Abstract
The safe use of intraoperative blood salvage (IBS) in cancer surgery remains controversial. Here, we investigated the killing effect of cisplatin combined with hyperthermia on human hepatocarcinoma (HepG2) cells and erythrocytes from IBS in vitro. HepG2 cells were mixed with concentrated erythrocytes and pretreated with cisplatin (50, 100, and 200 μg/ml) alone at 37 °C for 60 min and cisplatin (25, 50, 100, and 200 μg/ml) combined with hyperthermia at 42 °C for 60 min. After pretreatment, the cell viability, colony formation and DNA metabolism in HepG2 and the Na+-K+-ATPase activity, 2,3-diphosphoglycerate (2,3-DPG) concentration, free hemoglobin (Hb) level, osmotic fragility, membrane phosphatidylserine externalization, and blood gas variables in erythrocytes were determined. Pretreatment with cisplatin (50, 100, and 200 μg/ml) combined with hyperthermia (42 °C) for 60 min significantly decreased HepG2 cell viability, and completely inhibited colony formation and DNA metabolism when the HepG2 cell concentration was 5×104 ml−1 in the erythrocyte (P<0.01). Erythrocytic Na+-K+-ATPase activity, 2,3-DPG level, phosphatidylserine externalization, and extra-erythrocytic free Hb were significantly altered by hyperthermia plus high concentrations of cisplatin (100 and 200 μg/ml) (P<0.05), but not by hyperthermia plus 50 μg/ml cisplatin (P>0.05). In conclusion, pretreatment with cisplatin (50 μg/ml) combined with hyperthermia (42 °C) for 60 min effectively eliminated HepG2 cells from IBS but did not significantly affect erythrocytes in vitro.
Keywords: Erythrocytes, HepG2 cells, Intraoperative blood salvage, Cisplatin, Hyperthermia
1. Introduction
The use of intraoperative blood salvage (IBS) has increased in recent years. IBS involves suction, collection, filtration, and washes of blood from the surgical field and reinfusion of red cells. The technique has become widely used in a variety of surgical procedures and is highly effective in saving blood and reducing complications (Adias et al., 2006). Oncologic surgery is often accompanied by blood loss that requires massive blood transfusion (Kumar et al., 2014). Allogeneic blood transfusion (ABT) is often used for replenishing blood lost in tumor surgery. However, ABT is associated with many complications such as postoperative infection, pulmonary complications, and poor outcomes, especially the promotion of tumor recurrence in oncological surgery (Kumar et al., 2014).
The applicability of IBS in cancer surgery is controversial. Concerns arise from the hypothesis that tumor cells would be present in the shed blood and concentrated during the procedure, and that these tumor cells could lead to diffusion and metastasis after reinfusion (Waters et al., 2012). This possible risk of IBS seems to be real on the basis of some reports that tumor cells were found in blood salvaged from patients undergoing gynecological surgery (Catling et al., 2008), and that viability, proliferation capacity, invasiveness, and tumorigenicity were demonstrated in these tumor cells from the surgical field (Hansen et al., 1995). Whether tumor cells in the shed blood have malignant potential is uncertain, but eliminating tumor cells from IBS before reinfusion is safer for patients.
Leukoreduction filters and irradiation are the two commonly accepted ways of removing tumor cells from salvaged blood (Edelman et al., 1996; Catling et al., 2008), but their effects on tumor cells (>104) in the salvaged blood are questionable (Waters and Donnenberg, 2009). Gamma irradiation (50 Gy) is also useful for reducing tumor cell contamination from IBS (Hansen et al., 1999). However, irradiation may not be available because the use of irradiators is not popular in most hospitals (Waters and Donnenberg, 2009). Therefore, the development of a relatively cheap and effective alternative method for eliminating tumor cells from salvaged blood might be the key to the implementation of IBS in cancer surgery.
The study of Wu et al. (2006) showed that hyperthermia at 42–47 °C for 40 min killed tumor cells in a temperature-dependent manner, but also decreased the activity of Na+-K+-ATPase in erythrocytes. Therefore, the use of hyperthermia needs to be modified before it is used to eliminate tumor cells in IBS. The fact that heat treatment enhances the effect of anti-tumor drugs, such as cisplatin (Sugarbaker, 2007; Harrison et al., 2008), supports the use of hyperthermia in IBS. Recently, it was found that a combination of cisplatin (200 μg/ml) with hyperthermia (42 °C, 30 min) inhibited hepatoma cell proliferation in the blood, and did not markedly inhibit the function of erythrocytes (Zhou et al., 2011). However, the concentration of cisplatin in such a combination treatment was too high and needs to be optimized.
The current study was carried out to explore the effect of hyperthermia (42 °C for 30, 60, or 120 min) on eliminating human hepatocarcinoma (HepG2) cells in combination with different doses of cisplatin (25, 50, 100, or 200 μg/ml), and to develop an appropriate combined treatment for eliminating HepG2 cells in IBS while preserving erythrocyte functions.
2. Materials and methods
2.1. Ethics statement
All human experimental protocols were approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University and the Chinese Clinical Trial Register (No. ChiCTR-OCS-13003599). Written informed consents were obtained from 29 cancer patients who were scheduled for liver cancer surgeries. Erythrocytes were collected from the salvaged surgical field blood by Cell Saver5 (Haemonetics Co., Boston, MA, USA). The patients were all informed that the salvaged blood would not be transfused back into them. Concentrated erythrocytes from each patient were randomly divided into three series as described below.
2.2. Tumor cells
The HepG2 cell line was obtained from the Shanghai Institute of Cell Biology, China. HepG2 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% (0.1 g/ml) fetal bovine serum (FBS), along with 100 U/ml penicillin and 100 mg/ml streptomycin, at 37 °C in a humidified incubator containing 5% CO2. All media and FBS were purchased from Gibco (Grand Island, NY, USA). Cell viability was tested using trypan blue. All the cell numbers given in this study refer to viable cells.
2.3. Experimental protocols
2.3.1. The first series: determining the effect of hyperthermia on killing HepG2 cells and erythrocytes
HepG2 cells, cultured at 50%–60% confluence, were mixed with erythrocytes, and the final concentration of HepG2 cells was 5×104 ml−1 (Hansen et al., 1995). Mixed cells were divided into the following groups: a control group incubated at 37 °C for 30 min; hyperthermia groups incubated at 42 °C for 30, 60, or 120 min.
2.3.2. The second series: determining the effect of a combination of cisplatin and hyperthermia for 60 min on killing HepG2 cells and erythrocytes
Mixed cells were obtained as described above and were divided into the following groups: a control group incubated at 37 °C for 60 min; cisplatin groups incubated at 37 °C with cisplatin (Hansoh Pharmaceutical Co., Lianyungang, China) at 50, 100, or 200 μg/ml for 60 min; cisplatin in combination with hyperthermia groups incubated at 42 °C with cisplatin at 25, 50, 100, or 200 μg/ml for 60 min.
2.3.3. The third series: determining the effect of a combination of cisplatin and hyperthermia for 30 min on killing HepG2 cells and erythrocytes
Mixed cells were divided into the following groups: a control group incubated in 37 °C for 30 min; cisplatin groups incubated at 37 °C with cisplatin at 100 or 200 μg/ml for 30 min; cisplatin in combination with hyperthermia groups incubated at 42 °C with cisplatin at 100 or 200 μg/ml for 30 min.
2.4. Isolation of tumor cells
After treatment, HepG2 cells were isolated from the erythrocyte using a single-step density gradient centrifugation of Percoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) (Hansen et al., 1995). The density of Percoll was 1.063 g/ml, which provides a significantly greater separation of cancer cells from the blood with maximal reduction in leukocyte contamination (Hansen et al., 1995). The separated HepG2 cells and erythrocytes were evaluated as follows.
2.5. MTT assay
After the separated HepG2 cells were seeded on 96-well plates in quintuplicate for 24 h at 37 °C in a humidified incubator containing 5% CO2, cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Inc., St. Louis, MO, USA). The percentage of viable cells for each group was determined by measuring absorbance (optical density) at 490 nm (OD490) using a microplate reader (BioRad, Hercules, CA, USA) and normalized to the control group.
2.6. EdU incorporation assay
After the separated HepG2 cells were seeded on 96-well plates in triplicate for 24 h at 37 °C in a humidified incubator containing 5% CO2, they were incubated for 2 h with 50 μmol/L 5-ethynyl-2'-deoxyuridine (EdU; RiboBio, Guangzhou, China), according to the manufacturer’s instruction, to detect DNA replication. The numbers of EdU-positive cells were counted by Hoechst staining of nuclei.
2.7. Assay of plate colony formation in HepG2 cells
After the separated HepG2 cells were seeded on 6-well plates in triplicate for 14 d at 37 °C in a humidified incubator containing 5% CO2, colony formation in HepG2 cells was determined using Giemsa staining (Hansen et al., 1999).
2.8. Assay of 2,3-DPG and free Hb levels, and Na+-K+-ATPase activity in erythrocytes
After treatment, erythrocytes were separated from the mixed cells. Free hemoglobin (Hb) and 2,3-diphosphoglycerate (2,3-DPG) levels were determined using Quantitative Human Competitive ELISA kits (Hermes Criterion Biotechnology, Vancouver, Canada). Na+-K+-ATPase activity in erythrocytes was determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
2.9. Osmotic fragility test in erythrocytes
After treatment, erythrocytes were separated from the mixed cells. Erythrocyte osmotic fragility analysis was carried out according to the method described by Hasegawa et al. (2012). Briefly, erythrocytes were incubated in a series of NaCl concentrations ranging from 0.24% to 0.68%. The absorbance at 540 nm was measured to indicate Hb concentration in the supernatant. The erythrocyte group treated with normal saline was used as a negative control (0% hemolysis), while the group treated with distilled water was used as a positive control (100% hemolysis).
2.10. Assay of phospholipid bilayer integrity in erythrocytes
After treatment, erythrocytes were separated from the mixed cells. The physical integrity of the erythrocyte membrane was evaluated by measuring the amount of extracellular phosphatidylserine stained with fluorescein isothiocyanate-annexin V (BD Biosciences Pharmingen, San Diego, CA, USA) using flow cytometry according to the manufacturer’s instructions.
2.11. Assay of blood gases in the solution of erythrocytes
After treatment, erythrocytes were separated from the mixed cells. The K+ and Na+ concentrations, pH, and 50% of the haemes saturated with oxygen (P50) of the solution of erythrocytes were measured using an automated blood gas analyzer (Roche Diagnostics Cobas b123, Shanghai, China).
2.12. Statistical analysis
Continuous variables were expressed as the mean±standard deviation (SD). Phosphatidylserine levels were analyzed by paired Student’s t-test. Categorical variables were analyzed by chi-square tests. Other data were analyzed by one-way analysis of variance (ANOVA) followed by the Newman-Keuls test. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). A value of P<0.05 was considered to be statistically significant.
3. Results
3.1. Clinical data of patients
The baseline characteristics of patients, including their tumor types, sex ratio, ages, and clinical data, did not differ significantly among all the experimental series (Table 1).
Table 1.
Baseline characteristics and clinical data of patients
| Series | Sex (male/female) | Age (year) | Blood salvaged (ml) | Blood shed (ml) | Liver cancer | Receipt in the surgery (ml) |
|
| Crystalloids | Colloids | ||||||
| First | 5/4 | 62.56±11.08 | 135±126 | 217±158 | 9 | 1625±518 | 875±231 |
| Second | 6/4 | 58.39±12.41 | 122±115 | 210±149 | 10 | 1600±516 | 900±211 |
| Third | 5/5 | 59.30±9.44 | 125±117 | 215±195 | 10 | 1550±499 | 850±242 |
The first series: the effects of hyperthermia on killing HepG2 cells and erythrocytes; the second series: the effects of cisplatin combined with hyperthermia for 60 min on killing HepG2 cells and erythrocytes; the third series: the effects of cisplatin combined with hyperthermia for 30 min on killing HepG2 cells and erythrocytes. Data are expressed as mean±SD, except for sex and liver cancer (number)
3.2. Effects of hyperthermia on inhibiting HepG2 cell survival
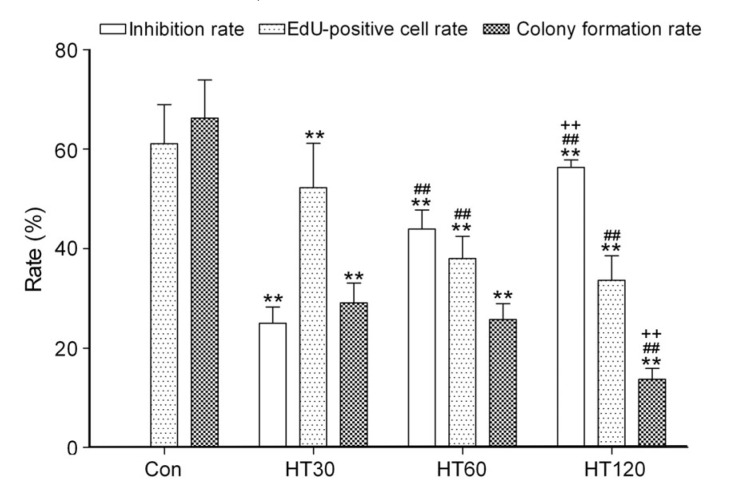
HepG2 cell viability, colony formation, and DNA metabolism were all significantly, but not completely, inhibited by hyperthermia in a time-dependent manner (42 °C for 30, 60, and 120 min) compared with the control group (P<0.01; Fig. 1). In addition, we found that cell viability, DNA metabolism, and colony formation in the HepG2 cell line without erythrocytes (data not shown) were slightly decreased, but showed no significant difference compared with those in the HepG2 cell line mixed with erythrocytes under the same hyperthermia, cisplatin, and hyperthermia plus cisplatin treatments.
Fig. 1.
Effects of hyperthermia on inhibiting HepG2 cell survival
Data are expressed as mean±SD, with n=9 in each experiment. ** P<0.01 vs. control (treated at 37 °C for 30 min); ## P<0.01 vs. HT30; ++ P<0.01 vs. HT60. HT30, HT60, and HT120: hyperthermia for 30, 60, and 120 min, respectively
3.3. Effects of hyperthermia on erythrocytes
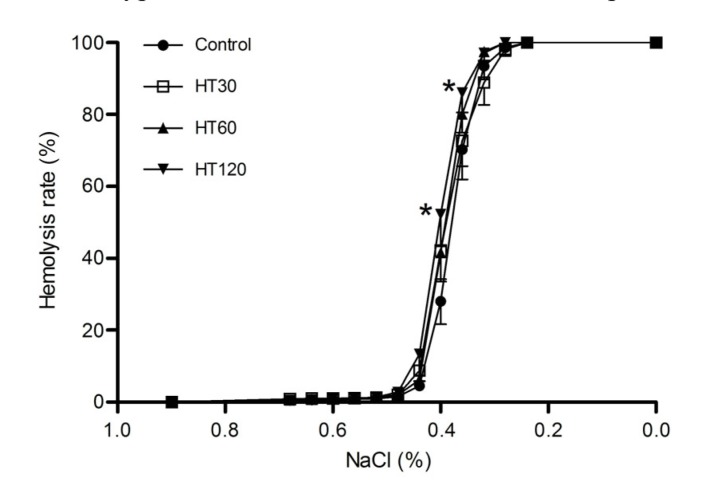
In erythrocytes, the osmotic fragility, Na+-K+-ATPase activity, 2,3-DPG level, free Hb level, or the blood gas variables were not significantly altered by pretreating with hyperthermia for 30 or 60 min (Fig. 2 and Table 2). However, extra-erythrocytic K+, free Hb, and osmotic fragility were markedly increased in the group treated with hyperthermia for 120 min (P<0.05 vs. control).
Fig. 2.
Effects of hyperthermia on the osmotic fragility of erythrocytes
Data are expressed as mean±SD, with n=9 in each experiment. * P<0.05 vs. control (treated at 37 °C for 30 min). HT30, HT60, and HT120: hyperthermia for 30, 60, and 120 min, respectively
Table 2.
Effects of hyperthermia on cell membrane and blood gas variables in erythrocytes
| Treatment | Hb (mg/ml) | P50 (mmHg) | pH | K+ (mmol/L) | Na+ (mmol/L) | ATPase (U/107 cells) | 2,3-DPG (g/L) | Free Hb (mg/ml) |
| Control | 60.53±11.61 | 19.08±4.96 | 7.46±0.13 | 1.47±0.53 | 150.22±1.11 | 0.080±0.018 | 9.68±1.77 | 0.089±0.004 |
| HT30 | 60.85±11.52 | 20.73±6.29 | 7.44±0.13 | 1.77±0.63 | 150.27±1.12 | 0.068±0.023 | 9.05±1.41 | 0.091±0.006 |
| HT60 | 59.90±10.72 | 20.14±6.11 | 7.42±0.13 | 2.01±0.68 | 150.11±1.59 | 0.067±0.021 | 8.47±1.29 | 0.091±0.008 |
| HT120 | 58.71±11.52 | 20.12±6.08 | 7.41±0.13 | 2.62±0.75** | 149.63±1.28 | 0.059±0.017 | 8.07±1.55 | 0.098±0.005** |
Data are expressed as mean±SD, with n=9 in each experiment
P<0.01 vs. control (treated at 37 °C for 30 min)
HT30, HT60, and HT120: hyperthermia for 30, 60, and 120 min, respectively
3.4. Effects of cisplatin combined with hyperthermia for 60 min on HepG2 cell survival
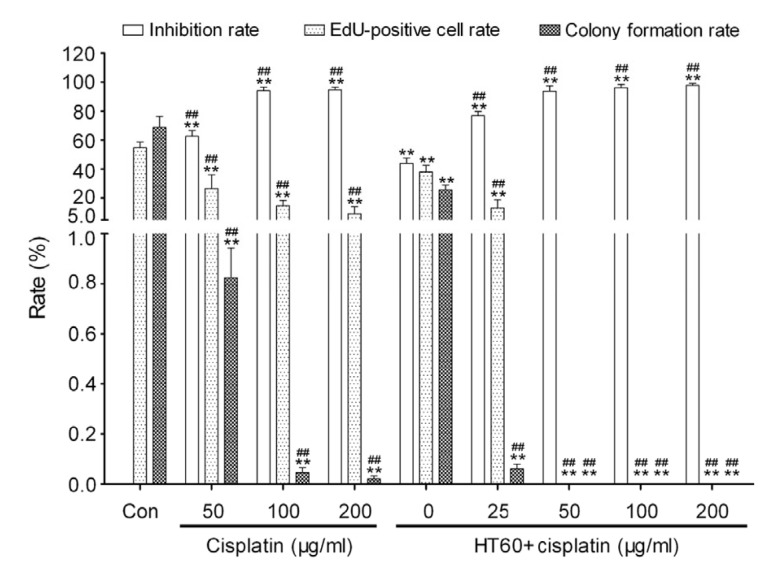
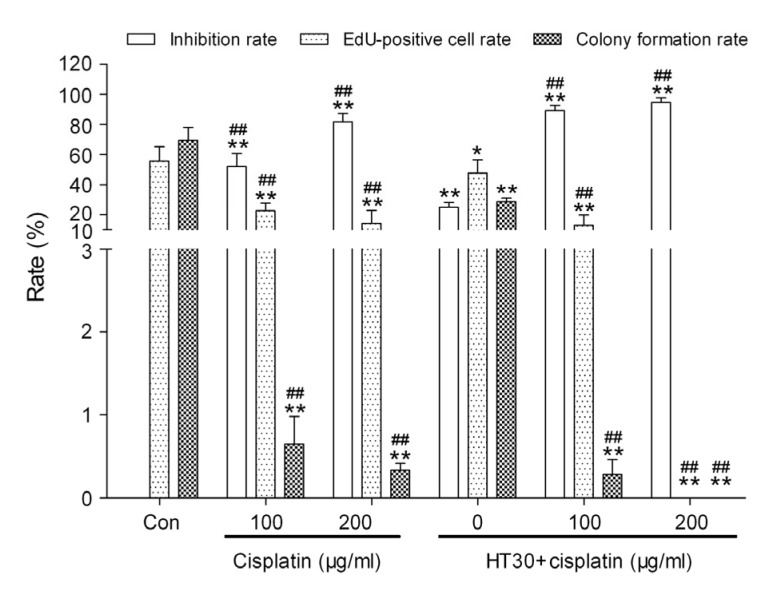
HepG2 cell death was significantly increased and colony formation and DNA metabolism were significantly inhibited in all cisplatin groups compared with the control group (P<0.01; Fig. 3). No EdU-positive cell or colony formation was found in HepG2 cells pretreated with a combination of cisplatin (50, 100, or 200 μg/ml) and hyperthermia for 60 min (Fig. 3).
Fig. 3.
Effects of cisplatin combined with hyperthermia for 60 min (HT60) on HepG2 cell survival
Data are expressed as mean±SD, with n=10 in each experiment. ** P<0.01 vs. control (treated at 37 °C for 60 min); ## P<0.01 vs. HT60
3.5. Effects of cisplatin combined with hyperthermia for 60 min on erythrocytes
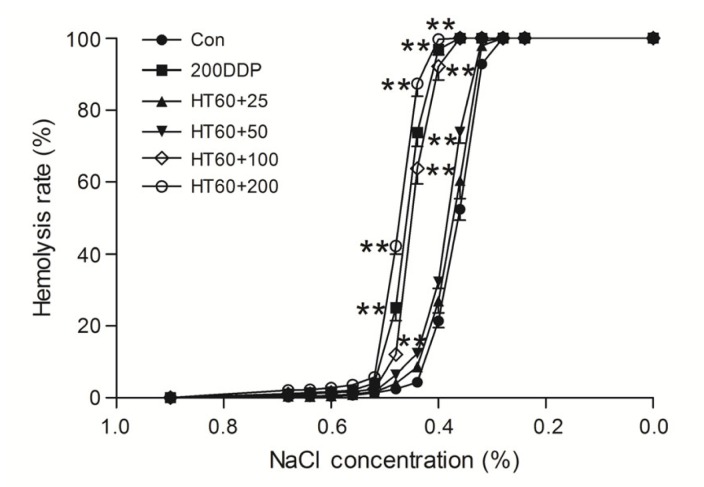
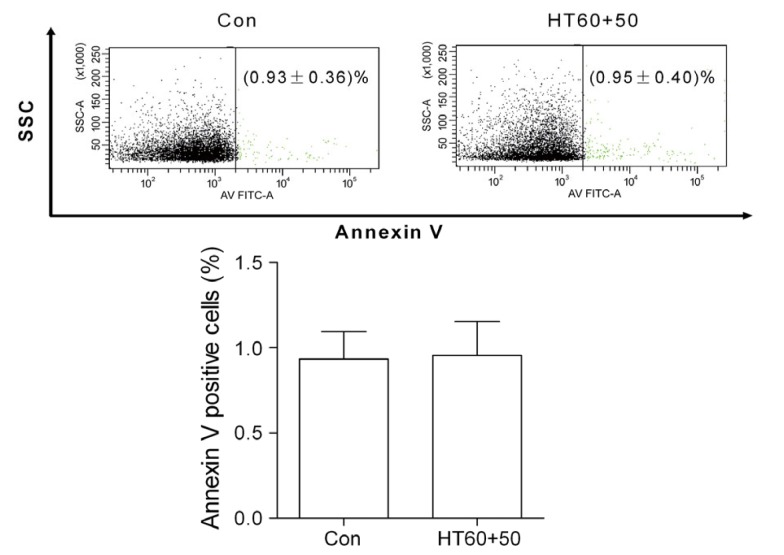
Osmotic fragility, Na+-K+-ATPase activity, 2,3-DPG, free Hb, pH value, and extracellular K+ and Na+ were all significantly altered by pretreating with a high dose of cisplatin (200 μg/ml) alone and cisplatin (100 or 200 μg/ml) combined with hyperthermia for 60 min (P<0.05 vs. control), but not by a low dose of cisplatin (50 or 100 μg/ml) alone or cisplatin (25 and 50 μg/ml) combined with hyperthermia (P>0.05 vs. control) (Fig. 4 and Table 3). The amount of phosphatidylserine outside of the erythrocyte membranes was not significantly increased by pretreating with cisplatin (50 μg/ml) combined with hyperthermia for 60 min (Fig. 5).
Fig. 4.
Effects of cisplatin combined with hyperthermia for 60 min (HT60) on the osmotic fragility of erythrocytes
Data are expressed as mean±SD, with n=10 in each experiment. ** P<0.01 vs. control (treated at 37 °C for 60 min). HT60+25, HT60+50, HT60+100, and HT60+200: 25, 50, 100, and 200 μg/ml cisplatin combined with hyperthermia for 60 min, respectively. 200DDP: 200 μg/ml cisplatin
Table 3.
Effects of a combination of cisplatin and hyperthermia for 60 min (HT60) on cell membrane and blood gas variables in erythrocytes
| Treatment | Hb (mg/ml) | P50 (mmHg) | pH | K+ (mmol/L) | Na+ (mmol/L) | ATPase (U/107 cells) | 2,3-DPG (g/L) | Free Hb (mg/ml) |
| Control | 61.68±2.69 | 18.58±8.76 | 7.44±0.17 | 1.43±0.48 | 148.71±2.74 | 0.078±0.022 | 9.67±1.74 | 0.089±0.005 |
| Cisplatin (μg/ml) | ||||||||
| 50 | 59.58±1.61 | 19.36±8.45 | 7.43±0.14 | 1.96±0.51 | 152.58±5.63 | 0.069±0.020 | 8.92±1.12 | 0.091±0.005 |
| 100 | 58.95±5.20 | 21.86±7.09 | 7.37±0.14 | 2.11±0.54 | 153.55±3.87 | 0.063±0.017 | 8.47±1.16 | 0.094±0.004 |
| 200 | 58.47±4.76 | 16.91±6.20 | 7.26±0.09* | 2.42±0.67** | 154.91±6.84* | 0.055±0.016* | 7.63±1.22* | 0.098±0.006* |
| HT60+cisplatin (μg/ml) | ||||||||
| 0 | 59.90±3.60 | 19.16±8.53 | 7.43±0.08 | 1.96±0.68 | 150.92±3.94 | 0.071±0.014 | 8.46±1.18 | 0.090±0.007 |
| 25 | 59.47±3.76 | 19.48±6.25 | 7.41±0.13 | 1.98±0.46 | 150.00±3.74 | 0.068±0.012 | 8.31±1.14 | 0.094±0.006 |
| 50 | 59.17±4.94 | 19.98±6.96 | 7.41±0.14 | 2.01±0.53 | 153.08±5.11 | 0.065±0.018 | 8.08±1.19 | 0.097±0.006 |
| 100 | 58.28±5.04 | 17.20±8.18 | 7.27±0.06* | 2.33±0.58* | 154.88±3.27* | 0.056±0.011* | 7.60±1.14* | 0.104±0.008** |
| 200 | 56.47±6.25 | 14.72±8.14 | 7.23±0.08** | 2.64±0.61** | 157.45±4.28** | 0.049±0.012** | 7.51±1.72* | 0.106±0.008** |
Data are expressed as mean±SD, with n=10 in each experiment
P<0.05 vs. control (treated at 37 °C for 60 min)
P<0.01 vs. control (treated at 37 °C for 60 min)
Fig. 5.
Effects of cisplatin (50 μg/ml) combined with hyperthermia for 60 min (HT60+50) on phosphatidylserine outside of the erythrocyte membranes
The amount of phosphatidylserine outside of the erythrocyte membranes is expressed as the percentage of annexin V-positive cells in total erythrocytes. Data are expressed as mean±SD, with n=10 in each experiment
3.6. Effects of cisplatin combined with hyperthermia for 30 min on HepG2 cell survival
HepG2 cell viability, colony formation, and DNA metabolism were significantly inhibited by pretreating with hyperthermia for 30 min, cisplatin (100 or 200 μg/ml) alone, and cisplatin (100 or 200 μg/ml) combined with hyperthermia for 30 min (P<0.05 vs. control; Fig. 6). No EdU-positive cells or colonies were found in HepG2 cells pretreated with cisplatin (200 μg/ml) combined with hyperthermia for 30 min (Fig. 6).
Fig. 6.
Effects of cisplatin combined with hyperthermia for 30 min (HT30) on HepG2 cell survival
Data are expressed as mean±SD, with n=10 in each experiment. * P<0.05, ** P<0.01 vs. control (treated at 37 °C for 30 min); ## P<0.01 vs. HT30
3.7. Effects of cisplatin combined with hyperthermia for 30 min on erythrocytes
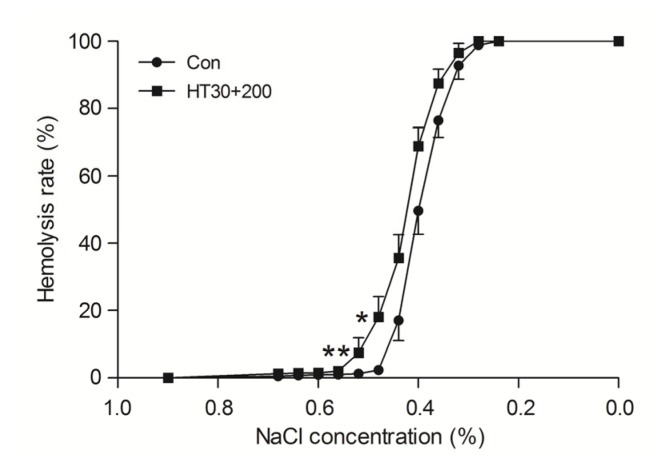
Osmotic fragility, Na+-K+-ATPase activity, 2,3-DPG, free Hb, pH value, and extracellular K+ and Na+ were all significantly altered by pretreating with a high dose of cisplatin (200 μg/ml) combined with hyperthermia for 30 min (P<0.05 vs. control), but not by other treatments (Fig. 7 and Table 4).
Fig. 7.
Effects of cisplatin (200 μg/ml) combined with hyperthermia for 30 min (HT30+200) on the osmotic fragility of erythrocytes
Data are expressed as mean±SD, with n=10 in each experiment. * P<0.05, ** P<0.01 vs. control (treated at 37 °C for 30 min)
Table 4.
Effects of a combination of cisplatin and hyperthermia for 30 min (HT30) on cell membrane and blood gas variables in erythrocytes
| Treatment | Hb (mg/ml) | P50 (mmHg) | pH | K+ (mmol/L) | Na+ (mmol/L) | ATPase (U/107 cells) | 2,3-DPG (g/L) | Free Hb (mg/ml) |
| Control | 62.20±15.87 | 20.28±7.81 | 7.45±0.11 | 1.46±0.56 | 148.28±0.46 | 0.080±0.012 | 9.67±2.09 | 0.089±0.008 |
| Cisplatin (μg/ml) | ||||||||
| 100 | 61.93±15.66 | 25.78±2.88 | 7.42±0.09 | 1.91±0.51 | 149.33±1.23 | 0.069±0.015 | 8.94±2.07 | 0.092±0.008 |
| 200 | 60.53±14.18 | 27.40±3.59 | 7.29±0.14* | 2.05±0.46 | 150.25±1.14 | 0.061±0.022 | 8.17±2.20 | 0.096±0.008 |
| HT30+cisplatin (μg/ml) | ||||||||
| 0 | 63.55±14.03 | 25.70±3.99 | 7.44±0.14 | 1.74±0.49 | 148.63±0.64 | 0.068±0.032 | 9.09±2.13 | 0.091±0.005 |
| 100 | 61.73±14.03 | 27.00±3.71 | 7.35±0.16 | 2.12±0.68 | 149.95±1.16 | 0.059±0.023 | 8.01±2.10 | 0.099±0.008* |
| 200 | 59.65±15.69 | 27.90±3.15 | 7.25±0.12** | 2.29±0.60* | 155.45±1.13* | 0.051±0.015* | 7.71±1.79 | 0.102±0.009** |
Data are expressed as mean±SD, with n=10 in each experiment
P<0.05 vs. control (treated at 37 °C for 30 min)
P<0.01 vs. control (treated at 37 °C for 30 min)
4. Discussion
Hepatoma is the third leading cause of death due to cancer worldwide and accounts for more than 50% of cancer deaths in China (Jemal et al., 2010). The use of IBS is necessary in hepatoma surgery because of the shortage of allogeneic blood supply in China. However, how to remove tumor cells effectively from IBS without injuring erythrocytes remains controversial. In the current work, we found that hyperthermia (42 °C) alone was not enough to completely kill HepG2 cell lines, but a combination of cisplatin (50 μg/ml) and hyperthermia (42 °C) for 60 min effectively purged HepG2 cell lines without significantly injuring the mixed erythrocytes. However, this approach of purging tumor cells from salvaged blood needs to be tested in some form of preclinical in vivo system before considering its application in cancer surgery.
It is known that hyperthermia damages the fluidity and stability of cellular membranes, inactivates membrane proteins, and ultimately induces cell death (Hildebrandt et al., 2002). Furthermore, it causes reorganization of the cytoskeleton, degradation of RNA and DNA, and a reduction in protein synthesis, all of which damage the tumor vasculature and kill tumor cells (Hildebrandt et al., 2002). Because of these effects, hyperthermia is commonly used as an auxiliary method to kill tumor cells in vitro. For hyperthermia, the American Association of Blood Banks suggested that the maximum temperature of warmed blood should be limited to 42 °C (Herron et al., 1997). In the current work, we applied hyperthermia (42 °C) for 30, 60, and 120 min and found that cell viability, DNA metabolism, and colony formation in HepG2 cells were inhibited in a time-dependent manner. However, extra-erythrocytic K+ and free Hb concentrations were both increased significantly by hyperthermia for 120 min, indicating serious hemolysis. Hence, we carried out the subsequent experiments using hyperthermia (42 °C) for 60 min.
Though hyperthermia (42 °C for 60 min) itself did not completely eliminate HepG2 cells in the current study, a combination of hyperthermia and chemotherapy has already been reported to effectively inhibit tumor cells (Issels, 2008). It is known that the cytotoxic effect of platinum compounds, commonly used in chemotherapy, is linearly enhanced with increasing temperature from 37 °C to over 40 °C (Westermann et al., 2001). Cisplatin is a broad-spectrum anticarcinogen, which is also used in combination with hyperthermia (Issels, 2008). The tumor cell inhibition of high doses of cisplatin (80 and 120 μg/ml) at 24 h in vitro is above 80% (Hartmann et al., 2014; Su et al., 2012). Anucleated erythrocytes are little damaged by cisplatin in theory because the key molecular target of cisplatin is eukaryotic DNA (Issels, 2008). Here, a combination of cisplatin and hyperthermia has been verified to effectively purge HepG2 cells in erythrocytes.
Cell colony formation and EdU incorporation as a measurement of DNA metabolism are used to evaluate the proliferation capacity of malignant cells. Our results showed that DNA metabolism and colony formation at the 14th day in HepG2 cells (5×104 ml−1 mixed in erythrocytes) were totally inhibited by pretreating with a combination of cisplatin (50, 100, or 200 μg/ml) and hyperthermia (42 °C) for 60 min. This result is consistent with that of a previous study showing that the half maximal inhibitory concentration (IC50) of cisplatin in human colon cells was about 25 μg/ml (Moretto et al., 2011). However, hyperthermia plus cisplatin (50 μg/ml) for 60 min in the current study did not significantly affect the oxygen-carrying function of erythrocytes. P50 is an index of oxygen affinity (Winslow, 2007) that is influenced by temperature, pH, and ATP concentration, and the key biomarker of oxygen-carrying function in erythrocytes is 2,3-DPG (Winslow, 2007; Wang et al., 2012). The membrane-bound protein Na+-K+-ATPase maintains a Na+ and K+ gradient across the plasma membrane and inhibits erythrocytic fragility. This gradient is necessary to preserve the normal morphology and oxygen-carrying function of erythrocytes (Fӧller et al., 2010). Therefore, preserving Na+-K+-ATPase activity, the 2,3-DPG concentration and the subsequent P50 in erythrocytes might be vital to the application of IBS in cancer surgery. All these erythrocytic parameters were not significantly damaged by hyperthermia plus cisplatin (50 μg/ml). Together with the finding that erythrocytic phosphatidylserine externalization, reflecting disintegration of the membrane phospholipid bilayer (Kiefer and Snyder, 2000; Sachar and Saxena, 2011), did not increase in the hyperthermia plus cisplatin (50 μg/ml) treated group, we suppose that a proper pretreatment with cisplatin combined with hyperthermia might be feasible in the application of IBS to cancer surgery.
We also found that cisplatin (200 μg/ml) combined with hyperthermia for 30 min completely inhibited HepG2 cell proliferation, which is consistent with the study of Zhou et al. (2011). However, this treatment significantly increased erythrocytic fragility, extra-erythrocytic free Hb and K+ levels, and markedly decreased erythrocytic Na+-K+-ATPase activity and pH. It seems that a high cisplatin concentration combined with hyperthermia (42 °C) for 30 min was not suitable for eliminating tumor cell contamination from IBS.
5. Conclusions
Pretreatment with cisplatin (50 μg/ml) combined with hyperthermia (42 °C) for 60 min effectively inhibited the survival of HepG2 cells but did not significantly affect erythrocytes in vitro. However, further work in a preclinical in vivo system is needed to evaluate whether this approach is feasible in clinical applications.
Footnotes
Project supported by the Scientific Research from Chinese Ministry of Health-Zhejiang Health Department, China (Nos. WKJ2008-2-021 and WKJ2013-2-019)
Compliance with ethics guidelines: Jin-ting YANG, Li-hui TANG, Yun-qing LIU, Yin WANG, Lie-ju WANG, Feng-jiang ZHANG, and Min YAN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
References
- 1.Adias TC, Jeremiah Z, Uko E, et al. Autologous blood transfusion—a review. S Afr J Surg. 2006;44(3):114–116. 118. [PubMed] [Google Scholar]
- 2.Catling S, Williams S, Freites O, et al. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63(12):1332–1338. doi: 10.1111/j.1365-2044.2008.05637.x. [DOI] [PubMed] [Google Scholar]
- 3.Edelman MJ, Potter P, Mahaffey KG, et al. The potential for reintroduction of tumor cells during intraoperative blood salvage: reduction of risk with use of the RC-400 leukocyte depletion filter. Urology. 1996;47(2):179–181. doi: 10.1016/S0090-4295(99)80411-7. [DOI] [PubMed] [Google Scholar]
- 4.Fӧller M, Braun M, Qadri SM, et al. Temperature sensitivity of suicidal erythrocyte death. Eur J Clin Invest. 2010;40(6):534–540. doi: 10.1111/j.1365-2362.2010.02296.x. [DOI] [PubMed] [Google Scholar]
- 5.Hansen E, Wolff N, Knuechel R, et al. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130(4):387–393. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 6.Hansen E, Knuechel R, Altmeppen J, et al. Blood irradiation for intraoperative autotransfusion in cancer surgery: demonstration of efficient elimination of contaminating tumor cells. Transfusion. 1999;39(6):608–615. doi: 10.1046/j.1537-2995.1999.39060608.x. [DOI] [PubMed] [Google Scholar]
- 7.Harrison LE, Bryan M, Pliner L, et al. Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann Surg Oncol. 2008;15(5):1407–1413. doi: 10.1245/s10434-007-9718-8. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann S, Kriegebaum U, Küchler N, et al. Correlation of MAGE-A tumor antigens and the efficacy of various chemotherapeutic agents in head and neck carcinoma cells. Clin Oral Investig. 2014;18(1):189–197. doi: 10.1007/s00784-013-0936-0. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa A, Shimizu R, Mohandas N, et al. Mature erythrocyte membrane homeostasis is compromised by loss of the GATA1-FOG1 interaction. Blood. 2012;119(11):2615–2623. doi: 10.1182/blood-2011-09-382473. [DOI] [PubMed] [Google Scholar]
- 10.Herron DM, Grabowy R, Connolly R, et al. The limits of bloodwarming: maximally heating blood with an inline microwave bloodwarmer. J Trauma. 1997;43(2):219–228. doi: 10.1097/00005373-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56. doi: 10.1016/S1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 12.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44(17):2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Center MM, Desantis C. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 14.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7(2):113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Chen Y, Zaw AS, et al. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol. 2014;15(1):e33–e41. doi: 10.1016/S1470-2045(13)70245-6. [DOI] [PubMed] [Google Scholar]
- 16.Moretto J, Chauffert B, Ghiringhelli F, et al. Discrepancy between in vitro and in vivo antitumor effect of a new platinum(II) metallointercalator. Invest New Drugs. 2011;29(6):1164–1176. doi: 10.1007/s10637-010-9461-z. [DOI] [PubMed] [Google Scholar]
- 17.Sachar S, Saxena RK. Cytotoxic effect of poly-dispersed single walled carbon nanotubes on erythrocytes in vitro and in vivo . PLoS ONE. 2011;6(7):e22032. doi: 10.1371/journal.pone.0022032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su XY, Yin HT, Li SY, et al. Intervention effects of nedaplatin and cisplatin on proliferation and apoptosis of human tumour cells in vitro . Asian Pac J Cancer Prev. 2012;13(9):4531–4536. doi: 10.7314/APJCP.2012.13.9.4531. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23(5):431–442. doi: 10.1080/02656730701455318. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Ji B, Zhang Y, et al. Comparison of the effects of three cell saver devices on erythrocyte function during cardiopulmonary bypass procedure—a pilot study. Artif Organs. 2012;36(10):931–935. doi: 10.1111/j.1525-1594.2012.01494.x. [DOI] [PubMed] [Google Scholar]
- 21.Waters JH, Donnenberg AD. Blood salvage and cancer surgery: should we do it? Transfusion. 2009;49(10):2016–2018. doi: 10.1111/j.1537-2995.2009.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waters JH, Yazer M, Chen YF, et al. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 2012;52(10):2167–2173. doi: 10.1111/j.1537-2995.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 23.Westermann AM, Grosen EA, Katschinski DM, et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer. 2001;37(9):1111–1117. doi: 10.1016/S0959-8049(01)00074-0. [DOI] [PubMed] [Google Scholar]
- 24.Winslow RM. The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol. 2007;158(2-3):121–127. doi: 10.1016/j.resp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Wu JB, Lei WF, Zhao LY, et al. Hyperthermia: its effectiveness in killing tumor cells and influence on Na+-K+-ATPase activities in erythrocytes. Chin J Anesthesiol. 2006;26(3):258–260. (in Chinese) [Google Scholar]
- 26.Zhou JF, Wang P, Lei WF. Effectiveness of cis-diamminedichloroplatinum combined with hyperthermia in killing liver tumor cells and its influence on erythrocytes in vitro . Chin J Anesthesiol. 2011;31(2):193–196. (in Chinese) [Google Scholar]