Abstract
Background
The DA1 gene family is plant-specific and Arabidopsis DA1 regulates seed and organ size, but the functions in soybeans are unknown. The cultivated soybean (Glycine max) is believed to be domesticated from the annual wild soybeans (Glycine soja). To evaluate whether DA1-like genes were involved in the evolution of soybeans, we compared variation at both sequence and expression levels of DA1-like genes from G. max (GmaDA1) and G. soja (GsoDA1).
Results
Sequence identities were extremely high between the orthologous pairs between soybeans, while the paralogous copies in a soybean species showed a relatively high divergence. Moreover, the expression variation of DA1-like paralogous genes in soybean was much greater than the orthologous gene pairs between the wild and cultivated soybeans during development and challenging abiotic stresses such as salinity. We further found that overexpressing GsoDA1 genes did not affect seed size. Nevertheless, overexpressing them reduced transgenic Arabidopsis seed germination sensitivity to salt stress. Moreover, most of these genes could improve salt tolerance of the transgenic Arabidopsis plants, corroborated by a detection of expression variation of several key genes in the salt-tolerance pathways.
Conclusions
Our work suggested that expression diversification of DA1-like genes is functionally associated with adaptive radiation of soybeans, reinforcing that the plant-specific DA1 gene family might have contributed to the successful adaption to complex environments and radiation of the plants.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-015-0519-0) contains supplementary material, which is available to authorized users.
Keywords: Evolution, Gene expression, Ortholog, Paralog, Soybean, Transgenic analysis
Background
The cultivated soybean (Glycine max) is a staple crop offering multiple proteins and oils for humans. It was suggested that the cultivated soybean was domesticated from its annual wild relative Glycine soja. Wild soybeans have a wide geographical distribution, and a wide range of genetic variations have been accumulated with adaption to geographic, abiotic and biotic environmental conditions during evolution [1, 2]. Although the domestication process has endowed cultivated soybeans with many advantages in morphological and physiological traits, studies have revealed that wild soybeans had more genetic diversity, especially for stress resistance, than the soybean cultivars [3]. Therefore, the wild soybean germplasm should be a potential genetic reservoir for the improvement of cultivated soybeans. It is an effective way to facilitate agricultural development through exploring and importing excellent genes from wild species [4]. Several agricultural traits of wild soybeans have already been introduced into cultivated soybeans [5, 6].
Salt stress has posed a great threat to agricultural development around the world. Soil salinity can inhibit plant growth and decrease crop yields [7]. More than 800 million hectares of lands have been affected by salinity per year in the world [8]. In Arabidopsis thaliana, the ionic signaling pathway is transduced via the salt-overly-sensitive (SOS) pathway where a calcium-responsive SOS3-SOS2 protein kinase complex controls the expression and activity of ion transporters [9, 10]. The complex of serine/threonine protein kinase SOS2 and the myristoylated calcium-binding protein SOS3 can be activated by a salt-stress-elicited calcium signal, and then the activated protein kinase complex phosphorylates and activates various ion transporters, such as the plasma membrane Na+/H+ antiporter SOS1 [10], which improves salt-tolerance. In response to osmotic stress caused by salt, plants can accumulate metabolites that act as compatible solutes to lower the cellular osmotic potential without affecting normal metabolic reactions [11]. Delta 1-pyrroline-5-carboxylate synthetase gene (P5CS1) encodes the rate-limiting enzyme involved in proline synthesis [12, 13]; this enzyme can increase the rate of proline synthesis, which results in proline accumulation and increased osmotic pressure, thus increasing salt tolerance in plants [14]. Some other genes, such as the alcohol dehydrogenase gene (ADH) and FIERY1 (FRY1) gene, that were involved in salt tolerance were related to the phytohormone abscisic acid (ABA) [15, 16]. Salt tolerance mechanisms in soybeans are unknown, but they have been proposed to consist of the maintenance of ion homeostasis, adjustment in response to osmotic stress, restoration of oxidative balance and other metabolic and structural adaptations [17].
Arabidopsis DA1 and DA1-related 1 (DAR1) genes, as representatives of the DA1 gene family, have a function in regulating organ and seed size [18, 19]. The DA1 expression is induced by ABA [18], implying that the DA1-like genes may be involved in the response to abiotic stresses. This assumption has been further corroborated by observations of another DAR gene; i.e., the chilling sensitive 3 (CHS3) gene, which plays a role in biotic and abiotic resistance responses [20, 21]. Therefore, DA1-like genes have multiple functions in plants. A genome-wide evolutionary study suggested that the DA1 gene family encodes a group of proteins belonging to the LIM (from lin-11, isl-1 and mec-3) domain superfamily, and that this gene family is plant-specific and may play a role in the successful radiation of plants in diverse environments during evolution [22].
Since wild soybeans are distributed in a variety of adverse natural environments, they may act as a source for improving phenological, agronomic traits, and the resistances and tolerances to biotic and abiotic stresses of cultivated soybeans [2, 23]. Comparative study of wild and cultivated soybeans at gene, genomic and transcriptomic levels might provide multiple genetic resources to improve the yield and quality of cultivated soybeans. Such studies will also provide valuable insights into the molecular basis of soybean domestication, in turn further directing genetic improvement of modern soybean cultivars. Whether the DA1-like genes were involved in the evolution / domestication of soybeans is unknown. In the present study, we evaluated this potentiality via comparison of sequences and expressions of the DA1 gene family between G. max (GmaDA1) and G. soja (GsoDA1). Little sequence divergence was observed between the Gma-GsoDA1 orthologous protein pairs while a relative high divergence was found among paralogous proteins in a species. The expression of their genes variously responded to diverse abiotic stresses. Moreover, overexpressing some GsoDA1 genes could improve salt-tolerance of the transgenic Arabidopsis plants. Therefore, our results suggested that expression variation of the DA1-like genes may be largely attributed to the adaptive evolution of soybeans.
Results
Sequencing analyses of the GmaDA1 and GsoDA1 genes
The soybean genome encodes 11 DA1-like genes [22]. We first cloned their cDNAs in the cultivated soybean Suinong 14 and the wild accession ZYD00006. To show the orthology between the cultivated and the wild soybeans, an unrooted phylogenetic tree was constructed using the amino acid sequences. In line with our previous work [22], the DA1-like genes in soybeans diverged into Class I and Class II (Fig. 1a). For each member, the two sequences from the cultivated and wild soybeans gathered together to form a pair (Fig. 1a), suggesting their orthology, and their inheritance from common ancestors (Fig. 1b). The sequence identities of the 11 orthologous pairs ranged from 95.8 to 100 % (Additional file 1: Table S1), indicating a high conservation of these orthologous pairs. Four orthologous pairs were identical in Class I (DA1-1 and DA1-9) and Class II (DA1-7 and DA1-11), and the rest contained a few amino acid substitutions and indels (Fig. 1a; Additional file 1: Table S2). Nonetheless, sequence identities of DA1-like paralogs in a soybean showed a large difference ranging from 44.1 to 97.7 % (Additional file 1: Table S1). These results suggested that the 11 DA1-like paralogs in a soybean species originated in soybean common ancestors (Fig. 1b) became more diversified than the orthologous pairs between the cultivated and wild soybeans during evolution.
Fig. 1.
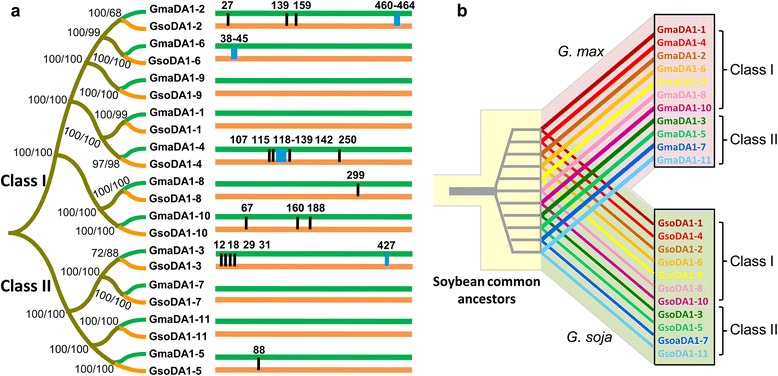
The phylogenetic relationship and sequence divergence of DA1-like proteins from G. soja (GsoDA1) and G. max (GmaDA1). a The ML (maximum likelihood) and NJ (neighbor-joining) trees were constructed using protein sequences. The green lines represent GmaDA1 proteins, and the orange lines represent GsoDA1 proteins. The position of amino acid substitution sites (vertical black lines) and of indels (vertical blue lines) is shown (details in Additional file 1: Table S2). b Schematic representation of the evolutionary process of DA1-like genes during the speciation of soybeans
To estimate the potential effects of amino acid substitutions on protein functions, we performed SNAP and PROVEAN analyses (see Methods). The results of SNAP showed that all substitutions were neutral with an expected accuracy of more than 60 %, suggesting that these substitutions would be unlikely to change the protein functions (Additional file 1: Table S2). Furthermore, the PROVEAN analysis also predicted that almost all of the substitutions might not influence protein functions except for position 299 in DA1-8. The predicted score of substitution in DA1-8 was −5.7, which was less than the cutoff value (−2.5), implying that the substitution may be deleterious to the protein functions (Additional file 1: Table S2). The inconsistent prediction of DA1-8 in these two analyses may be caused by different referential databases of the two methods. Besides the amino acid substitutions, the indels could also affect the protein functions. Therefore, we used PROVEAN analysis to predict the functions of these indels, and showed that all the indels were neutral. Meanwhile, prediction of subcellular locations showed that there was no divergence in the distributions of these Gma-GsoDA1 orthologous pairs. Nonetheless, subcellular locations of different DA1-like members (paralogs) in a soybean species were diverged (Additional file 1: Table S2). These predictions need to be further clarified experimentally, but they may reflect the putative functional variation resulting from the coding sequence variation in these DA1-like genes. Besides this, the divergence of DA1-like genes may occur in their expression levels.
The DA1-like expression in various organs of soybeans
qRT-PCR was used to detect the relative expression levels of DA1-like genes in various tissues of soybeans. Owing to high sequence identities, the degenerate primers of DA1-8 and DA1-10 were designed and named DA1-8/10. The tissue-specific expression of GmaDA1 genes in the cultivated soybean Suinong 14 was previously investigated [22]. Here we investigated the expression of GsoDA1 genes in various organs in wild soybean ZYD00006 (Fig. 2). GsoDA1 genes presented a higher expression level in roots, stems and leaves than the reproductive organs such as the flowers and the developing fruits. Moreover, the expression levels of these genes overall declined during fruit development with few fluctuations. The overall extensive expression of the DA1-like genes in various organs implied that they may have universal roles in soybean development. We further compared the expression profile of GmaDA1 [22] and GsoDA1 (Fig. 2) orthologous gene pairs and we did not find a significant divergence (P > 0.09) between these 11 orthologous gene pairs, while GmaDA1 (P = 0.009) or GsoDA1 (P = 0.04) paralogs in a species were different in all the detected organs, indicating an organ-biased role for each soybean DA1-like gene.
Fig. 2.
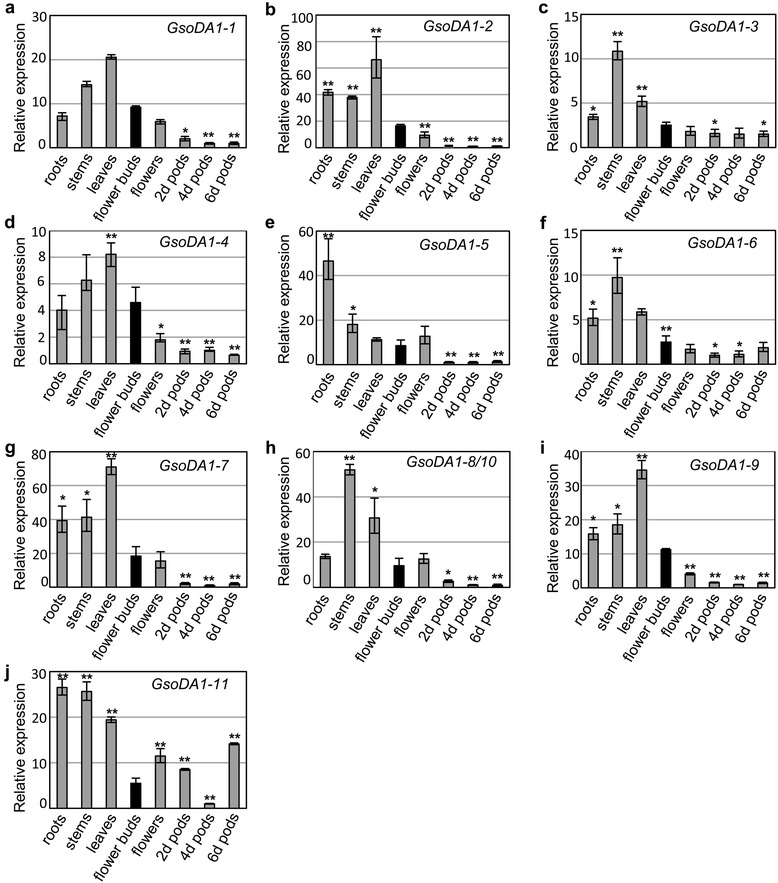
Expression of the GsoDA1 genes during soybean development. (a-j) The expression of GsoDA1-1 (a), GsoDA1-2 (b), GsoDA1-3 (c), GsoDA1-4 (d), GsoDA1-5 (e), GsoDA1-6 (f), GsoDA1-7 (g), GsoDA1-8/10 (h), GsoDA1-9 (i) and GsoDA1-11 (j). The total RNAs were isolated from roots, stems, leaves of 14-day-old seedlings, unfertilized flower buds, flowers, and 2-, 4- and 6-day-old fruits after fertilization. The ACTIN gene was used as an internal control. The experiments were repeated using three independent biological samples. Error bar: standard deviation. The significance was tested in comparison with the expression of each gene in flower buds (black column). The * means significance at a P < 0.05 level, and the ** represents the significance at a P < 0.01 level
Soybean DA1-like expression in response to various abiotic stresses
We next investigated the potential evolutionary patterns of expression variation of soybean DA1-like genes in adaptation to natural environments, fulfilled by applying various external stimuli to the soybean seedlings. Divergence of GmaDA1 and GsoDA1 genes in response to salt stresses was first analyzed using qRT-PCR (Fig. 3). Overall, the expression of Gma-GsoDA1 orthologous pairs was relatively conserved except for DA1-8/10 (P = 0.02) when challenged with different strengths of salt stresses. Considering the details, these genes actually showed different patterns in tendency and magnitude in response to salt stresses. Some Gma-GsoDA1 orthologous gene pairs shared a similar expression variation pattern, such as DA1-4, DA1-5, DA1-6, DA1-8/10 and DA1-11 (Fig. 3d–f, h, j). The expression level of DA1-4 was elevated with a peak at 200 mM (P < 0.002), but was down-regulated at 250 mM in the wild and cultivated soybeans (P < 0.03; Fig. 3d). DA1-6 and DA1-11 were consistently induced in wild and cultivated soybeans (P < 0.01; Fig. 3f, j). DA1-5 and DA1-8/10 were also significantly induced in soybeans to different extents (P < 0.002; Fig. 3e, h). We also observed different expression variations between Gma-GsoDA1 gene pairs under different strengths of salt stress. The most striking pattern was that the expression trends of the orthologous gene pairs were opposite and showed that the expressions of DA1-1, DA1-3 and DA1-7 were significantly suppressed in cultivated soybeans, but were intensely induced in wild soybeans (Fig. 3a, c, g), and the DA1-9 orthologous pair had an opposite tendency (Fig. 3i), suggesting a divergence in Gma-GsoDA1 orthologous gene pairs in response to salt stresses. Nevertheless, the variation extents were different in different genes. For example, GmaDA1-3 and GmaDA1-7 were repressed only at 200 mM NaCl (P < 0.006), but GmaDA1-1 was declined (P < 0.03) except for the 150 mM NaCl stress condition (P = 0.25). GsoDA1-3 and GsoDA1-7 were only increased in 200 mM and 250 mM (P < 0.0001), but GsoDA1-1 was elevated under all the NaCl concentrations (P < 0.03). The DA1-2 gene showed complex and different expression variations in the two soybeans (Fig. 3b). With the salt concentration increasing, GmaDA1-2 was basically unchanged (P > 0.12) except for a decline at 200 mM (P = 0.003), but the GsoDA1-2 was basically repressed except for a peak at 200 mM (P < 0.009). In a dramatic contrast, the GsoDA1 or GmaDA1 paralogs in a soybean showed extremely diverse variations in both tendency and magnitude of gene expression (P < 0.02). These observations suggested that the DA1 genes might have different roles in response to salt, and they may have diverged in wild and cultivated soybeans.
Fig. 3.
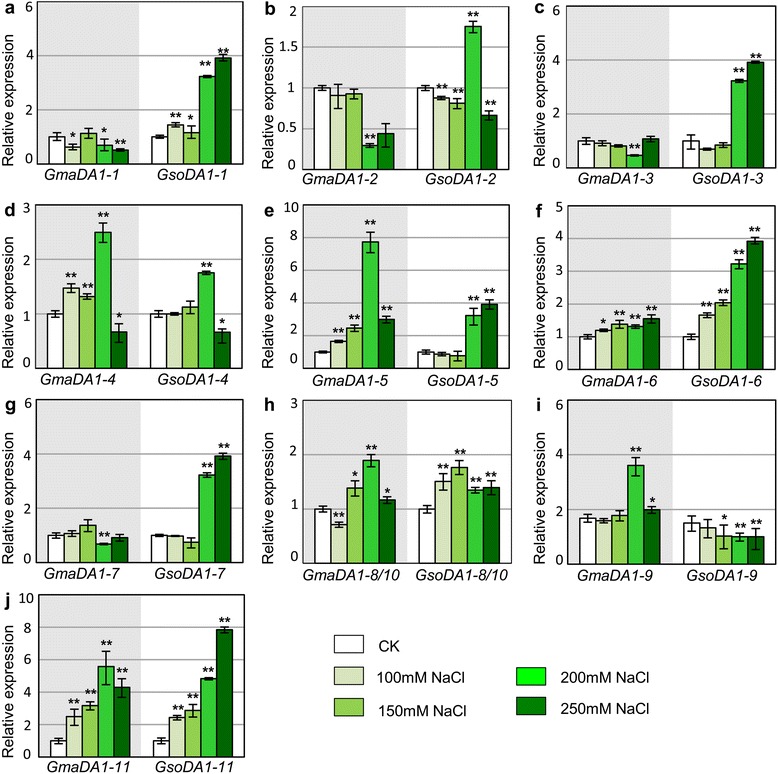
Expression of the GmaDA1 and GsoDA1 genes in response to salt stress. (a-j) The expression of Gma-GsoDA1 orthologous gene pairs: DA1-1 (a), DA1-2 (b), DA1-3 (c), DA1-4 (d), DA1-5 (e), DA1-6 (f), DA1-7 (g), DA1-8/10 (h), DA1-9 (i) and DA1-11 (j). Total RNAs from roots after 4 h treatment for different NaCl stresses were subjected to qRT-PCR analyses. Expression of each gene in the non-treated conditions (white column) was used as a control (CK) and was set as 1. The expression of GmaDA1 challenging 200 mM NaCl has been reported [22]. The ACTIN gene was used as an internal control. The experiments were performed using three independent biological samples. Error bar: standard deviation. The * means significance at a P < 0.05 level, and the ** represent the significance at a P < 0.01 level. For easy comparison of Gma-GsoDA1 orthologous gene pairs, the GmaDA1 expression was displayed in a gray ground
Besides salinity, these genes also differentially responded to drought, acidic and alkali-stresses and ABA treatment (Additional file 1: Dataset S1; Additional file 1: Figures S1–S3). Thus, the expression variation of soybean DA1-like genes in response to various stresses suggested that they might also exert roles in stress-tolerance in soybeans associated with the adaptation evolution of soybeans.
Overexpressing GsoDA1 affected seed germination under salinity
To get further clues about the role of DA1-like genes in the development and the adaptation of soybeans, we overexpressed some of these genes in Arabidopsis. GsoDA1-1, GsoDA1-2, GsoDA1-3, GsoDA1-7, GsoDA1-8, GsoDA1-9 and GsoDA1-11 were manipulated and three independent T3 transgenic Arabidopsis plants for each GsoDA1 gene were analyzed (Additional file 1: Figure S4). The phenotypic variations of transgenic lines of each GsoDA1 gene, such as seedling morphology, seed size and germination rate, were not deviated from those of the wild-type Arabidopsis under normal conditions (Additional file 1: Figure S5; Fig. 4a). However, different seed germination capabilities of transgenic plants from the wild-type seeds were observed in salinity conditions. Vernalization was performed to synchronize seed germination. No significant difference in seed germination was observed between transgenic plants and the wild-type Arabidopsis growing under normal conditions (Fig. 4). The germination rate of the wild-type seeds was significantly reduced under salinity conditions (green column, red stars in Fig. 4b–h); however, the transgenic Arabidopsis seeds on salt-containing medium had statistically higher germination rates than the wild-type Arabidopsis seeds (black stars in Fig. 4b–h). Thus, overexpression of GsoDA1 genes could reduce the sensibility of seed germination to salt in transgenic Arabidopsis.
Fig. 4.
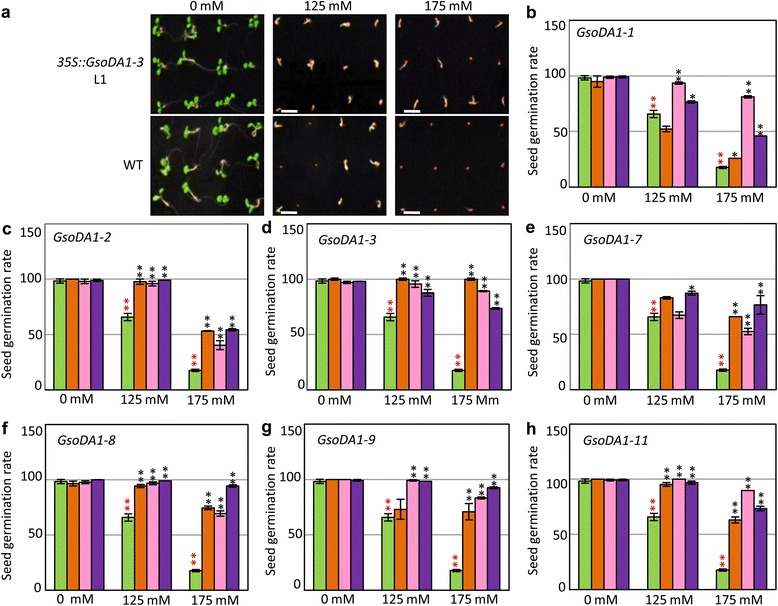
Determination of seed germination rates of transgenic Arabidopsis plants (a) Seeds of the wild-type (WT) and the L1 transgenic Arabidopsis plants (35S::GsoDA1-3) were germinated for five days. These seeds were treated by 0 mM, 125 mM and 175 mM NaCl, respectively. Bars = 2.5 mm. b-h Germination rates of WT and transgenic Arabidopsis seeds under salinity. The green column represents the WT, and the orange, pink and purple columns represent the transgenic lines L1, L2 and L3, respectively. Error bar: standard deviation. The red ** represent the significance at a P < 0.01 level in comparison to WT without treatment. The black * means significance at a P < 0.05 level, and the black ** represents the significance at a P < 0.01 level in comparison with WT at the same condition
GsoDA1 transgenic Arabidopsis plants showed an enhanced salt tolerance
Salt tolerance of GsoDA1 transgenic plants was further evaluated. Four-week old seedlings were watered with 250 mM saline solution (one time on five days). The wild-type control plants and 35S::GsoDA1-9 transgenic lines came to exhibit much more bleaching and wilt than other transgenics under the same salt treatment after 15 days of treatment. Hence, chlorophyll contents were determined in wild-type controls as well as in transgenic plants at this time. The chlorophyll content of 35S::GsoDA1-9 transgenics was significantly reduced, while a relatively and significantly high content of chlorophyll was observed in other salt-tolerant transgenic plants in comparison to the wild-type control plants (Fig. 5a). In line with this, we observed that the aboveground biomasses of all the transgenic GsoDA1-1, GsoDA1-2, GsoDA1-3, GsoDA1-7 and GsoDA1-8 plants were significantly enhanced; nonetheless, no significant difference in biomasses was observed between the wild-type control and most transgenic lines of 35S::GsoDA1-9 and 35S::GsoDA1-11 under salinity conditions (Fig. 5a). Around the 20th-day treatment, the wild-type Arabidopsis and the transgenic lines harboring GsoDA1-9 died, while the other 35S::GsoDA1 transgenic Arabidopsis plants, 35S::GsoDA1-3 plants as an example, survived (Fig. 5b). Thus, overexpressing GsoDA1 genes might reduce salt injury to plants, and improve the salt tolerance of plants.
Fig. 5.
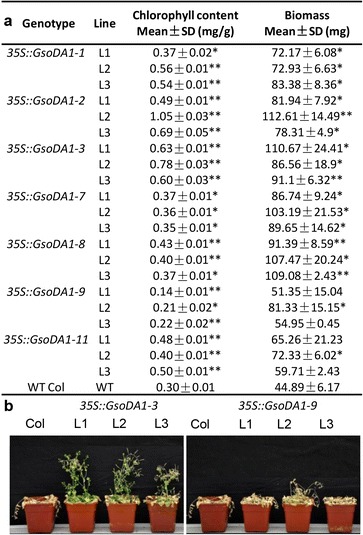
Improved salt tolerance of GsoDA1-transgenic plants a) Determination of chlorophyll content after the 15th-day treatment andmeasurement of the aboveground biomass after the 30th-day treatment. SD means standard deviation. The star means significant difference between the transgenic plants and wild type Arabidopsis. The * and ** represent the significance at a P < 0.05 level and at a P < 0.01 level, respectively. b Comparison of GsoDA1-transgenic and wild-type plants under salt stress. The 4-week-old seedlings of transgenic and wild-type plants were treated with 250 mM NaCl. Plants were photographed after treatment for 20 days. Two representatives are shown. Left: 35S::GsoDA1-3, right: 35S::GsoDA1-9
The salt-tolerance pathway was affected in GsoDA1 transgenic Arabidopsis plants
To further understand the role of GsoDA1 genes in salt stress signaling pathways, the expression of SOS2, SOS3, ADH, P5CS1 and FRY1 genes, which are known in salt tolerance pathways, were investigated in transgenic plants. Transgenic Arabidopsis plants of 35S::GsoDA1-8, 35S::GsoDA1-9 and 35S::GsoDA1-11 were compared with the wild-type Arabidopsis. The SOS2 expression in all these transgenic lines was significantly down-regulated (Fig. 6a). The SOS3 expression in 35S::GsoDA1-11 transgenic lines was reduced (Fig. 6b). The ADH expression in these transgenic plants were not statistically changed (Fig. 6c). The P5CS1 expression in different lines of 35S::GsoDA1-9 transgenic plants were significantly suppressed (Fig. 6d). The expression of FRY1 was significantly suppressed in the 35S::GsoDA1-11 and 35S::GsoDA1-8 transgenic Arabidopsis plants but not changed in the 35S::GsoDA1-9 transgenic Arabidopsis plants (Fig. 6e). We further investigated the effect of GsoDA1 genes on these salt-tolerance genes in transgenic Arabidopsis plants under salinity conditions. For this purpose, 35S::GsoDA1-8 transgenic plants were treated with 250 mM NaCl. The expression of SOS2, SOS3 and FRY1 genes was further repressed by salt stress, while both ADH and P5CS1 expressions were enhanced (Fig. 6f). Thus, overexpressing different GsoDA1 genes differentially affected the expression of salt-tolerance related genes in transgenic Arabidopsis plants.
Fig. 6.
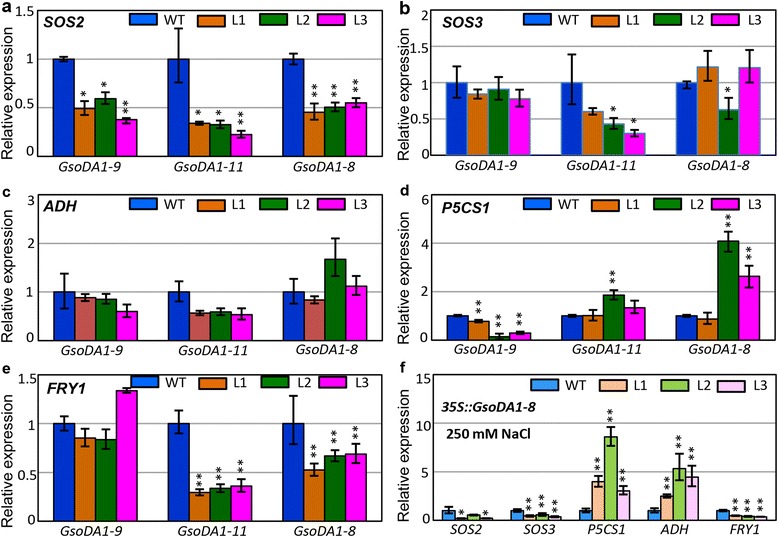
Expression of salt tolerance-related genes in wild-type and transgenic Arabidopsis. a–e The expression variation of SOS2 (a), SOS3 (b), ADH (c), P5CS1 (d) and FRY1 (e) in the indicated transgenic Arabidopsis lines in comparison with wild-type Arabidopsis (WT). f The 35::GsoDA1-8 transgenic Arabidopsis seedlings were also treated by 250 mM NaCl. Expressions of each gene in wild-type Arabidopsis (dark blue and light blue columns) were set as controls (CK). The ACTIN gene was used as an internal control. The experiments were performed using three independent biological samples. Error bar: standard deviation. The * means significance at a P < 0.05 level, and the ** represent the significance at a P < 0.01 level
Discussion
The DA1 gene family is plant-specific functioning in organ size control [18, 19] and has been suggested to play multiple roles in plants during plant development and evolution [22]. To further expand this knowledge, in the present study, we comparatively investigated the sequences and expression patterns of the DA1 gene family in cultivated and wild soybeans, providing further insights into the roles of this gene family in soybean development and evolution.
Soybean DA1 genes have roles in challenging stresses
ABA plays essential roles in many physiological processes, such as embryogenesis, seed dormancy, leaf transpiration and stress tolerance [24, 25]. The expression of Arabidopsis DA1 was induced by ABA [18] and soybean GmaDA1 genes also responded to various stresses, including ABA [22], suggesting that DA1-like genes might be involved in multiple developmental and physiological processes. In the present study, we found that all these genes from G. max and G. soja were substantially variably expressed in response to different abiotic stresses and ABA, further suggesting a role for these genes related to stress tolerance.
Salt stress is one of the main abiotic stresses that significantly inhibits plant growth and reduces crop production. The mechanism underlying salt tolerance has been extensively investigated in the model plants Arabidopsis and rice [10, 26–28]. However, owing to ancient allotetraploid with a large and highly duplicated genome, the absence of an extensive mutant collection and the inefficient transformation system of soybeans, the information available on salt stress responses and possible tolerance mechanisms in soybean is preliminary [29, 30], and such roles for soybean genes are usually inferred from transgenic Arabidopsis [31, 32]. Although the link between gene expression in response to stresses in soybeans and phenotypic variations (in morphology, gene expression and physiology) of transgenic Arabidopsis harboring cDNA of each gene could not be clearly established in the present work, our transgenic Arabidopsis analyses revealed that overexpressing some GsoDA1-like genes could improve the salt-tolerance of transgenic Arabidopsis plants, suggesting that the soybean DA1-like genes may be involved in the salt tolerance signal pathways. Furthermore, we found that the expressions of SOS2 [10], SOS3 [10], ADH [15], P5CS1 [14] and FRY1 [16] genes, which were involved in the salt-tolerance pathways, were differentially affected in GsoDA1 transgenic Arabidopsis plants in a complex manner. The downregulation of FRY1 and the elevated expression of SOS2, SOS3, ADH and P5CS1 can increase salt tolerance in plants [10, 14–16]. Therefore, the alteration of these genes might, in part, account for the alteration of salt-tolerance of transgenic Arabidopsis plants. Arabidopsis DA1-like genes were found to be involved in the ubquitination processes [18, 19], while how they affected gene expression at a transcription level remained unknown. Our observations further suggested that different DA1 paralogs in a soybean species might be involved in different stress signal pathways. Nonetheless, the role of soybean DA1-like genes in both development and challenging stresses needs further substantiation in native hosts.
The role of the DA1 gene family in the evolution of soybeans
Wild and cultivated soybeans were regarded to have common progenitors, and the domestication has endowed the cultivated soybean with many advantages in morphological and physiological traits [3], including retention of seed on the seed head, reduction in lateral branching, reduction in seed dormancy, an increase in seed size, and shifts in flowering time and grain composition [33]. A growing number of major-effect domestication and crop improvement genes have been investigated, including the indeterminate gene 1 (Dt1) in soybean [34], fascinated gene (fas) in tomato [35], shattering gene 1 (Sh1) in sorghum [36] and FLOWERING LOCUS T gene (HaFT1) in sunflower [37]. These studies revealed a diversity of underlying causative mutations affecting phenotypes important in plant domestication, including coding sequence substitutions, copy number variation, transposon activation leading to novel gene structures and expression patterns, diversification following gene duplication, and polyploidy leading to altered combinatorial capabilities [38]. To exploit the role of DA1-like genes in soybean domestication, we comparatively analyzed the sequences and expressions of these genes among cultivated and wild soybeans. Although Arabidopsis DA1-like genes are involved in seed size control [18, 19], we did not find any evidence supporting such a role for DA1-like genes in soybeans. The differential expressions of DA1-6, DA1-8/10 and DA1-11 between the cultivated and wild soybeans in the reproductive organs hinted at a potential role in seed development, but the transgenic Arabidopsis plants harboring GsoDA1 genes did not affect seed size. Manipulating GmaDA1 genes in transgenic Arabidopsis might clarify this. Nonetheless, the role of DA1-like genes in organ size control might be specific to the Brassica species or be a gain-of-function related to the da1-1 mutation (R358K) in Arabidopsis [18], since all isolated soybean DA1-like genes encoded putative proteins having a conserved 358 site (R).
Domesticated genes often have large phenotypic effects and are relatively insensitive to genetic background and environmental effects [33]. However, human selections put the progenitors of the cultivated soybeans in conditions that were totally different from natural conditions, thus effects in the physiological response to stresses during soybean domestication could be estimated. The sequences of Gma-GsoDA1 orthologous gene pairs originated from common ancestors were extremely conserved, and their mRNA expression profiles during development were similar overall. However, responses of some orthologous gene pairs to different abiotic stresses were different, indicating potential effects of domestication on soybean genomes. Both sequences and expression among the DA1-like paralogs in a soybean species changed dramatically. In particular, the expression variation in response to various stresses was tremendous. These variations might have contributed to soybean adaptation during evolution. This assumption was further supported by our observations that the transgenic Arabidopsis harboring GsoDA1 genes showed enhanced salt-tolerance. The underlying evolutionary mechanisms of these sequence and expression variations need further investigation in population level of both G. max and G. soja; nonetheless, the DA1-like genes seem to be involved in the adaptive evolution of soybeans.
Conclusions
Soybean DA1-like genes were inherited from common ancestors, and comparative studies of the DA1 gene family in wild and cultivated soybeans have suggested that the sequences of DA1-like orthologous pairs are more conserved than the paralogous copies in soybean species, but the expression of these genes varies when challenging different abiotic stresses. Overexpressing GsoDA1 genes did not alter seed sizes of transgenic Arabidopsis, but improved salt tolerance of the transgenic plants, thus elevating expression of some genes may improve stress-tolerance of soybeans. The observed divergence of the DA1-like genes in cultivated soybean compared to wild soybean is part of the genetic variation influenced by human breeding, while the variations in paralogous copies in a species, particularly in wild soybean are largely associated with the evolution of soybeans under natural environments. This study further suggested that the plant-specific DA1 gene family might have contributed to the successful adaption to complex environments and radiation of the plants.
Methods
Plant materials and growth conditions
The cultivated soybean ‘Suinong14’ and the wild accession ‘ZYD00006’ were grown in a greenhouse of the Institute of Botany (Beijing, China) under short-day conditions (16 h dark / 8 h light at 23–25 °C). The flower buds, mature flowers, and 2-, 4- and 6-day post-fertilization fruits were harvested at same time point to study gene expression. To evaluate gene expression in response to stresses, soybeans were cultured with 50 % Hoagland solution in a growth chamber under long-day conditions (16 h light / 8 h dark at 23–25 °C). The roots, stems and leaves were collected from 2-week-old seedlings at same time point. The harvested tissues were immediately stored in liquid N2 and then stored at −80 °C for total RNA extraction using TRIzol reagent (Invitrogen).
Abiotic stress treatments
For stress treatment, two-week-old ‘Suinong14’ and ‘ZYD00006’ seedlings growing in a chamber were transferred to modified 50 % Hoagland solution containing NaCl (100 mM, 150 mM, 200 mM or 250 mM), polyethylene glycol (PEG6000 10 %, 15 %, 20 % or 25 %), acids (pH2, pH3, pH4 or pH5), and alkaline (pH7, pH8, pH9 or pH10) for 4 h, respectively. To analyze ABA responsiveness, the seedlings were transferred to the Hoagland solution containing 10 μM ABA for 1 h, 3 h, 6 h or 12 h. Soybean seedlings without any treatment were used as controls. The roots were harvested at the appropriate times for expression studies.
Quantitative RT-PCR analyses
Two micrograms of total RNA were treated with DNase I (Sigma-aldrich, USA) and used to synthesize the first strand cDNA using a M-MLV cDNA Synthesis Kit (Invitrogen). Quantitative RT-PCR (qRT-PCR) was conducted using SYBR Premix Ex Taq™ (TaKaRa) in an Mx3000P QPCR system (Stratagene). ACTIN (Glyma.18G290800) was used to as an internal control. Each experiment was performed using three independent biological samples. PCR was performed in a 25.0 μL reaction mixture containing 12.5 μL 2 × SYBR Premix Ex Taq (TaKaRa), 50 ng cDNA template, 0.5 μL of each primer (10.0 μM) and 10.5 μL of double distilled H2O (dd H2O). The optimized operational procedure was performed as follows: 30 s at 95 °C (1 cycle), 5 s at 95 °C and 40 s at 60 °C (40 cycles) and then 60 s at 95 °C, 30 s at 55 °C and 30 s at 95 °C (1 cycle for melting curve analysis). Relative gene expression was evaluated as previously described [39].
Generation of transgenic Arabidopsis
The pSUPER1300-35S-GsoDA1 plasmids were constructed and were respectively transformed into Agrobacterium tumefaciens strain GV3101. Transformation of Arabidopsis thaliana ecotype Columbia (Col-0) was performed with a floral dipping method [40]. The transgenic plants were selected on Murashige & Skoog (MS) medium containing 40 mg/L hygromycin (Sigma-aldrich, USA), and confirmed further by PCR analysis.
Analyses of transgenic plants
Seeds of Col-0 and T3 transgenic plants were sterilized by soaking in 70 % ethanol (v/v) for 5 min in 15 % NaClO (v/v) for 10 min. They were then rinsed four to five times with sterile ddH2O. The seeds were plated on Agar medium and incubated for 3 days at 4 °C in darkness. Half seeds of each line were plated onto solidified 1/2 MS medium containing 3 % (w/v) sucrose (pH = 5.8), and then were transferred to a growth chamber under long-day conditions (16 h light / 8 h dark at 23–25 °C). Another set of half seeds were plated on 1/2 MS medium containing various concentrations of NaCl (125 mM and 175 mM). After five days, germination rates were calculated. To evaluate the stress-tolerance of transgenic Arabidopsis seedlings, seeds were germinated and grown on 1/2 MS medium for one week, and the seedlings were transferred to soil to grow for three weeks before treatments. A total of 250 mM NaCl was imposed for 20 days (once for five days) until a lethal effect was observed on most of the wild-type plants. The content of chlorophyll at the 15th-day treatment and the aboveground dry biomass at the 30th-day treatment was measured.
Determination of chlorophyll content
Rosette leaves of the Col-0 and the transgenic A. thaliana plants were collected and crushed in absolute alcohol. The mixture was rapidly shaken and left standing in the dark for 2 h. The debris was pelleted down by centrifugation and the supernatant was used for spectrophotometric determination. The optical densities of the supernatant were determined using a NanoDrop spectrophotometer (NanoDrop 2000, Thermo Fisher, USA). Chlorophyll contents were calculated using MacKinney’s specific absorption coefficients [41].
Statistical analyses
Each experiment / measurement was performed using three independent biological replicates or repeated three times unless stated otherwise. A Student’s two-tailed t-test was used for statistical analysis in the present study.
Sequencing analyses
The DA1-like genes in Williams 82 was characterized in our previous work [22]. The gene-specific primers were designed to get the full cDNA sequences in the soybeans Suinong 14 (GmaDA1) and ZYD00006 (GsoDA1). The unrooted phylogenetic tree was constructed using amino acid sequences. Both of the neighbor-joining (NJ) and maximum likelihood (ML; JTT model, bootstrap 100) methods were implemented in the software MEGA5 [42]. The tree was displayed using Treeview v0.4 [43]. In-frame insertions and deletions were detected by PROVEAN [44]. The consequence of sequence divergence between Gma-GsoDA1 orthologous pairs was predicted by SNAP and PROVEAN [44, 45]. All inserted fragments in the derived constructs were commercially sequenced in Beijing Genomic Institute (BGI, Beijing, China). Gene-specific primers for each analysis in the present work (Additional file 1: Table S3) were commercially synthesized in BGI (Beijing, China).
Availability of supporting data
All relevant supporting data can be found within the additional files accompanying this article. Phylogenetic data supporting the results of this article are available in the TreeBASE repository at http://purl.org/phylo/treebase/phylows/study/TB2:S17527. Sequence data described in this article can be found in GenBank (http://www.ncbi.nlm.nih.gov) under the accessions of KR261655-KR261665 and KR349314-KR349324.
Acknowledgements
This work was supported by the grant (XDA08010105) from the Chinese Academy of Sciences.
Abbreviations
- cDNA
Complementary DNA
- qRT-PCR
Quantitative RT-PCR
- ABA
Abscisic acid
- SOS
Salt-overly-sensitive
- NJ
Neighbor-joining
- ML
Maximum likelihood
- GmaDA1
Glycine max DA1
- GsoDA1
Glycine soja DA1
Additional file
Sequence identities of GsoDA1 and GmaDA1 proteins. Table S2. Divergence of GsoDA1 and GmaDA1 proteins. Table S3. Primers used in the present work. Figure S1. Expression of the GmaDA1 and GsoDA1 genes in response to drought. Figure S2. Expression of the GmaDA1 and GsoDA1 genes in response to acid and base stresses. Figure S3. Expression of the GmaDA1 and GsoDA1 genes in response to ABA. Figure S4. PCR-confirmation of 35S::GsoDA1 transgenic Arabidopsis.1: Figure S5. Seed size of 35S::GsoDA1 transgenic Arabidopsis. Dataset S1. Soybean DA1-like expression in response to various abiotic stresses.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CH and MZ conceived and designed the experiments. MZ performed the experiments. YG, LH and QC participated in stress treatments and plant material preparation. MZ performed evolutionary analyses. MZ and CH analyzed the data. MZ and CH drafted the manuscript. All authors have read and approved the manuscript.
Contributor Information
Man Zhao, Email: zhaoman09@sina.com.
Yongzhe Gu, Email: yongzhe@ibcas.ac.cn.
Lingli He, Email: linglihe@ibcas.ac.cn.
Qingshan Chen, Email: qshchen@126.com.
Chaoying He, Phone: +86-10-62836085, Email: chaoying@ibcas.ac.cn.
References
- 1.Li FS. Studies on the ecological geographical distribution of the Chinese resources of wild soybean. Sci Agr Sin. 1993;26:47–55. [Google Scholar]
- 2.Wen Z, Ding Y, Zhao T, Gai J. Genetic diversity and peculiarity of annual wild soybean (G.soja Sieb. et Zucc.) from various eco-regions in China. Theor Appl Genet. 2009;119:371–81. doi: 10.1007/s00122-009-1045-y. [DOI] [PubMed] [Google Scholar]
- 3.Wang KJ, Li XH. Phylogenetic relationships, interspecific hybridization and origin of some rare characters of wild soybean in the subgenus Glycine soja in China. Genet Resour Crop Evol. 2012;59:73–85. doi: 10.1007/s10722-011-9669-6. [DOI] [Google Scholar]
- 4.Upadhyaya H, Reddy K, Singh S, Gowda C. Phenotypic diversity in Cajanus species and identification of promising sources for agronomic traits and seed protein content. Genet Resour Crop Evol. 2013;60:39–59. doi: 10.1007/s10722-012-9864-0. [DOI] [Google Scholar]
- 5.Concibido VC, La Vallee B, Mclaird P, Pineda N, Meyer J, Hummel L, Yang J, Wu K, Delannay X. Introgression of a quantitative trait locus for yield from Glycine soja into commercial soybean cultivars. Theor Appl Genet. 2003;106:575–82. doi: 10.1007/s00122-002-1071-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang K, Liu Y, Dong K, Dong J, Kang J, Yang Q, Zhou H, Sun Y. The effect of NaCl on proline metabolism in Saussurea amara seedlings. Afr J Biotech. 2011;10:2886–93. [Google Scholar]
- 7.Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–21. [Google Scholar]
- 8.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 9.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A. 2002;99:8436–41. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–5. doi: 10.1016/S1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–99. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 12.Delauney AJ, Verma DPS. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coliandis found to be osmoregulated. Mol Gen Genet. 1990;221:299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- 13.Hu CAA, Delauney AJ, Verma DPS. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci U S A. 1992;89:9354–8. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armengaud P, Thiery L, Buhot N, Grenier-De MG, Savoure A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant. 2004;120:442–50. doi: 10.1111/j.0031-9317.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 15.de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 1996;111:381–91. doi: 10.1104/pp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong L, Lee B, Ishitani M, Lee H, Zhang C, Zhu JK. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001;15:1971–84. doi: 10.1101/gad.891901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phang TH, Shao G, Lam HM. Salt tolerance in soybean. J Integr Plant Biol. 2008;50:1196–212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Zheng L, Corke F, Smith C, Bevan MW. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008;22:1331–6. doi: 10.1101/gad.463608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li Y. The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. Plant Cell. 2013;25:3347–59. doi: 10.1105/tpc.113.115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi D, Johnson KC, Zhu Z, Huang Y, Chen F, Zhang Y, Li X. Mutations in an atypical TIR-NB-LRR-LIM resistance protein confer autoimmunity. Front Plant Sci. 2011;2:71. doi: 10.3389/fpls.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J. 2010;63:283–96. doi: 10.1111/j.1365-313X.2010.04241.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, He LL, Gu Y, Wang Y, Chen QS, He CY. Genome-wide analyses of a plant-specific LIM-domain gene family implicate its evolutionary role in plant diversification. Genome Biol Evol. 2014;6:1000–12. doi: 10.1093/gbe/evu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuyen DD, Lal SK, Xu DH. Identification of a major QTL allele from wild soybean (Glycine soja Sieb. & Zucc.) for increasing alkaline salt tolerance in soybean. Theor Appl Genet. 2010;121:229–36. doi: 10.1007/s00122-010-1304-y. [DOI] [PubMed] [Google Scholar]
- 24.Koornneef M, Leon-Kloosterziel K, Schwartz SH, Zeevaart JAD. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem. 1998;36:83–9. doi: 10.1016/S0981-9428(98)80093-4. [DOI] [Google Scholar]
- 25.Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 26.Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiporter in Arabidopsis. Science. 1999;285:1256–8. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 27.Gao JP, Chao DY, Lin HX. Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. J Integr Plant Biol. 2007;49:742–50. doi: 10.1111/j.1744-7909.2007.00495.x. [DOI] [Google Scholar]
- 28.Moller IS. Salinity tolerance of Arabidopsis: a good model for cereals? Trends Plant Sci. 2007;12:534–40. doi: 10.1016/j.tplants.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Du S, Liu Y, Yao B, Bai C, Miao X, Liu C, Chen Q, Hu G. Optimization of soybean transformation system and transferring Dof 4 gene into soybean. Soybean Sci. 2010;29:398–402. [Google Scholar]
- 30.Schlueter JA, Lin JY, Schlueter SD, Vasylenko-Sanders IF, Deshpande S, Yi J, et al. Gene duplication and paleopolyploid in soybean and the implications for whole genome sequencing. BMC Genomics. 2007;8:33. doi: 10.1186/1471-2164-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Zou HF, Wang HW, Zhang WK, Ma B, Zhang JS, Chen SY. Soybean GmMYB76, GmMYB92 and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 2008;18:1047–60. doi: 10.1038/cr.2008.280. [DOI] [PubMed] [Google Scholar]
- 32.Hao YJ, Wei W, Song QX, Chen HW, Zhang YQ, Wang F, Zou HF, Lei G, Tian AG, Zhang WK, Ma B, Zhang JS, Chen SY. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011;68:302–13. doi: 10.1111/j.1365-313X.2011.04687.x. [DOI] [PubMed] [Google Scholar]
- 33.Doust A, Lukens L, Olsen KM, Mauro-Herrera M, Meyer A, Rogers K. Beyond the single gene: How epistasis and gene-by-environment effects influence crop domestication. Proc Natl Acad Sci U S A. 2013;111:6178–83. doi: 10.1073/pnas.1308940110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Z, Wang X, Lee R, Li Y, Specht JE, Nelson RL, McClean PE, Qiu L, Ma J. Artificial selection for determinate growth habit in soybean. Proc Natl Acad Sci U S A. 2010;107:8563–8. doi: 10.1073/pnas.1000088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cong B, Barrero LS, Tanksley SD. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat Genet. 2008;40:800–4. doi: 10.1038/ng.144. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z, Li X, Shannon LM, Yeh C-T, Wang ML, Bai G, Peng Z, Li J, Trick HN, Clemente TE, Doebley J, Schnable PS, Tuinstra MR, Tesso TT, White F, Yu J. Parallel domestication of the Shattering1 genes in cereals. Nat Genet. 2012;44:720–724. doi: 10.1038/ng.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackman BK, Strasburg JL, Raduski AR, Michaels SD, Rieseberg LH. The role of recently derived FT paralogs in sunflower domestication. Curr Biol. 2010;20:629–35. doi: 10.1016/j.cub.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen KM, Wendel JF. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu Rev Plant Biol. 2013;64:47–70. doi: 10.1146/annurev-arplant-050312-120048. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 41.MacKinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–22. [Google Scholar]
- 42.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page RD. TreeView: An application to display phylogenetic trees on personal computers. CABIOS. 1996;12:357–8. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 44.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS One. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35:3823–35. doi: 10.1093/nar/gkm238. [DOI] [PMC free article] [PubMed] [Google Scholar]


