand, the cerebellum plays a major role in motor learning, forming a forward dynamic model that is involved in the predicted outcomes of a motor action.19
Some results from the current study are remarkably similar to another study from the MAPP neuroimaging consortium, performed in men with urologic chronic pelvic pain syndrome (UCPPS).5 Functional connectivity of PelMotor with pontine (possibly including the inferior olive) and cerebellar regions was shown to be altered, suggesting changes in the cortico-pontine-cerebellar pathway in male UCPPS patients affecting neuromotor control. Similar to men with UCPPS, female IC/PBS patients had altered PelMotor connectivity with a similar subregion within the cerebellum in the current study. While altered connectivity between PelMotor and pontine regions was not found in the current study, altered connectivity between PelMotor and a relay center between PelMotor and the inferior olive (the red nucleus) was demonstrated. When viewed together, these studies suggest that both men with UCPPS and women with IC/PBS have altered cortical control of the cerebellum and brainstem structures known to be involved in micturition.15 Altered neuromotor function in IC/PBS is also consistent with a previous study demonstrating deficits in sensorimotor gating in women with IC/PBS.24
Although similarities exist between the current study and the MAPP study of men with UCPPS discussed above5, the findings of the current study differ from findings in other persistent pain conditions, such as irritable bowel syndrome, vulvodynia, fibromyalgia, and low back pain, that have demonstrated pain-related abnormalities in classical “pain regions”, including anterior insula, anterior cingulate cortex, and thalamus.3,4 Possibly, alterations in the activity of typical pain regions in pain syndromes are only seen during evoked or spontaneous pain, not during resting conditions. Alternatively, IC/PBS may be a different condition with different brain abnormalities, which will need to be addressed in future studies.
Limitations
The separation of frequency bands used in this analysis was based on the observation that behaviorally relevant brain oscillations have linearly distributed center frequencies on the natural logarithmic scale;9 however, little is known about the functional relevance of these bands. Given that activation of brain regions through a task has been shown to shift frequency distribution towards higher frequencies,25 one possible interpretation of theincreased LF power in regions within motor cortices is that these regions are less active in women with IC/PBS. However, local field potential studies show that lower frequency oscillations are found in deeper cortical layers.26 As the BOLD signal within a brain voxel combines signals from all layers of the cortex, another possibility is that the contribution of the signal from deeper cortical layers, including layer V where the cortico-rubral pathway originates, is enhanced in women with IC/PBS, consistent with the observed increase in functional connectivity between motor cortices and red nucleus.
Possible clinical implications
Although no assessment of pelvic floor dysfunction was performed in the current study, several clinical observations support the concept of increased pelvic floor contractions in IC/PBS. Numerous studies have reported the high prevalence of pelvic floor dysfunction in IC/PBS in terms of pain during physical palpitation.18,27 In addition, pelvic MRI has demonstrated a shorter puborectal distance and levator lengths in IC/PBS patients, consistent with hypertonicity.28 Physical therapy of the pelvic floor has been a successful therapy for IC/PBS as shown in a recent random controlled study.29 Finally, animal studies have shown that somatic dysfunction can induce visceral hypersensitivity.30 However, the demonstrated altered activity and connectivity of cortical control regions of the pelvic floor in women with IC/PBS could be a primary or secondary abnormality. For example, a persistent or recurrent sensation of urgency and bladder fullness resulting from increased viscerosensory input to the insular cortex could result in increased engagement of cortical control regions to voluntarily suppress the micturition reflex. Longitudinal studies are needed to demonstrate if therapies targeted at abnormal motor control (such as biofeedback, muscle relaxation and pelvic floor exercises) will normalize the central motor networks, and if this normalization is associated with reduction in symptoms. In addition, further brain imaging studies may help to identify biologically distinct symptom based subgroups such as those with and without pain during bladder filling.
Supplementary Material
Acknowledgments
Funding: Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316.). In addition, this work was supported in part by: R01 DK04835 and K01 DK085133.
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens JQ. Male and female pelvic pain disorders--is it all in their heads? J Urol. 2008;179:813. doi: 10.1016/j.juro.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Mayer E, Bushnell M. Functional pain disorders: Time for a paradigm shift? In: Mayer E, Bushnell M, editors. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press; 2009. [Google Scholar]
- 4.Farmer MA, Chanda ML, Parks EL, et al. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutch J, Yani M, Cossand L, et al. Altered neuromotor connectivity in men with urologic chronic pelvic pain syndrome: A Trans-MAPP neuroimaging study. Journal of Urology. submitted. [Google Scholar]
- 6.Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 10.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrum A, Wolff S, van der Horst C, et al. Motor cortical representation of the pelvic floor muscles. J Urol. 2011;186:185. doi: 10.1016/j.juro.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Dinov ID, Labus J, et al. Sex-Related Differences of Cortical Thickness in Patients with Chronic Abdominal Pain. PLoS One. 2013;8:e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett M, Anton JL, Valabregue R, et al. Region of interest analysis using an SPM toolbox. Eighth International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 15.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 20.Zermann DH, Ishigooka M, Doggweiler R, et al. Central autonomic innervation of the lower urinary tract--a neuroanatomy study. World J Urol. 1998;16:417. doi: 10.1007/s003450050094. [DOI] [PubMed] [Google Scholar]
- 21.Lewin RJ, Dillard GV, Porter RW. Extrapyramidal inhibition of the urinary bladder. Brain Res. 1967;4:301. doi: 10.1016/0006-8993(67)90160-6. [DOI] [PubMed] [Google Scholar]
- 22.Jing YY, Wang JY, Li XL, et al. Nerve growth factor of red nucleus involvement in pain induced by spared nerve injury of the rat sciatic nerve. Neurochem Res. 2009;34:1612. doi: 10.1007/s11064-009-9950-7. [DOI] [PubMed] [Google Scholar]
- 23.Moulton EA, Schmahmann JD, Becerra L, et al. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65:14. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick LA, Ornitz E, Ibrahimovic H, et al. Gating of sensory information differs in patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;184:958. doi: 10.1016/j.juro.2010.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baria AT, Baliki MN, Parrish T, et al. Anatomical and Functional Assemblies of Brain BOLD Oscillations. J Neurosci. 2011;31:7910. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maier A, Adams GK, Aura C, et al. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassaly R, Tidwell N, Bertolino S, et al. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22:413. doi: 10.1007/s00192-010-1301-3. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman AL, Lee UJ, Tan N, et al. Alterations in the pelvic floor musculature on pelvic MRI in patients with Interstitial Cystitis. Journal of Urology. 2012;187:e337. [Google Scholar]
- 29.FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187:2113. doi: 10.1016/j.juro.2012.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster R, Jung J, Farooq A, et al. Sciatic nerve injury induces functional pro-nociceptive chemokine receptors in bladder-associated primary afferent neurons in the rat. Neuroscience. 2011;183:230. doi: 10.1016/j.neuroscience.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.