Abstract
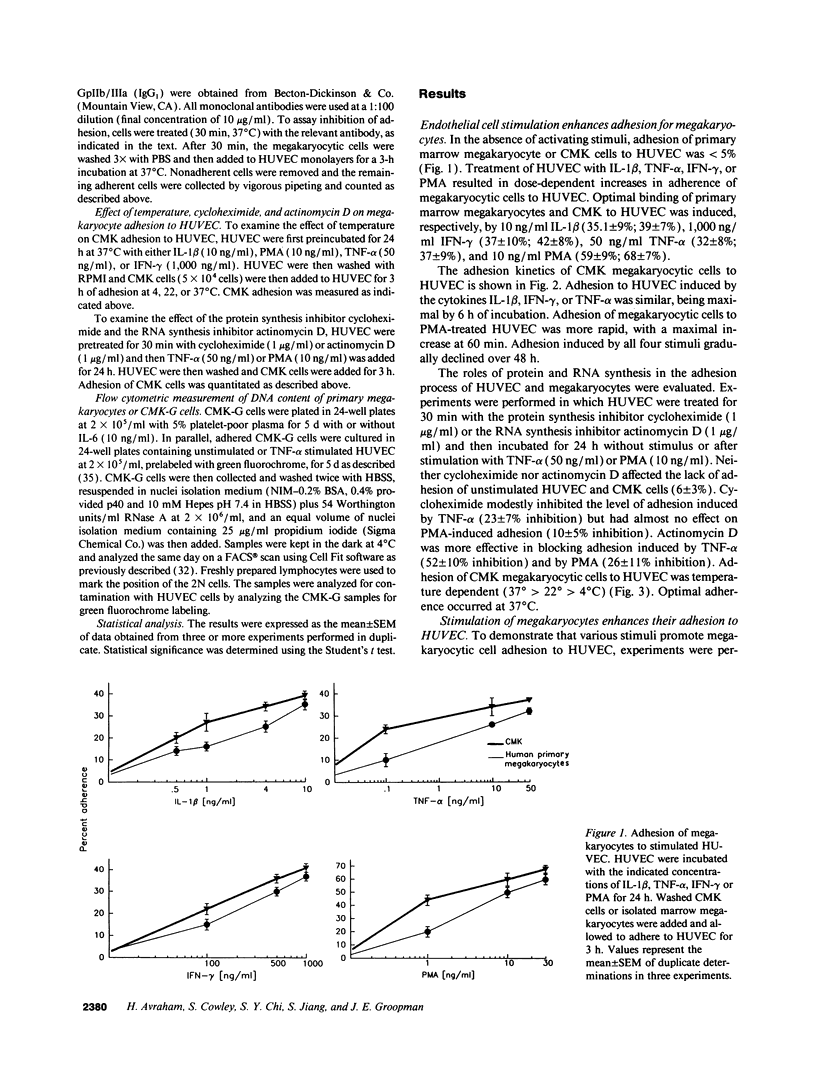
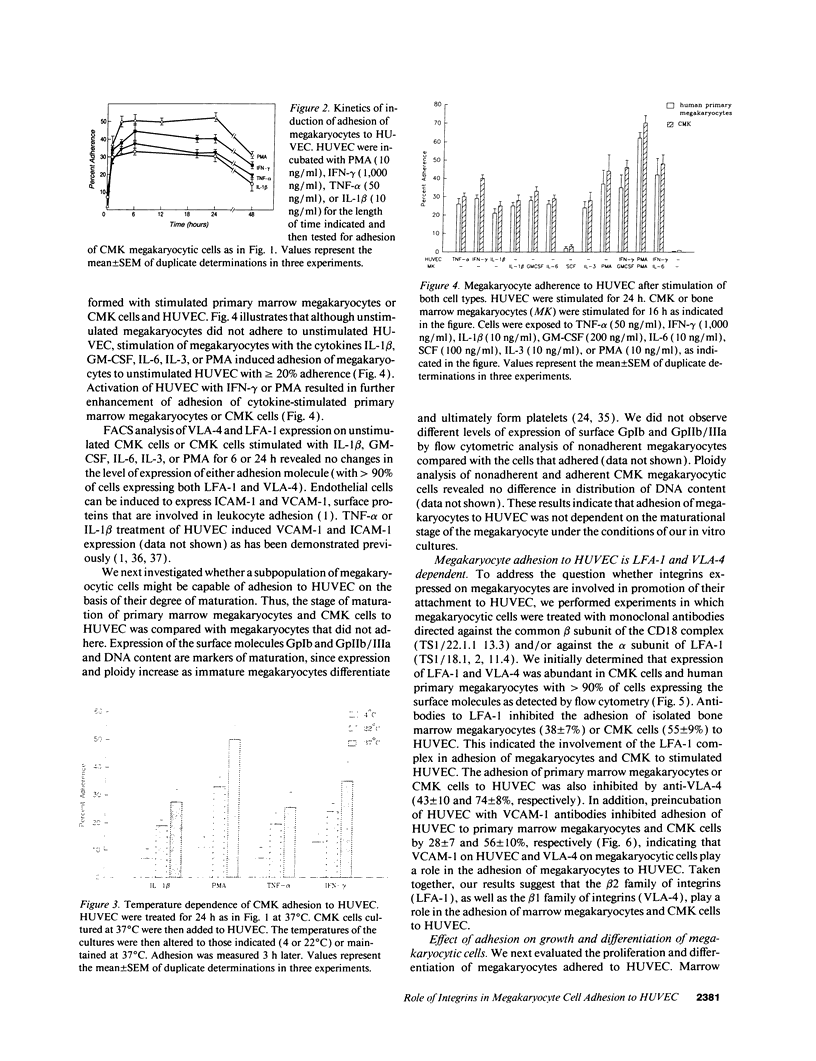
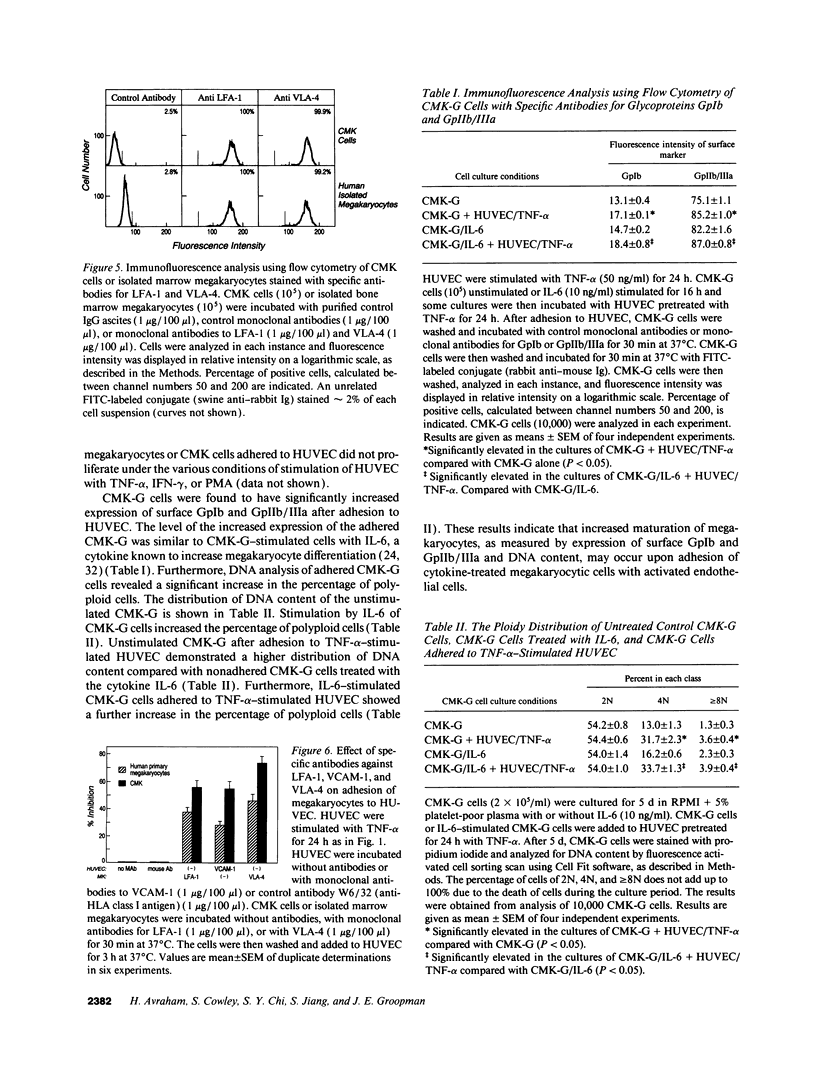
Cell-cell adhesion is essential for many immunological functions and is believed to be important in the regulation of hematopoiesis. Adhesive interactions between human endothelial cells and megakaryocytes were characterized in vitro using the CMK megakaryocytic cell line as well as marrow megakaryocytes. Although there was no adhesion between unactivated human umbilical vein endothelial cells (HUVEC) and megakaryocytes, treatment of HUVEC with inflammatory cytokines such as IL-1 beta, tumor necrosis factor alpha, INF-gamma, or the phorbol ester phorbol myristate acetate (PMA) resulted in a time- and dose-dependent increase in adhesion. Stimulation of marrow megakaryocytes or CMK cells with the cytokines IL-1 beta, GM-CSF, IL-6, IL-3, or PMA augmented their adhesion to endothelium. Monoclonal antibodies against the LFA-1 subunit of the leukocyte adherence complex CD18 inhibited the binding of marrow megakaryocytes or CMK cells to HUVEC. Adhesion blocking experiments also demonstrated that the VLA-4/VCAM-1 pathway was important for megakaryocyte attachment to HUVEC. Adhesion promoted maturation of megakaryocytic cells as measured by increased expression of glycoproteins GpIb and GpIIb/IIIa and by increased DNA content. These observations suggest that alterations in megakaryocyte adhesion may occur during inflammatory conditions, mediated by certain cytokines, resulting in augmented megakaryocyte maturation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Scadden D. T., Chi S., Broudy V. C., Zsebo K. M., Groopman J. E. Interaction of human bone marrow fibroblasts with megakaryocytes: role of the c-kit ligand. Blood. 1992 Oct 1;80(7):1679–1684. [PubMed] [Google Scholar]
- Avraham H., Vannier E., Chi S. Y., Dinarello C. A., Groopman J. E. Cytokine gene expression and synthesis by human megakaryocytic cells. Int J Cell Cloning. 1992 Mar;10(2):70–79. doi: 10.1002/stem.5530100203. [DOI] [PubMed] [Google Scholar]
- Avraham H., Vannier E., Cowley S., Jiang S. X., Chi S., Dinarello C. A., Zsebo K. M., Groopman J. E. Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood. 1992 Jan 15;79(2):365–371. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., Peachell P. T., Brown K. E., Schleimer R. P. Adherence of human basophils to cultured umbilical vein endothelial cells. J Clin Invest. 1988 May;81(5):1355–1364. doi: 10.1172/JCI113463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Cowley S. A., Groopman J. E., Avraham H. Effects of transforming growth factor beta on megakaryocytic cell fusion and endomitosis. Int J Cell Cloning. 1992 Jul;10(4):223–231. doi: 10.1002/stem.5530100405. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Regulation of megakaryocytopoiesis. Blood. 1989 Sep;74(4):1196–1212. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Komatsu N., Suda T., Moroi M., Tokuyama N., Sakata Y., Okada M., Nishida T., Hirai Y., Sato T., Fuse A. Growth and differentiation of a human megakaryoblastic cell line, CMK. Blood. 1989 Jul;74(1):42–48. [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Lapidot T., Pflumio F., Doedens M., Murdoch B., Williams D. E., Dick J. E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992 Feb 28;255(5048):1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Eldor A., HyAm E., Gamliel H., Fuks Z., Vlodavsky I. Megakaryocyte interaction with subendothelial extracellular matrix is associated with adhesion, platelet-like shape change, and thromboxane A2 production. Blood. 1985 Sep;66(3):570–576. [PubMed] [Google Scholar]
- Lichtman M. A., Chamberlain J. K., Simon W., Santillo P. A. Parasinusoidal location of megakaryocytes in marrow: a determinant of platelet release. Am J Hematol. 1978;4(4):303–312. doi: 10.1002/ajh.2830040402. [DOI] [PubMed] [Google Scholar]
- Long M. W. Blood cell cytoadhesion molecules. Exp Hematol. 1992 Mar;20(3):288–301. [PubMed] [Google Scholar]
- Lowe J. B., Stoolman L. M., Nair R. P., Larsen R. D., Berhend T. L., Marks R. M. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990 Nov 2;63(3):475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. H., Nuccie B. L., Abboud C. N., Winslow J. M. Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest. 1991 Sep;88(3):995–1004. doi: 10.1172/JCI115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Taichman D. B., Cybulsky M. I., Djaffar I., Longenecker B. M., Teixidó J., Rice G. E., Aruffo A., Bevilacqua M. P. Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul. 1991 May;2(5):347–355. doi: 10.1091/mbc.2.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Ishida Y., Kaneko T., Matsumoto N. Isolation of human megakaryocytes by immunomagnetic beads. Br J Haematol. 1989 Sep;73(1):18–22. doi: 10.1111/j.1365-2141.1989.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Aoki M. Localization of megakaryocytes in the bone marrow. Blood Cells. 1989;15(1):3–14. [PubMed] [Google Scholar]
- Tavassoli M., Aoki M. Migration of entire megakaryocytes through the marrow--blood barrier. Br J Haematol. 1981 May;48(1):25–29. doi: 10.1111/j.1365-2141.1981.00025.x. [DOI] [PubMed] [Google Scholar]
- Walsh G. M., Mermod J. J., Hartnell A., Kay A. B., Wardlaw A. J. Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol. 1991 May 15;146(10):3419–3423. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]



