Abstract
Objective: An engineered skin substitute is produced to accelerate wound healing by increasing the mechanical strength of the skin wound via high production of collagen bundles. During the remodeling stage of wound healing, collagen deposition is the most important event. The collagen deposition process may be altered by nutritional deficiency, diabetes mellitus, microbial infection, or radiation exposure, leading to impaired healing. This study describes the fabrication of an engineered bilayer skin substitute and evaluates its effectiveness for the production of collagen bundles in an impaired healing model.
Approach: Rats were exposed to 10 Gy of radiation. Two months postirradiation, the wounds were excised and treated with one of three skin replacement products: bilayer engineered skin substitutes, chitosan skin templates, or duoderm©. The collagen deposition was analyzed by hematoxylin and eosin staining.
Results: On day 21 postwound, the irradiated wounds displayed increased collagen bundle deposition after treatment using bilayer engineered skin substitutes (3.4±0.25) and chitosan skin templates (3.2±0.58) compared with duoderm (2.0±0.63).
Innovation: We provide the first report on the fabrication of bilayer engineered skin substitutes using high density human dermal fibroblasts cocultured with HFSCs on chitosan skin templates.
Conclusion: The high density of fibroblasts significantly increases the penetration of cells into chitosan skin templates, contributing to the fabrication of bilayer engineered skin substitute.

A.B. Mohd Hilmi, PhD
Introduction
The great importance of and high demand for skin replacement products have encouraged researchers worldwide to focus on developing biomaterials for skin substitutes. Three criteria must be satisfied for all tissue-engineered skin substitute bioconstructs. First, they must be safe for patient use. Second, they must be clinically effective. Third, they must be easy to handle and apply. Skin substitutes are classified by their anatomical structure (epidermal, dermal or composite), their duration of use (permanent, semi-permanent, or temporary), source (autologous, allograft, xenograft, natural, or synthetic), and composition (cellular or acellular matrix).1 Skin substitute bioconstructs are designed to replace autografts, which are harvested from a patients' own skin for burn or wound therapy.2
Currently, numerous potential applications for artificial skin products are available. Artificial skin products or skin substitutes are important to increase rates of healing and inhibit secondary complications. In addition to heal the wound, artificial skin products or skin substitutes may be used for living skin grafts, for example breast reconstruction in which skin coverage is insufficient or for complex surgical procedure in which patients are compromised in wound healing. Partial and full-thickness burns that involve critical condition may be benefited after treatment using artificial skin products or skin substitutes instead of autografts or allografts. Most importantly, the artificial skin products or skin substitutes are beneficial for the incidence of rare dermatological disease such as bullous that affects large areas of skin breakdown.
Many studies have proven that composite engineered skin that contains epithelial cells directly contributes to the repair of severe skin injuries. However, as epithelial stem cells slowly grow on the surface of matrices and are liable to differentiate, the post-transplantation survival rate is less than satisfactory.3 Enhancing the adherence of epithelial cells on chitosan skin templates and promoting growth and proliferation on the surface of the chitosan skin template has been challenging. Therefore, cocultured epithelial cells with fibroblast are often used.
According to the American Medical Association, tissue-engineered skin substitute also known as artificial skin product is referred to as bioengineered skin product derived from autologous or allogeneic human tissue; xenogeneic animal cell or tissue; artificial or synthetic biomaterial, and also composites of this biomaterial. Therefore, tissue-engineered skin substitutes are cellular, acellular, and composition based. Cellular tissue-engineered skin substitute is composed of living cells such as fibroblasts, keratinocytes, and stem cells within matrices. Meanwhile, acellular skin substitutes, for example cadaveric skin, chitosan sponge, or collagen gel referred to matrices or scaffolds without living cells or tissue and normally contain growth factor or extra cellular matrix such as collagen and fibronectin. Composite tissue-engineered skin substitutes referred to the combination of cellular and acellular skin substitutes.
In this study, we sought to fabricate bilayer engineered skin substitutes composed of dermal and epidermal component for permanent wound covering in impaired full-thickness wound. We demonstrated that chitosan skin templates are suitable for composite skin, which can contain hair follicle stem cells (HFSCs) and fibroblasts as a strategy for repairing full-thickness wounds in impaired healing model.
Clinical Problem Addressed
Impaired healing resulting from radiation exposure is caused by inhibition of fibroblasts renewal in the wound. High dose skin irradiation creates cellular dysfunction. The tissue appears to be antiproliferative with aberrant molecular signaling, which impairs the onset of healing. The use of an ideal matrix for wound healing may accelerate the healing process in both impaired and acute wounds.
Materials and Methods
Signed informed consent was obtained before harvesting the scalp samples. The patients or parents (if the subject was below 18 years) were given an explanation about this study before signing the consent form. All experiments were approved by the Research Ethics Committee (Human) of Universiti Sains Malaysia. Approval code: USMKK/PPP/JEPeM [212.3(15)].
Isolation of HFSCs and Human Dermal Fibroblasts
Isolation of HFSCs was performed using previously published methods.4 Briefly, epidermis must be first separated from the dermis. The cleaned pieces of scalp were incubated overnight at 4°C in 0.1% (w/v) dispase (Gibco) and 1% (v/v) antimycotic (Gibco). The epidermis was discarded, subsequently the remaining dermis was incubated overnight in a 37°C incubator-roller in a growth medium supplemented with 0.1% collagenase type I (Gibco). HFSCs were collected by centrifugation for 5 min (Hettichzentrifugen) at 320 g and cultivated in a T25 flask (Orange Scientific) containing CnT-07 supplemented with 1% antimycotic.
Human dermal fibroblasts (HDFs) were isolated as described previously.5 The HDFs were collected by centrifugation and cultured in T25 flasks containing Dulbecco's Modified Eagle's Medium (DMEM; Gibco) enriched with 10% (v/v) fetal bovine serum (FBS; Gibco) and 1% antimycotic. The culture medium of HDFs was changed twice per week and the images of HDF cultures were captured for monitoring.
Three-dimensional cultures of HDFs in chitosan skin templates
The evaluation of HDFs seeded into chitosan skin templates were assessed in three-dimensional (3D) cultures. The 3D cultures were performed according to a previous study.6 The chitosan discs 5 mm in diameter and 2 mm thick were placed in 96-well plates and then were seeded with 40 μL of HDFs at a density of 0.5×106 to 3×106 cells/cm2 in culture medium consisting Dulbecco's Modified Eagle's Medium/Ham's F12 (DMEM/F12; Invitrogen) supplemented with 10% (v/v) FBS. Initially, the first 20 μL of cells were dispensed using a micropipette in the center of each chitosan disc. Subsequently, the remaining cells were dispensed at the edges of each chitosan.
At 2 h postculture, another 100 μL of culture medium was added to each chitosan. At 6 h postculture, the chitosan discs were transferred into 24-well plates and medium was added adequately as previously explained7 followed by incubation under 5% CO2 at 37°C. The growth medium was changed every day. On day 14 postculture, the 3D chitosan discs were collected for analysis.
Cocultures of HFSCs and HDFs using chitosan skin templates for fabrication of engineered skin substitute
To fabricate bilayer engineered skin substitutes, the 1 cm by 1 cm chitosan skin templates were seeded with HDFs at a density of 3×106 cells/cm2. The cell culture procedures were similar to those described previously.6 After 2 weeks, the HDFs cultured in chitosan were seeded with HFSCs at a density of 1×106 cells/cm2. HFSCs/HDFs cocultures were carried out for 1 week using a combination of DMEM/F12 (Invitrogen) supplemented with 5% FBS and CnT07 medium (CellnTech) at a ratio of 1:2. After 1 week of coculture, bilayer engineered skin substitutes were harvested for hematoxylin and eosin (H&E) staining. The bilayer engineered skin substitutes were then used for wound healing experiments.
In vivo study
The in vivo study was approved by the Animal Ethics Committee of Universiti Sains Malaysia. Approval code: USM/Animal Ethics Approval/2009/(44)(133). The animal procedures were conducted in accordance with the guidelines of the Animal Research and Service Centre, USM. Rats were divided into irradiated (n=15) and nonirradiated (n=15) groups. Every single rat of these two groups received chitosan bilayer engineered skin substitutes-treated wound (n=5), chitosan skin templates-treated wound (n=5), and duoderm-treated wound (n=5).
Rat irradiation
To impede the healing process rats were exposed to radiation. The radiation exposure was carried out according to a previous protocol.8 Briefly, the dorsal area was given a single 10 Gy dose of radiation. Before radiation, a 1.5 cm tissue equivalent of bolus material was placed over the rat to bring the full radiation dose to the dorsal surface.9
Excisional wound
Three full-thicknesses wounds 1 cm by 1 cm in size were created on the irradiated skin after 2 months of radiation exposure. The wounds were then excised based on a previous protocol.8 Briefly, full-thickness wounds were created beneath the panniculus carnosus muscle extending to the deep fascia layer.10 Each of the wounds was randomly covered with three different types of biomaterials immediately after defect creation. This procedure is known as primary dressing. Then, the secondary dressing was performed by using Hypafix® and TG fix® before covering the wound with a bandage.
Wound analysis
Rats from each group were sacrificed on postoperative days 7, 14, and 21 using an intramuscular injection of ketamine (90 mg/kg) and xylazine (5 mg/kg) followed by exsanguination. The excised area included the wound bed and the intact skin. The specimens were fixed with 10% formalin for histological analysis by H&E staining. The images of the stained samples were captured, measured, and analyzed using a Mirax Desk Scanner (Zeiss). The collagen deposition was scored according to a previous protocol.11 Scores were as follows: 1 if poor, 2 if moderate, 3 if good, and 4 if excellent.
Statistical analysis
Analyses were performed using SPSS® (version 20.0; IBM). Statistically significant differences were determined by a one-way analysis of variance with independent t-tests. Statistical significance was set at p≤0.05. Data were presented as mean values±standard deviations.
Results
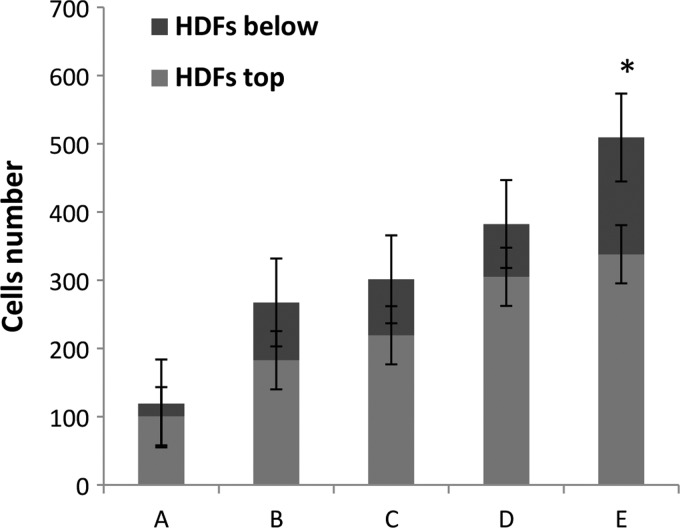
HDFs seeded at densities from 0.5×106 to 2.5×106 cells/cm2 migrated and attached to the surface of chitosan. However, they did not penetrate into the chitosan. Meanwhile, HDFs at a density of 3×106 cells/cm2 migrated and attached to the surface and penetrated into the chitosan. This cell density (Figs. 1 and 2) was preferred for skin substitute preparation.
Figure 1.
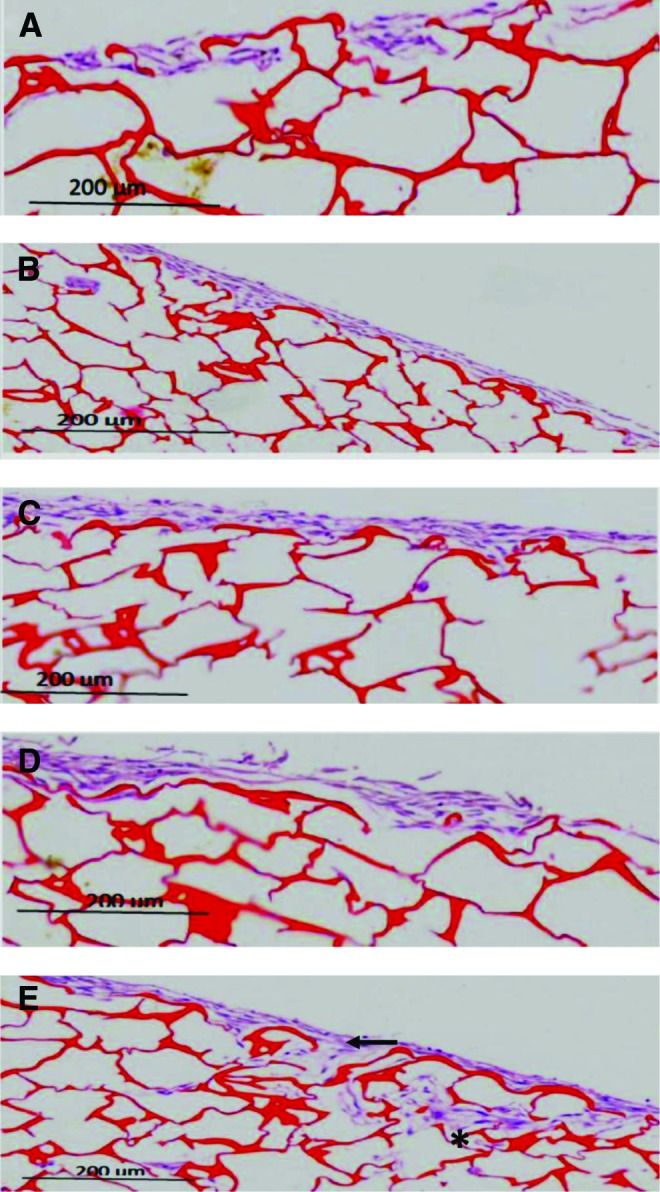
Representative histology sections of human dermal fibroblasts (HDFs) proliferating on chitosan skin templates on day 14. The chitosan skin templates stained red and the HDFs stained purple. Different densities of HDFs (A) 0.5×106 cells/cm2 (B) 1×106 cells/cm2 (C) 2×106 cells/cm2 (D) 2.5×106 cells/cm2 and (E) 3×106 cells/cm2 showed differences in cell migration. The cells robustly penetrated the chitosan skin template (asterisk) and migrated (arrow) in (E), n=3 per condition. Scale bar=200 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 2.
Quantification of HDFs migration (HDFs top) and penetration (HDFs below) into the chitosan skin template at different densities: (A) 0.5×106 cells/cm2 (B) 1×106 cells/cm2 (C) 2×106 cells/cm2 (D) 2.5×106 cells/cm2 and (E) 3×106 cells/cm2. In (E), the number of HDFs penetrating into chitosan was higher than all other densities (*p<0.001), n=3 per condition. Significance was determined using an analysis of variance with the Bonferroni's multiple comparisons test for post hoc analysis.
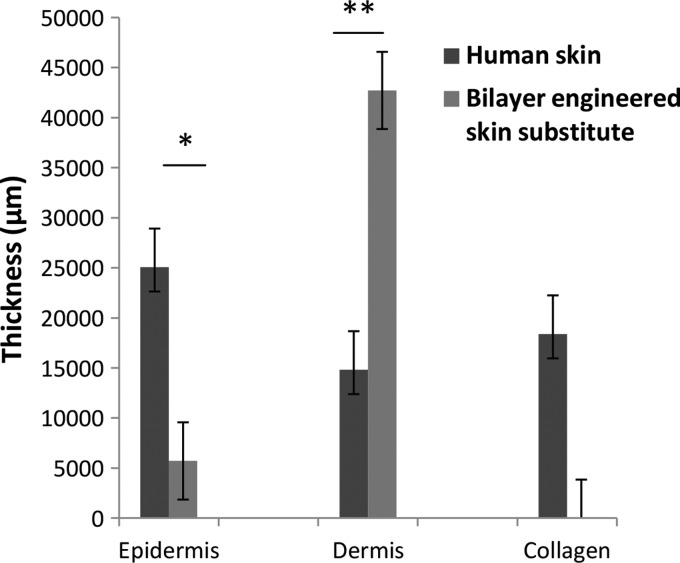
Bilayer engineered skin substitutes were harvested after 2 weeks of HDFs culture in chitosan and then cultured for another 1 week with HFSCs. After 3 weeks, the bilayer engineered skin substitutes were embedded in paraffin. H&E analysis showed that the bilayer engineered skin substitutes (Fig. 3B) were similar to human skin (Fig. 3A) based on the thin layer formation of epithelial cells and migration of fibroblasts beneath the epithelium. However, collagen was not present (Fig. 4).
Figure 3.
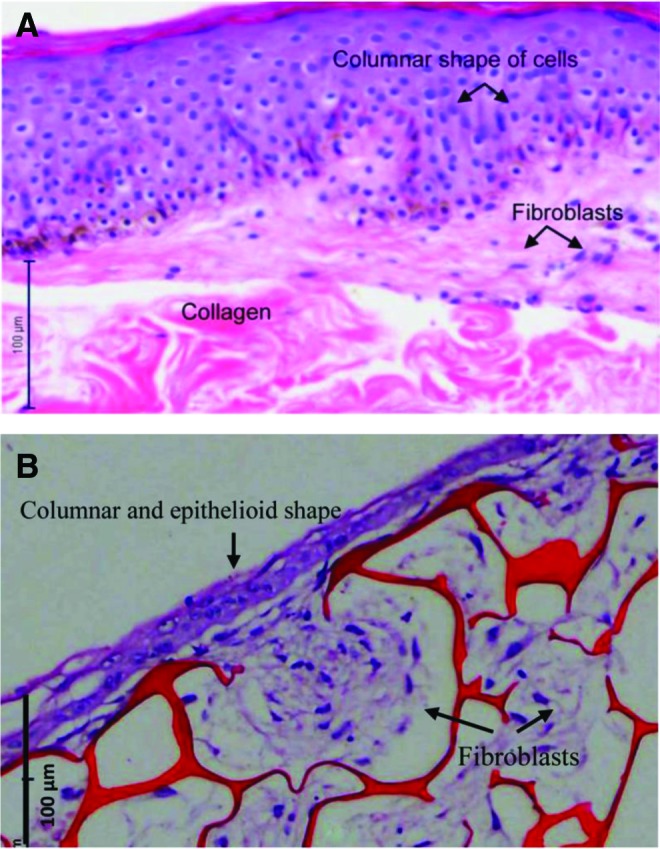
Representative histology sections of human skin (A) and bilayer engineered skin substitutes (B). The bilayer engineered skin substitute had a thin layer of epidermis with columnar, epithelioid cells. The fibroblasts migrated beneath the epithelial layer to form a dermis-like layer, n=5 per condition. Scale bar=100 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 4.
Average thickness measurements of the epidermis, dermis, and collagen between the human skin and the bilayer engineered skin substitute, n=3 per condition. The epidermis was thicker for the native human skin, than the bilayer engineered skin substitute (*p=0.001). The bilayer engineered skin substitute has no collagen and is filled with undifferentiated fibroblasts. As a result, the dermis in the bilayer engineered skin substitute is thicker than human skin (**p=0.013). Significance was determined using independent t-tests.
In the irradiated skin, the epithelial layer showed sub-epidermal edema and the collagen bundles appeared thicker. In the nonirradiated skin, the epidermis and collagen bundles had normal morphologies (Fig. 5).
Figure 5.
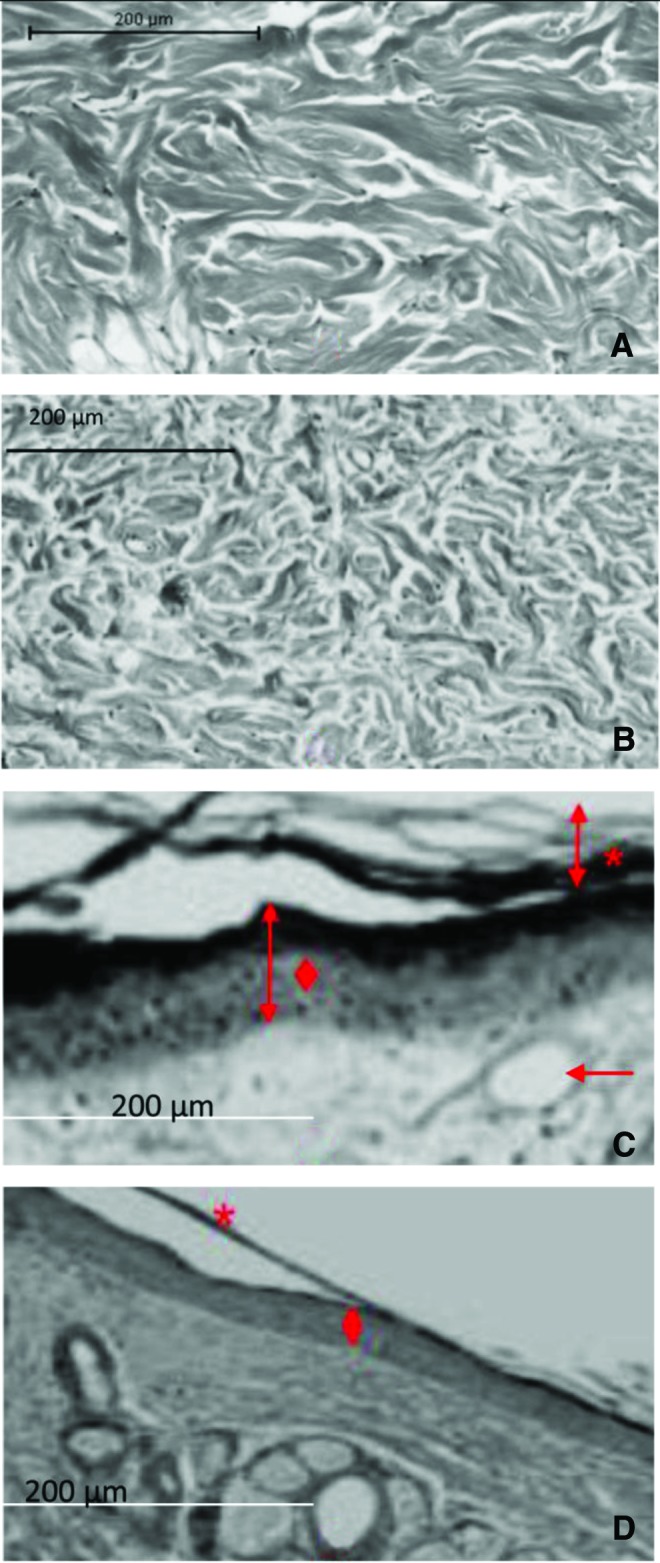
In the irradiated dermis (A), the density of the dermal collagen was increased in a horizontal compact arrangement. The nonirradiated dermis (B) showed interlacing collagen bundles. The irradiated skin (C) presented with thickened regions of the stratum corneum and hyperkeratosis (*). In the epidermis, epidermal hyperplasia and squamatization of basal cells were observed (♦). In the dermis, fibrosis was observed (arrow). Nonirradiated skin (D) presented with normal configurations of the stratum corneum (*) epidermis, and dermis (♦), n=3 per condition. Scale bar=200 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
On day 7, the collagen was poorly deposited in the irradiated wounds after treatment with the three biomaterials. In the nonirradiated group, both the chitosan skin template-treated wounds and the duoderm®-treated wounds showed moderate collagen deposition. Meanwhile, the bilayer engineered skin substitute-treated wounds showed poor collagen deposition. On day 14, the wounds treated with the three biomaterials showed good collagen deposition both in the irradiated and in the nonirradiated groups. On day 21, the bilayer engineered skin substitute-treated wounds and chitosan skin template-treated wounds showed good collagen deposition for both the irradiated and nonirradiated groups. Meanwhile, the duoderm-treated wound showed moderate collagen deposition. The collagen deposition analysis is summarized in Table 1.
Table 1.
Average collagen deposition in irradiated and nonirradiated wounds after treatment using the three different biomaterials, n=5 for each treatment
| Mean±SD | ||
|---|---|---|
| Biomaterials | Irradiated rat | Nonirradiated rat |
| Day 7 | ||
| Bilayer engineered skin substitute | 1±0.00 | 1.4±0.25 |
| Chitosan skin template | 1±0.00 | 1.6±0.25 |
| Duoderm® | 1±0.00 | 1.6±0.40 |
| Day 14 | ||
| Bilayer engineered skin substitute | 3.0±0.00 | 3.0±0.48 |
| Chitosan skin template | 3.2±0.20 | 3.2±0.20 |
| Duoderm | 3.0±0.55 | 3.6±0.25 |
| Day 21 | ||
| Bilayer engineered skin substitute | 3.4±0.25 | 3.4±0.40 |
| Chitosan skin template | 3.2±0.58 | 2.8±0.37 |
| Duoderm | 2.0±0.63 | 2.2±0.74 |
SD, standard deviations.
Discussion
Human fibroblasts can secrete various soluble cytokines, growth factor, and extra cellular matrix components, which promote proliferation of epithelial cells. In fact, the direct contact of fibroblast and epithelial cells plays an important role in preventing differentiation of epithelial cells.12 Coculture with fibroblasts on the same side of the matrix enhances epithelial cell proliferation, inhibits apoptosis, prevents epithelial differentiation, and increases adherence between epithelial cells and the surface of the matrix, thus increasing the post-transplantation survival rate of the composite skin.6 This is the reason why coculture was used in our study. Based on the collagen deposition analysis, the dermis in nonirradiated wounds was moderately developed by day 7. This supports the notion that remodeling normally starts 7 days after injury.13 However, in the irradiated wound, the dermis remains poorly developed. This is because healing will not initiate unless new and functioning blood vessels are present to provide oxygen and nourishment to the wound. Radiation exposure, however, suppresses neovascularization 14 and results in impairment of macrophages that signal blood vessels to begin regeneration. Wounds filled with new vessels are pink or red in appearance. In contrast to the adjacent tissues, the wound remains much redder in appearance throughout the healing process. Blood supply initiates the migration of epithelial cells from the periphery toward the wound bed. Epithelial cells seek out moist and oxygen-rich tissues.13 The important role of a scab is to protect the healing process especially epithelialization from damage and reduce pain locally.15 However, the migration epithelial tongue may retard owing to lack of oxygen supply. Thus, the dressing material must be oxygen permeable and concurrently may avoid wound dehiscence. Normally, several weeks are required for the wound to recover and then form a multilayer before later differentiating into the functional epidermis.16
During wound healing, collagen deposited to initiate remodeling. Its function is to increase the mechanical strength of the wound. Within 3 weeks of injury, the mechanical strength is restored to nearly 20% of normal skin.13 Consistent with this timeline, on day 21 the wounds that incorporated the chitosan skin template or the bilayer engineered skin substitute showed good scaffold retention. In contrast, the duoderm-treated wounds exhibited only moderate matrix formation. This result suggests that bilayer engineered skin substitute and chitosan skin template are suitable biomaterials for permanent skin repair.
This study used cellular composite chitosan-based bilayer engineered skin substitute. Historically, cellular composite collagen-based bilayer engineered skin has been used for full-thickness wound repair. This material is composed of cutaneous fibroblasts17 and keratinocytes.18 Fibroblasts from the collagen matrix are seeded into collagen gel. Two weeks later, dermal equivalents develop in vitro. Collagen gels can support the growth and differentiation of fibroblasts, owing to their origin microenvironments. Subsequently, keratinocytes can be seeded onto the dermal equivalents where they differentiate into epidermis after 21 days of coculture. Eventually, the bilayer engineered skin substitutes composed of collagen gel can be used for grafting in rats. After 1 month, the wounds heal completely; however, neither hairs nor sebaceous glands are present up to 2 years later.19
The fabrication of bilayer engineered skin substitutes has also been achieved using collagen sponges.20 The application of this type of skin substitute, however, is not permanent and thus must be removed from the wound bed due to its inability to degrade over time. Alternatively, the permanent bilayer engineered skin substitute used in this study is biodegradable. It is used only once during treatment and may decrease the cost. The idea to use sponge biomaterials derived from chitosan has been broadly applied to skin repair especially for full-thickness wounds with either normal or impaired healing. Mainly, these materials are used because chitosan is abundantly available and easily obtained from crustaceans. Additionally, a recent study found that platelets significantly adhere and aggregate on chitosan to promote hemostasis.21 This result supports the previous finding that chitosan reduces the inflammatory response, which later improves the inflammation stage to accelerate wound healing.22 As a result, proliferation will increase and enhanced collagen deposition will occur during remodeling. Most importantly, chitosan biomaterials are antimicrobial.23 Prevention of infection in wounds minimizes scar formation and promotes wound healing. The use of chitosan as a biomaterial is not limited to skin repair however, it can also enhance nerve24 and spinal cord25 reconstruction.
Commercially, available chitosan can be fabricated in various forms for various wound applications. Chitosan hydrogels for example, have poor biomechanical properties26 (they are unable to return to their normal shape after deformation) but they are very useful for daily changes of dressings and treatments of superficial wounds. Chitosan fiber, however, is poorly adherent to wounds and has poor exudate absorbing capacity;27 therefore, it is only suitable for partial thickness wounds. The current bilayer engineered skin substitutes and the chitosan skin templates are created as flexible elastic sponges for permanent use in full-thickness wounds. The ability of a biomaterial that can conform and return to its nominal shape or thickness is important for support surface systems.28
The current bilayer engineered skin substitutes are composed of fibroblasts and HFSCs. The advantage of this technique are that the interaction of these two cell types regulate skin homeostasis; control growth, migration, and differentiation of epithelial cells; and establish autoimmune activities.29 This enable us to produce bilayer skin substitutes that were similar to a previous study.30 In Figure 2, columnar and epithelioid cells were observed on the first layer of the skin substitute. Fibroblasts were found in the second layer. The HFSCs were overlaid on the fibroblasts after the fibroblasts were seeded onto chitosan for 2 weeks and then cocultured for an additional week. A high density of fibroblasts (3×106/cm2) was applied to promote cell attachment and infiltration throughout the construct. A previous study used a high density of cells in their engineered tissue and noted similar findings.31 HFSCs adhered to the surface of the scaffold and formed a confluent epidermis-like layer as seen in Fig. 2 and in the previous study.32 Similar performance was achieved with 7 days of coculture in a previous study.33 Another study showed that a period of 7 days is optimal for coculture between fibroblasts and epithelial cells.6 Our bilayer engineered skin substitutes were harvested after 3 weeks in culture. Based on the H&E analysis, typical epithelioid cell morphologies with clear nuclei and large cytoplasmic volumes were observed. Spindle-shaped fibroblasts were also found beneath the epithelial layer.
The histological changes in the dermis of the rat skin after radiation can be clearly seen in Fig. 3. After radiation exposure, thickened regions of the stratum corneum in the irradiated epidermis were present, which were not seen in the nonirradiated epidermal tissue. Additionally, thickened epidermis and compact collagen bundles were clearly visible. These findings were similar to previous findings.34 Most importantly, the previous study found that the collagen fibers were in a horizontal thickened orientation after radiation exposure. Kozikowski et al. described thickening of the stratum spinosum, as a characteristic of acanthosis on the epidermis in irradiated skin.35 This was similar to the results of our study.
The rats were properly handled and standardized to ensure that the impairment of wound healing was due to the radiation and not other bias factors. For example, the rats were required to be young and of the same sex, with symptoms of stress regularly observed, determined, and immediately prevented. Previous studies have mentioned that other factors may cause impaired wound healing such as diabetes mellitus, nutritional deficiency, and obesity.36–38
To date, the pharmaceutical and research sectors have focused on the fabrication of novel biomaterials. The innovation of biomaterials that employ new polymers or techniques as skin substitutes is ongoing. Currently, electrospinning is the latest technology for the fabrication of novel biomaterials.39 In comparison with conventional methods of freeze drying that have been employed for the fabrication of chitosan skin templates, electrospinning produces nanofibrillar structures. Nanofiber biomaterials provide better substrates for cell adhesion, proliferation, and differentiation in 3D culture. During skin repair and regeneration, nanofibers contribute to increased vascularization, reepithelialization, and enhanced granulation tissue formation. The major skin cells, particularly epithelial cells and fibroblasts grow well on this type of biomaterial.
Innovation
We have shown that chitosan skin templates are suitable scaffolds for human skin cells to develop bilayer engineered skin substitutes. Within 3 weeks of culture, epidermal layers were fabricated and dermal-like skin was observed beneath the epidermis. However, this same culture duration was unable to produce such a layer in a previous study.40 Although those researchers used an air–liquid interface culture, their study utilized only keratinocytes. Meanwhile, our study utilized HDFs in high density, which led to significantly thicker dermal layers.
KEY FINDING.
• Cocultures of HFSCs and HDFs develop into bilayer engineered skin substitutes
• Culturing HDFs at high densities allows penetration into the chitosan skin templates
• Bilayer engineered skin substitutes and chitosan skin templates are suitable for skin repair
Abbreviations and Acronyms
- 3D
three-dimensional
- DMEM/F12
Dulbecco's Modified Eagle's Medium/Ham's F12
- DMEM
Dulbecco's Modified Eagle's Medium
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- HDFs
human dermal fibroblasts
- HFSCs
hair follicle stem cells
Acknowledgments and Funding Sources
Fabrication of chitosan skin templates and overall study were funded by a Techno Fund grant from the Ministry of Science, Technology, and Innovation of Malaysia (304/PPSP/6150101) and a supported grant from the Universiti Sains Malaysia (1001/PPSP/8144012).
Author Disclosure and Ghostwriting
No competing financial interests exist. All authors are listed on the front page and no ghost writer exists.
About the Authors
Abu Bakar Mohd Hilmi, PhD, is a stem cell and tissue engineering scientist in the Reconstructive Science Unit. Previously, he has established the isolation of dental pulp stem cells derived from deciduous tooth. Those stem cells are used for cellular and molecular research for current postgraduate students. He is actively involved in regenerative medicine research using human or animal stem cells. With high skill in fluorescent imaging on cells and tissues he has embarked on his role of monitoring student data analysis. Currently, he is a senior lecturer at the Department of Diagnostic and Biomedicine, Universiti Sultan Zainal Abidin. Asma Hassan, MBBS, PhD, is a senior medical lecturer in the Department of Anatomy, School of Medical Sciences, Universiti Sains Malaysia. She is involved in bone marrow stem cells for bone tissue engineering research. Apart of stem cells, she is also involved in biomedicine-based research and has used her teaching experiences in anatomy to help review the school curriculum. Currently, she is an Anatomy Professor at Universiti Sultan Zainal Abidin. Ahmad Sukari Halim, MD, FCCP, is a Professor of Plastic and Reconstructive Surgery at School of Medical Sciences, Universiti Sains Malaysia. His research projects focus on biomaterial and tissue engineering related to wounds and major defects with special emphasis on development of advanced dressing products and reconstructive microsurgery.
References
- 1.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 2010;7:229–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovington L. Dressing and skin substitute. In: McCulloch J, Kloth L, eds. Wound Healing Evidence-Based Management, 4th ed. Philadelphia, PA: FA Davis, 2010:183–186 [Google Scholar]
- 3.Chakrabarty K, Dawson R, Harris P, et al. . Development of autologous human dermal–epidermal composites based on sterilized human allodermis for clinical use. Br J Dermatol 1999;141:811–823 [DOI] [PubMed] [Google Scholar]
- 4.Hilmi ABM, Halim AS, Noor NM, et al. . A simple culture method for epithelial stem cells derived from human hair follicle. Cent Eur J Biol 2013;8:432–439 [Google Scholar]
- 5.Hilmi M, Bakar A, Halim AS, et al. . In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. Springerplus 2013;2:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao S, Zhu S, Ma B, et al. . A new system for cultivation of human keratinocytes on acellular dermal matrix substitute with the use of human fibroblast feeder layer. Cells Tissues Organs 2007;187:123–130 [DOI] [PubMed] [Google Scholar]
- 7.Lim CK, Halim AS, Zainol I, Noorsal K. In vitro evaluation of a biomedical-grade bilayer chitosan porous skin regenerating template as a potential dermal scaffold in skin tissue engineering. Int J Polym Sci 2011;2011:Article ID 645820 [Google Scholar]
- 8.Mohd Hilmi AB, Halim AS, Jaafar H, Asiah AB, Hassan A. Chitosan dermal substitute and chitosan skin substitute contribute to accelerated full-thickness wound healing in irradiated rats. Biomed Res Int 2013;795458:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biel MA, Kim T, Trump MJ. Effect of radiation therapy and Photofrin® on tissue response in a rat model. Lasers Surg Med 1993;13:672–676 [DOI] [PubMed] [Google Scholar]
- 10.Richter GT, Fan CY, Ozgursoy O, McCoy J, Vural E. Effect of vascular endothelial growth factor on skin graft survival in Sprague-Dawley rats. Arch Otolaryngol Head Neck Surg 2006;132:637. [DOI] [PubMed] [Google Scholar]
- 11.Şakrak T, Köse AA, Kivanç Ö, et al. . The effects of combined application of autogenous fibroblast cell culture and full-tissue skin graft (FTSG) on wound healing and contraction in full-thickness tissue defects. Burns 2012;38:225–231 [DOI] [PubMed] [Google Scholar]
- 12.Kubo K, Kuroyanagi Y. A study of cytokines released from fibroblasts in cultured dermal substitute. Artif Organs 2005;29:845–849 [DOI] [PubMed] [Google Scholar]
- 13.Holloway S, Harding K, Stechmiller JK, Schultz G. Acute and chronic wound healing. In: Baranoski S, Ayello EA, eds. Wound Care Essential, 3rd ed. Ambler: Lippincott Williams & Wilkins, 2012:89 [Google Scholar]
- 14.Imaizumi N, Monnier Y, Hegi M, Mirimanoff R-O, Rüegg C. Radiotherapy suppresses angiogenesis in mice through TGF-βRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS One 2010;5:e11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy MA. The biology of scar formation. Phys Ther 1989;69:1014–1024 [DOI] [PubMed] [Google Scholar]
- 16.Mallefet P, Dweck AC. Mechanisms involved in wound healing. Biomed Sci 2008;9:609–615 [Google Scholar]
- 17.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 1979;76:1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell E, Sher S, Hull B, et al. . The reconstitution of living skin. J Invest Dermatol 1983;81(1 Suppl):2s–10s [DOI] [PubMed] [Google Scholar]
- 19.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981;211:1052–1054 [DOI] [PubMed] [Google Scholar]
- 20.Yannas I, Burke J, Orgill D, Skrabut E. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982;215:174–176 [DOI] [PubMed] [Google Scholar]
- 21.Periayah MH, Halim AS, Hussein AR, et al. . In vitro capacity of different grades of chitosan derivatives to induce platelet adhesion and aggregation. Inte J Biol Macromol 2013;52:244–249 [DOI] [PubMed] [Google Scholar]
- 22.Shah Jumaat MY, Ahmad Sukari H, Arman Zaharil MS, Hasnan J. Evaluation of the biocompatibility of a bilayer chitosan skin regenerating template, human skin allograft, and integra implants in rats. ISRN Mater Sci 2011;2011:Article ID 857483 [Google Scholar]
- 23.Jeon SJ, Oh M, Yeo W-S, Galvão KN, Jeong KC. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS One 2014;9:e92723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simões M, Gärtner A, Shirosaki Y, et al. . In vitro and in vivo chitosan membranes testing for peripheral nerve reconstruction. Acta Méd Port 2011;24:43–52 [PubMed] [Google Scholar]
- 25.Chen B, Bohnert D, Borgens RB, Cho Y. Pushing the science forward: chitosan nanoparticles and functional repair of CNS tissue after spinal cord injury. Injury 2013;5:7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albanna MZ, Bou-Akl TH, Blowytsky O, Walters Iii HL, Matthew HWT. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J Mech Behav Biomed Mater 2013;20:217–226 [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Yang H, Liu X, et al. . Potential of quaternization-functionalized chitosan fiber for wound dressing. Int J Biol Macromol 2013;52:327–332 [DOI] [PubMed] [Google Scholar]
- 28.Brienza DM, Geyer MJ, Sprigle S, Zulkowski K. Pressure redistribution: seating, positioning and support surface. In: Baranoski S, Ayello EA, eds. Wound Care Essentials: Practice Principles, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2012:265–294 [Google Scholar]
- 29.Skibinski G, Elborn JS, Ennis M. Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor. Am J Physiol Lung Cell Mol Physiol 2007;293:L69–L76 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang Y, Farhangfar F, Zimmer M, Zhang Y. Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PLoS One 2012;7:e40951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keogh MB, O'Brien FJ, Daly JS. A novel collagen scaffold supports human osteogenesis—applications for bone tissue engineering. Cell Tissue Res 2010;340:169–177 [DOI] [PubMed] [Google Scholar]
- 32.Keck M, Haluza D, Lumenta DB, et al. . Construction of a multi-layer skin substitute: simultaneous cultivation of keratinocytes and preadipocytes on a dermal template. Burns 2011;37:626–630 [DOI] [PubMed] [Google Scholar]
- 33.Dai N-T, Yeh M-K, Liu DD, et al. . A co-cultured skin model based on cell support membranes. Biochem Biophys Res Commun 2005;329:905–908 [DOI] [PubMed] [Google Scholar]
- 34.Almeida E, Caraca R, Adam R, et al. . Photodamage in feline skin: clinical and histomorphometric analysis. Vet Pathol Online 2008;45:327–335 [DOI] [PubMed] [Google Scholar]
- 35.Kozikowski RT, Smith SE, Lee JA, et al. . Comparative evaluation of differential laser-induced perturbation spectroscopy as a technique to discriminate emerging skin pathology. J Biomed Opt 2012;17:0670021–06700211 [DOI] [PubMed] [Google Scholar]
- 36.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–289 [DOI] [PubMed] [Google Scholar]
- 37.Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth AK, De la Garza M, Fang RC, Hong SJ, Galiano RD. Excisional wound healing is delayed in a murine model of chronic kidney disease. PLoS One 2013;8:e59979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm 2013;452:333–343 [DOI] [PubMed] [Google Scholar]
- 40.Garric X, Guillaume O, Dabboue H, Vert M, Molès J-P. Potential of a PLA–PEO–PLA-based scaffold for skin tissue engineering: in vitro evaluation. J Biomater Sci Polym Ed 2012;23:1687–1700 [DOI] [PubMed] [Google Scholar]