Abstract
The objective of this study was to characterize the diagnostic timelines and their predictors in people with amyotrophic lateral sclerosis (ALS). Patients were identified through ALS billing codes. Time from presenting symptom to first doctor visit, first doctor visit to suspected ALS diagnosis, suspected to confirmed ALS diagnosis, and presenting symptom to confirmed ALS diagnosis (total diagnostic time) were collected. Regression models were used to analyze the predictors of diagnostic delay. Three hundred and four ALS patients were included in the analysis. Median total diagnostic time was 11.5 months. Diagnostic timelines were longer in patients with age > 60 years (p < 0.001), sporadic ALS (p = 0.043), and limb onset (p = 0.010). The presence of fasciculations, slurred speech, and lower extremity weakness when symptoms were first noted were independent predictors of shorter time to ALS diagnosis (p = 0.04, p = 0.02, and p = 0.04, respectively). About half of the patients (52%) received an alternative diagnosis and each patient saw an average of three different physicians before ALS diagnosis was confirmed. In conclusion, diagnostic timelines in ALS are long, and patients see many physicians and receive multiple alternative diagnoses before the diagnosis of ALS is confirmed. Older age, sporadic disease, and limb onset can delay ALS diagnosis.
Keywords: Diagnosis, predictor, delay, timeline
Introduction
Total diagnostic time, defined as the time from symptom onset to confirmed diagnosis, has been reported to range from eight to 15 months in ALS (1–8). This delay in diagnosis represents a significant proportion of total disease duration, prolongs a period of uncertainty that adds to patients’ stress, and is a missed opportunity to begin treatment with riluzole and address ALS related symptoms. Furthermore, diagnostic delay prevents people with ALS from entering clinical trials at an early stage in their disease, when they may be more likely to benefit from potential experimental treatments.
The aim of the present study was to determine the timelines of the diagnostic process in a large cohort of ALS patients and to identify factors that contribute to the delay in diagnosis.
Materials and methods
Patients
ALS patients were identified through billing codes using the Partners Healthcare System Research Patient Data Registry (RPDR). The RPDR Query Tool was used to query for patients seen at the ALS clinic of Massachusetts General Hospital (MGH) between 2000 and 2011 with an ICD-9 code for ‘amyotrophic lateral sclerosis’. Charts were randomly selected for analysis and reviewed manually to confirm a final diagnosis of ALS. Three hundred and twenty-six patients with confirmed ALS diagnosis were identified and underwent detailed chart review. Twenty-two subjects were excluded because of missing symptom onset dates. The study protocol was approved by the Partners Institutional Review Board (IRB).
Statistical analysis
Variables collected for the study included demographic information, date of symptom onset, ALS presenting symptoms, site of onset, date of first doctor visit, date of suspected diagnosis, date of confirmed diagnosis, alternative diagnoses considered before the final ALS diagnosis, and medical specialty of the physician making ALS or alternative diagnoses. Date of symptom onset was recorded as the month and year the subject reported first experiencing ALS symptoms. Site of onset was defined as bulbar, limb, neck, respiratory, or multiple. Date of first doctor visit was recorded as the date of the visit when the patient first saw a physician to discuss ALS presenting symptoms. A suspected diagnosis of ALS was recorded as the date of the visit when ALS was first mentioned in any physician’s note. A confirmed diagnosis of ALS was recorded as the date of the visit when ALS diagnosis was confirmed and discussed with the patient or when riluzole was prescribed.
All analyses were performed using SAS statistical software (version 9.3, SAS Institute, Cary, NC). Frequencies, averages, medians, interquartile ranges (IQR), and standard deviations were used to describe demographic or clinical data and diagnostic timelines, as applicable. A generalized linear model assuming log-normally distributed times was used to compare diagnostic timelines between people with bulbar vs. limb onset, familial vs. non-familial, males vs. females, and between age groups. A generalized linear model assuming negative binomially distributed counts was used to compare number of specialists and number of alternative diagnoses for the same factors.
Results
Patients
Three hundred and four ALS patients were included in the analysis. Their demographic and clinical characteristics of the study cohort are summarized in Table I.
Table I.
Clinical and demographic cohort characteristics.
| Total cohort (n = 304) | |
|---|---|
| Female (n (%)) | 130 (43%) |
| FALS (n (%)) | 61 (20%) |
| Bulbar onset (n (%)) | 84 (28%) |
| Limb onset (n (%)) | 196 (64%) |
| Riluzole use (n (%)) | 156 (51%) |
| Age at Dx (yr) (Mean ± SD) | 58± 12.4 |
| FVC at Dx (% predicted) (Mean ± SD) | 82± 22.1 |
| ALSFRS-R at Dx (Mean ± SD) | 37± 6.6 |
| Physicians seen before confirmed Dx (Mean ± SD) | 3.0± 1.5 |
n: number; SD: standard deviation; FALS: familial ALS; Dx: diagnosis; ALSFRS-R: ALS Functional Rating Scale-revised; FVC: functional vital capacity percent of predicted. Patients with onset in multiple body regions (n = 17), neck (n = 2) and respiratory (n = 5) onset are not represented.
Diagnostic timelines
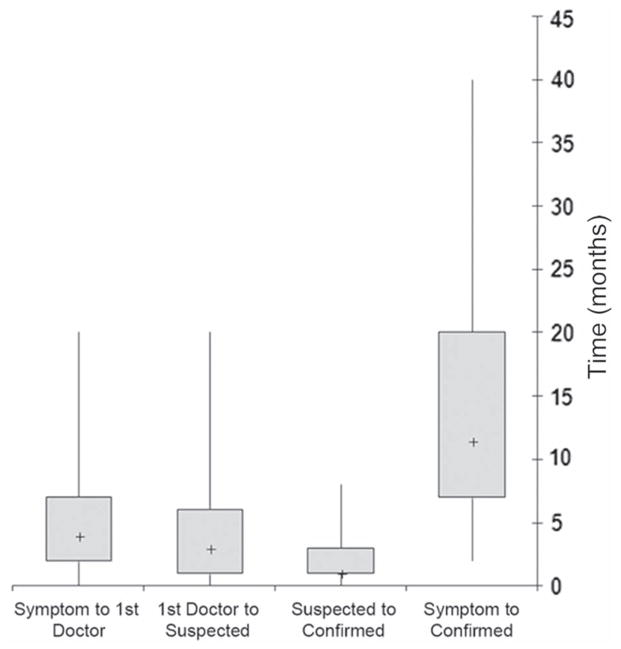
The median total diagnostic time in the whole cohort (n = 304) was 11.5 months (interquartile range, IQR, 7–20 months) (Figure 1). Interim diagnostic milestones were available for a subset of patients and included: median time from presenting symptom to first doctor visit (four months, IQR 2–7 months, n = 122), first doctor visit to suspected ALS diagnosis (three months, IQR 1–6, n = 100), and suspected to confirmed ALS diagnosis (one month, IQR 1–3, n = 183) (Figure 1). Median total diagnostic times were examined in patient sub-groups based on clinical and demographic characteristics (Figure 2). Older patients and people with sporadic disease and limb onset had longer diagnostic timelines (Figure 2).
Figure 1.
Diagnostic timelines. Box-and-whiskers plot representing the medians (+), inter-quartile ranges (boxes), and minimum and maximum values (whiskers) of the diagnostic intervals in months. The intervals represented include: time from presenting symptom to first doctor visit, time from first doctor visit to suspected ALS diagnosis, time from suspected ALS diagnosis to confirmed ALS diagnosis, and time from presenting symptom to confirmed ALS diagnosis.
Figure 2.
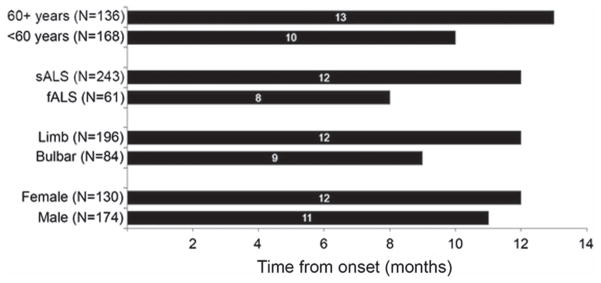
Total diagnostic time by patient sub-group. Total diagnostic time (from presenting symptom to confirmed diagnosis) was longer in patients over the age of 60 years compared to younger patients, in sporadic ALS (SALS) compared to familial ALS (FALS), and in limb compared to bulbar onset. Total diagnostic time was not different between genders. Time intervals are represented in months.
Predictors of diagnostic timelines
In a multiple regression model of baseline characteristics, the total time between symptom onset and confirmed ALS diagnosis was 51% longer in people older than 60 years compared to younger patients (p < 0.001), 46% longer in sporadic ALS (SALS) compared to familial ALS (FALS) (p = 0.043), and 45% longer in people with limb onset compared to bulbar onset (p = 0.010). Diagnostic time for females and males did not differ (p = 0.90). In a multiple regression model of presenting symptoms, the occurrence of fasciculations, slurred speech, and lower extremity weakness as part of the initial clinical presentation was associated with shorter time to ALS diagnosis relative to patients without these symptoms on initial presentation (fasciculations: 46% shorter (p = 0.04); slurred speech: 33% shorter (p = 0.02); lower extremity weakness: 27% shorter (p = 0.04)).
Alternative diagnoses and number of opinions
About half of the patients (52%) received at least one alternative diagnosis before ALS diagnosis was confirmed. The most common alternative diagnoses are given in Table II. Each patient saw an average of three physicians before ALS diagnosis was confirmed. In most patients the ALS diagnosis was first suspected by either a general neurologist or an ALS specialist (57% and 20% of cases, respectively). The diagnosis was confirmed by an ALS specialist in most cases (85%) or by a general neurologist (12%). Patients with FALS saw fewer specialists (p = 0.003) and received fewer incorrect diagnoses (p = 0.002) compared to patients with SALS. ALS patients older than 60 years saw more specialists (p = 0.04) compared to younger patients.
Table II.
Most common alternative diagnoses.
| Neuropathy | 28% |
| Spine disease | 18% |
| Vascular disease | 11% |
| Neurodegenerative disease | 11% |
| NMJ transmission disorder | 9% |
| ENT problem | 7% |
| Muscle disease | 6% |
| Other | 10% |
Fifty-two percent of patients in our cohort (n = 157) received at least one alternative diagnosis before ALS was confirmed. The ‘neurodegenerative disease’ category included Parkinson’s disease, hereditary spastic paraplegia, progressive supranuclear palsy, and progressive bulbar palsy. NMJ: neuromuscular junction. ENT: ear nose throat.
Discussion
Diagnostic timelines are long in ALS. In this large database, the median total diagnostic time was 11.5 months. This result is consistent with prior studies that reported median times from symptom onset to diagnosis of eight to 15 months (1–8). Our results expand on previous observations by defining the interim diagnostic milestones and their multiple clinical predictors (age, site of onset, family history, and presenting symptoms). Most previous studies were limited by a small sample size that precluded the analysis of interim diagnostic milestones and determinants of diagnostic delay (4–8). Even studies that analyzed large cohorts mostly focused on describing the overall length of the diagnostic process from onset to final diagnosis with no detailed characterization of time to see a physician, time to suspect vs. confirm the diagnosis, number of physicians seen and their specialty, and type of alternative diagnoses (2,3). Further, the only clinical determinants of diagnostic delay that were identified in prior studies were site of onset and presence of fasciculations (1–8). Our study provides a comprehensive description of the complex diagnostic process in one large cohort of people with ALS.
The implications of long delays in diagnosing ALS are significant, especially when one considers that they represent a significant proportion of total disease duration. During this complex diagnostic process, ALS patients may undergo unnecessary and sometimes painful diagnostic tests. Some patients may even receive invasive procedures such as spine surgeries. We could not estimate the number or cost of unnecessary interventions in our cohort; however, these would be extremely important factors to investigate in future studies. A prolonged diagnostic process is likely to have a negative impact on the psychological well-being of patients and their families and result in a period of stressful uncertainty and anxiety. Furthermore, delays in diagnosis represent a missed opportunity for patients to receive treatment, such as riluzole, medications for symptom management, rehabilitation interventions, nutritional counseling, and non-invasive ventilation (9,10). Identification of patients at early disease stages is a critical area of research and an unmet need not only in ALS but also in other neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. These pathologies are characterized by slow development with an asymptomatic or pauci-symptomatic period followed by gradual emergence of typical symptoms. As a consequence, these conditions are diagnosed when marked neuronal death has already occurred and therapeutic interventions are less likely to be effective. Importantly, patients diagnosed in later stages may have missed the window to enroll in clinical trials, many of which require an early ALS diagnosis as an entry criterion. Diagnostic delays also have a negative impact on research efforts revolving around the identification of biomarkers and mechanisms of early disease.
The causes of diagnostic delay in ALS are likely multifactorial. First, the time from symptom onset to first doctor visit was long (four months). This delay in seeking medical attention could be due to the slow and insidious onset of the disease and to the possibility that patients may wait until symptoms become more noticeable or cause a functional limitation before seeking medical attention. Secondly, ALS presenting symptoms can mimic a variety of other conditions (11). In our sample just over half the patients initially received alternative diagnoses, consistent with other reports which suggested that 27–61% of this patient population is misdiagnosed (1,3,5,7,12,13). Patients in this study saw an average of three physicians before reaching the final diagnosis. ALS diagnosis is primarily based on clinical assessment. It is therefore not surprising that confirmation of the diagnosis is often deferred to sub-specialists with expertise in this rare condition. We speculate that fast referral to general neurologists and dedicated ALS centers may shorten ALS diagnostic timeline. Future studies are needed to confirm this hypothesis. Further, research is needed to clarify the impact of other non-clinical factors (e.g. rural vs. urban setting, insurance coverage) in order to optimize referral patterns and prioritize resource allocation.
Acknowledgments
This study was funded in part by support from the Harvard NeuroDiscovery Center.
Footnotes
Declaration of interest: Sabrina Paganoni is funded by an NIH Career Development Award. Eric Macklin serves on Data Monitoring Committees for Lantheus Medical Imaging and Shire Human Genetic Therapies and was an unpaid consultant to Knopp Biosciences. Merit Cudkowicz has provided consulting for Neuraltus, Cytokinetics and Teva. Nazem Atassi receives fellowship grants from the American Academy of Neurology (AAN), Muscular Dystrophy Association (MDA), and the Anne Young Fellowship, has research grants from the Harvard NeuroDiscovery Center and ALS Therapy Alliance (ATA), and provided consulting for Biogen IDEC.
The authors alone are responsible for the content and writing of the paper.
References
- 1.Chio A. ISIS Survey: an international study on the diagnostic process and its implications in amyotrophic lateral sclerosis. J Neurol. 1999;246(Suppl 3):III1–5. doi: 10.1007/BF03161081. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JD, Callagher P, Gardham J, Mitchell C, Dixon M, Addison-Jones R, et al. Timelines in the diagnostic evaluation of people with suspected amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) – a 20-year review: Can we do better? Amyotroph Lateral Scler. 2010;11:537–41. doi: 10.3109/17482968.2010.495158. [DOI] [PubMed] [Google Scholar]
- 3.Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2012;114:550–4. doi: 10.1016/j.clineuro.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Donaghy C, Dick A, Hardiman O, Patterson V. Timeliness of diagnosis in motor neuron disease: a population based study. Ulster Med J. 2008;77:18–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Househam E, Swash M. Diagnostic delay in amyotrophic lateral sclerosis: what scope for improvement? J Neurol Sci. 2000;180:76–81. doi: 10.1016/s0022-510x(00)00418-4. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki Y, Ikeda K, Ichikawa Y, Igarashi O, Kinoshita M. The diagnostic interval in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2002;104:87–9. doi: 10.1016/s0303-8467(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer M, Buerger M, Berlit P. Diagnostic problems and delay of diagnosis in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2010;112:103–5. doi: 10.1016/j.clineuro.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Zoccolella S, Beghi E, Palagano G, Fraddosio A, Samarelli V, Lamberti P, et al. Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population based study. J Neurol Sci. 2006;250:45–9. doi: 10.1016/j.jns.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227–33. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry. 2003;74:1258–61. doi: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial ‘Clinical limits of amyotrophic lateral sclerosis’ workshop contributors. J Neurol Sci. 1994;124 (Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 12.Belsh JM, Schiffman PL. Misdiagnosis in patients with amyotrophic lateral sclerosis. Arch Intern Med. 1990;150:2301–5. [PubMed] [Google Scholar]
- 13.Belsh JM, Schiffman PL. The amyotrophic lateral sclerosis (ALS) patient perspective on misdiagnosis and its repercussions. J Neurol Sci. 1996;139 (Suppl):110–6. doi: 10.1016/0022-510x(96)00088-3. [DOI] [PubMed] [Google Scholar]