Abstract
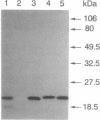
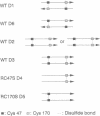
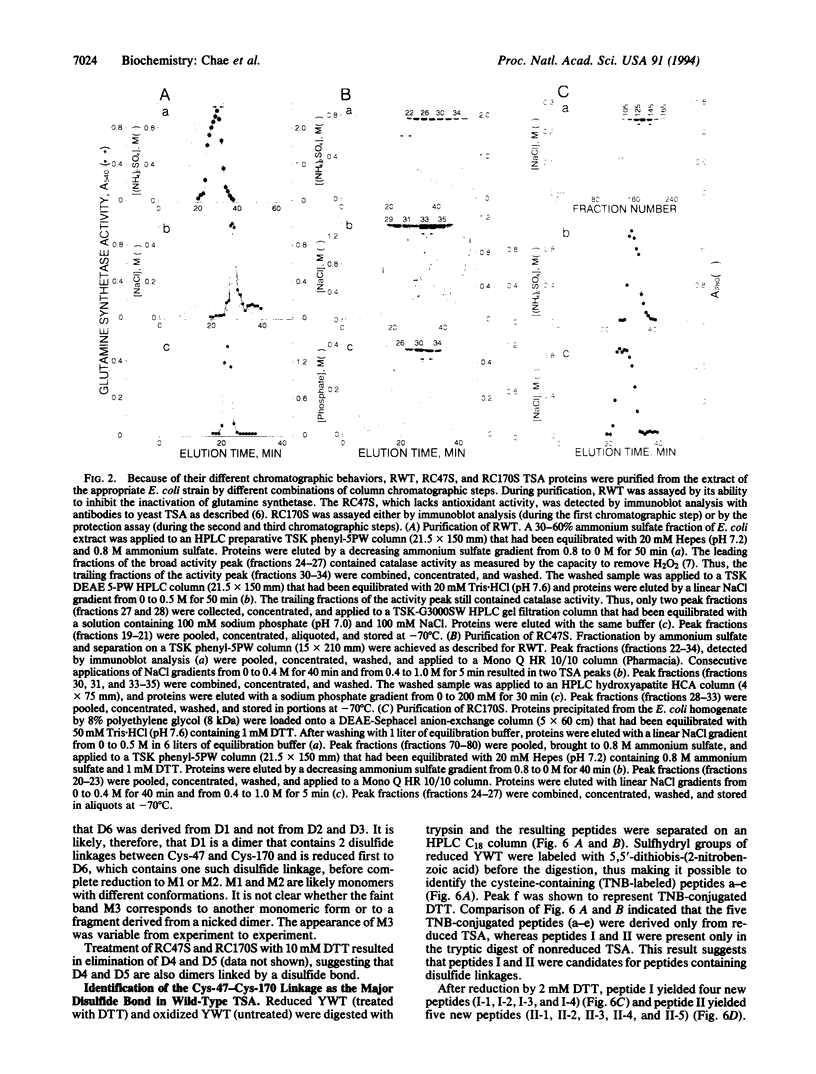
Thiol-specific antioxidant (TSA) from yeast contains cysteine residues at amino acid positions 47 and 170 but is not associated with obvious redox cofactors. These two cysteines are highly conserved in a family of proteins that exhibit sequence identity of 23-98% with TSA. The roles of Cys-47 and Cys-170 in yeast TSA were investigated by replacing them individually with serine and expressing the mutant TSA proteins (RC47S and RC170S, respectively), as well as wild-type TSA (RWT), in Escherichia coli. Wild-type TSA purified from yeast (YWT) and RWT were both shown to exist predominantly as dimers, whereas RC47S and RC170S existed mainly as monomers under a denaturing condition. This observation suggests that the dimerization of YWT and RWT requires disulfide linkage of Cys-47 and Cys-170. The presence of the Cys-47-Cys-170 linkage in YWT was directly shown by isolation of dimeric tryptic peptides, one monomer of which contained Cys-47 and the other contained Cys-170. A small percentage of YWT, RWT, RC47S, and RC170S molecules formed dimers linked by Cys-47-Cys-47 or Cys-170-Cys-170 disulfide bonds. The antioxidant activity of the various TSA proteins was evaluated from their ability to protect glutamine synthetase against the dithiothreitol/Fe3+/O2 oxidation system. YWT, RWT, and RC170S were equally protective, whereas RC47S was completely ineffective. Thus, Cys-47, but not Cys-170, constitutes the site of oxidation by putative substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae H. Z., Kim I. H., Kim K., Rhee S. G. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993 Aug 5;268(22):16815–16821. [PubMed] [Google Scholar]
- Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Jacobson F. S., Morgan R. W., Christman M. F., Ames B. N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989 Jan 25;264(3):1488–1496. [PubMed] [Google Scholar]
- Kim I. H., Kim K., Rhee S. G. Induction of an antioxidant protein of Saccharomyces cerevisiae by O2, Fe3+, or 2-mercaptoethanol. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6018–6022. doi: 10.1073/pnas.86.16.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. The isolation and purification of a specific "protector" protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988 Apr 5;263(10):4704–4711. [PubMed] [Google Scholar]
- Storz G., Jacobson F. S., Tartaglia L. A., Morgan R. W., Silveira L. A., Ames B. N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989 Apr;171(4):2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]