Abstract
Background
We have previously shown an increased incidence of intermittent hypoxemic events (IH) in preterm infants with severe retinopathy of prematurity (ROP). Animal models suggest that patterns of IH events may play a role in ROP severity as well. We hypothesize that specific IH patterns are associated with ROP in preterm infants.
Methods
Variability in IH duration, severity and the time interval between IH (≤80%, ≥10 seconds and ≤3min) along with the frequency spectrum of the oxygen saturation (SpO2) waveform were assessed.
Results
Severe ROP was associated with 1) an increased mean and standard deviation of the duration of IH (p<0.005), 2) more variability (Histogram Entropy) of the time interval between IH (p<0.005), 3) a higher IH nadir (p<0.05), 4) a time interval between IH of 1–20min (p<0.05) and 5) increased spectral power in the range of 0.002–0.008Hz (p<.05), corresponding to SpO2 waveform oscillations of 2–8 minutes in duration. Spectral differences were detected as early as 14 days of life.
Conclusions
Severe ROP was associated with more variable, longer, and less severe IH events. Identification of specific spectral components in the SpO2 waveform may assist in early identification of infants at risk for severe ROP.
Introduction
Retinopathy of prematurity (ROP), a vasoproliferative disorder of the retina that can produce significant loss of vision, continues to cause major morbidity in the preterm population. Reduction in oxygen levels has been only partially successful in reducing the incidence of this disease (1–3). The first phase of ROP is associated with hyperoxia, which is thought to down-regulate vascular endothelial growth factor (VEGF) and initiate avascularization or vascular regression. In the second phase the retina becomes hypoxic, up-regulating VEGF and other growth factors leading to rebound neovascularization. In animal models, short fluctuations in oxygen levels also play a role in neovascularization and retinal levels of VEGF (4–6).
We have previously shown that intermittent hypoxemic events (IH) are associated with severe ROP in preterm infants. In neonates of 24–28 weeks gestation, the occurrence of IH increases with advancing postnatal age. In infants with mild or no ROP, the incidence of IH events is relatively low during the first week of life, increases through the 5th week of life, followed by a decrease in IH thereafter (7). In contrast, after 5 weeks of age, the prevalence of IH remains elevated in infants with severe ROP. Anecdotally, there is a wide range in patterns of these events both within and between infants, as IH can occur in isolated episodes or clusters such as those accompanying periodic breathing. Timing of IH may affect the extent of neovascularization. Rodent data have shown that clustered IH result in increased neovascularization and higher levels of retinal VEGF when compared with a comparable number of equally dispersed IH (4). Therefore, the purpose of this study was to characterize the patterns of IH associated with severe ROP in preterm infants.
Results
The study population included 63 infants with mild or no ROP and 16 infants with severe ROP requiring laser therapy. As previously reported (7), the infants with severe ROP had lower birth weights (730±156 versus 863±180 g, p<0.01), lower gestational age (25.5±0.7 versus 26.2±1.2 weeks, p<0.001), and had a higher percentage of males (69% versus 36%, p<0.05). There was no difference between infant groups in multiple births (25% versus 21%) or race (63% versus 41% Caucasian). The infants with severe ROP received more days of supplemental oxygen therapy (51±12 versus 34±20 days, p<0.001) and ventilatory support (40±18 versus 14±15 days, p<0.001) and had fewer days on caffeine (34±16 versus 44±12 days, p<0.01), while exhibiting a higher incidence of IH events in the severe ROP group after 35 days of life (7).
As the higher number of IH episodes might be attributed to lower mean oxygen saturation in the ROP group, both the mean oxygen saturation and the mean baseline oxygen saturation, excluding IH events, were calculated. Mean and baseline oxygen saturation levels were significantly lower in the ROP group (p<0.05 and p<0.01, respectively, Figure 1) after 42 days of life (p<0.05). Interestingly, infants with severe ROP had decreased event severity (p=0.04), as defined by a higher nadir, after 14 days of life (p<0.05, Figure 2).
Figure 1.

Both A) mean SpO2 and B) baseline SpO2, excluding IH events, were significantly lower in the severe ROP group (p<0.05 and p<0.01, respectively). open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
Figure 2.

There was an increase in event severity with increasing age in both infant groups. Infants with severe ROP had less severe events, as defined by a higher nadir, after 14 days of life (p<0.05). open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
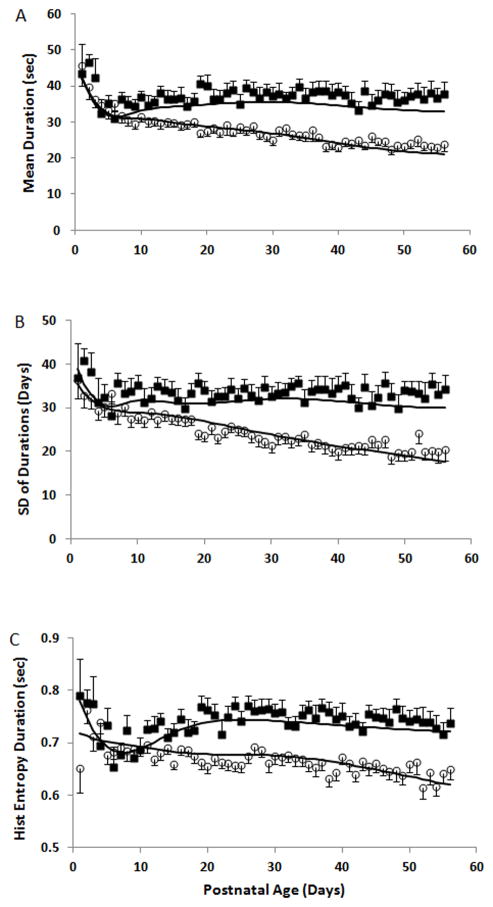
Duration of IH Events
The mean and SD of the duration of IH events decreased with increasing age in the no or mild ROP group. In contrast, both parameters remained constant in the ROP group with a longer mean and higher SD when compared to the no or mild ROP group (both p<0.005) with significantly longer mean durations ≥21 days of life (p<0.005) and higher SDs of the duration of IH events ≥28 days of life (p=0.004) (Figure 3). There were no differences in the CoV of the IH event durations between infant groups (not shown). Histogram Entropy of the durations decreased with increasing postnatal age in the no or mild ROP group, indicating a trend toward reduced variability and complexity in the IH event time series. In contrast, Histogram Entropy was higher in the severe ROP group (p<0.0001) and remained relatively constant revealing a significantly less predictable pattern of event durations in that infant cohort ≥14 days of life (p<0.05) (Figure 3c).
Figure 3.
The A) mean, B) standard deviation, and C) histogram (Hist) entropy of the duration of IH events decreased with increasing postnatal age in the No or Mild ROP group. In contrast, these parameters remained relatively constant in the severe ROP group, after the first week of life, with a longer mean (p<0.05), higher standard deviation (p<0.05) and higher histogram entropy (p<0.0001) when compared to the No or Mild ROP group. Histogram entropy may be a more sensitive marker with earlier differences identified between groups at ≥14 days of life (p<.05) followed by mean duration ≥ 21 days of life (p<0.005) and standard deviation of IH event durations at ≥ 28 days of life (p=0.004). open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
Time Interval between Events
The mean and standard deviation of the time interval between IH events declined over the first three weeks of life with no differences between infant groups, and the CoV remained constant (not shown). Histogram Entropy of the time intervals was higher in the severe ROP group (p<0.005) with significant differences between infant groups at 28 days life (p=0.033).
Due to the wide variability in the time interval between IH events, the number of IH events was stratified into three time interval windows: those that occurred with a time interval between events of <1min, 1–20 minutes and >20 minutes (Figure 4). A substantial number of events occurred <1min apart, however, there were no differences between infant groups at this time interval range. Relatively few IH events occurred with a time interval of >20 min between IH events also with no overall difference between infant groups. Interestingly, the higher number of IH in the severe ROP group was associated with a time interval of 1–20 minutes between IH significantly different at 28 days of life (p<0.05) and a trend towards a higher frequency of events at 21 and 35 days (both p=0.064).
Figure 4.
Stratification of events by a time interval of A) <1min, B) 1–20 minutes and C) >20 minutes. A) A substantial number of events occurred <1min apart, however, there were no differences between infant groups. B) A higher number of IH in the severe ROP group was associated with a time interval of 1–20 minutes between IH events with a significant difference at 28 days of life (p<0.05) and a trend towards a higher frequency of events at 21 and 35 days (both p=0.064). C) Relatively few IH events occurred with a time interval of >20 min between IH events with no overall difference between infant groups. open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
SpO2 Waveform Analysis
To detect differences between groups that may have been missed by the arbitrary thresholds chosen to define an IH event, SamEn and Wavelet analyses were applied to the entire raw SpO2 waveform. There was an increase in SamEn of the SpO2 waveform with increasing postnatal age in both infant groups with no significant difference between groups (Figure 5). Wavelet analysis revealed differences in spectral power between the two infant groups that were frequency and age dependent (Figure 6). At higher frequencies (D1: 0.125–0.250Hz, D2: 0.063–0.125Hz, and D3: 0.031–0.063Hz), the severe ROP infant group had significantly lower spectral power (all p<0.05) from 21–49 days of life at level D1 (p<0.05) and ≥14 days of life at levels D2 and D3 (both p<0.05). In contrast, there was a shift towards a significantly higher spectral power in the severe ROP group at the two lowest frequency levels (D6: 0.004–0.008Hz, 21 to 28 days of life (p<0.05), D7: 0.002–0.004Hz, 21 to 35 days of life (p<0.05)). Thus, the ROP group had lower power in SpO2 waveform oscillations of 4–30 s in duration and higher power in SpO2 waveform oscillations of 2–8 minutes in duration.
Figure 5.

There was an increase in Sample Entropy with increasing postnatal age in both infant groups. Although there was a slightly higher Sample Entropy in the ROP group there was no significant difference between groups. open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
Figure 6.
Wavelet analysis of the SpO2 waveform. At the higher frequencies levels, A) D1: 0.125–0.250Hz, B) D2: 0.063–0.125Hz, and C) D3: 0.031–0.063hz, the severe ROP infant group had significantly lower spectral power from 21–49 days of life at level D1 (p<0.05) and ≥14 days of life at levels D2 and D3 (all p<0.05). There were no differences between groups at mid frequencies D) D4: 0.016–0.031Hz, and E) D5: 0.008–0.016Hz. At the two lowest frequency levels F) D6: 0.004–0.008Hz and G) D7: 0.002–0.004Hz) there was a shift towards a significantly higher spectral power in the severe ROP group from 21 to 28 days of life at level D6 (p<0.05) and from 21 to 35 days of life at D7 (p<0.05). open circle, No or Mild ROP, black square, Severe ROP, solid line, covariate adjusted models. m±SEM.
Postmenstrual Age
In addition to the above analyses which were based on post natal age, we also compared IH events between ROP groups over postmenstrual age. There were IH events of longer duration at a post menstrual age of >28 weeks (p<0.05), and more IH events at 1–20 minutes intervals in the severe ROP group from 29–30 weeks (p<0.05). There was lower power in the D1 frequency range and higher power in the D7 frequency range in the severe ROP group at 29–33 weeks (p<0.05) and 29–30 weeks (p<0.05) postmenstrual age, respectively.
Discussion
Previous work by our group revealed more IH events in this cohort of preterm infants with severe ROP. This study gives novel new insight into the patterns of these events. Infants with severe ROP had longer, more variable, and less predictable IH durations. Standard statistical summary measures (mean and SD) were inadequate in their ability to distinguish group differences in IH time intervals but stratification of IH by time interval ranges revealed a higher number of IH events in the severe ROP group associated with 1–20 minute intervals between events. Sample Entropy, an unbiased measure of predictability based on the entire SpO2 waveform data, was found to be slightly but not significantly increased in the severe ROP group. Lastly, Wavelet analysis revealed time-frequency dependent differences in spectral power between infant groups. In summary, this study identifies potential tools that can be used to identify infants who may be at future risk for severe ROP based on dynamic temporal and time-frequency patterns of the oxygen saturation waveform.
Using standard statistical descriptors (mean and SD) and Histogram Entropy (8), we now show that infants with severe ROP have longer and less predictable IH durations. This may be due to decreased oxygen reserve destabilizing respiration. A mathematical model incorporating pulmonary uptake dynamics found that low pre-apneic alveolar PO2 caused early onset of desaturation (9). The lower baseline oxygen saturation levels in the severe ROP group, which suggest a decreased oxygen reserve, may have initiated an early onset of desaturation and prolonged the duration of IH in this infant cohort. The instability related to an immature respiratory system compounded by low baseline oxygen saturation may lead to more complex or variable duration patterns showing a higher Histogram Entropy value in the ROP group. Interestingly, even with longer, less predictable durations, the infants with ROP had less severe events. Nock et al. (10) showed an augmented ventilatory response to hypoxia in preterm infants with increased apnea. This might imply that IH accompanying apnea may initiate a downstream protective cascade, although the differential effects of this response on the duration and severity of IH are unknown.
Although ROP is associated predominantly with hyperoxia, infants with severe ROP were found to have a lower baseline SpO2 after 42 days of life. It may be that both infant groups were exposed to relative “hyperoxia” during early postnatal life, however the subsequent lower baseline oxygen saturation in the ROP group exacerbated the rebound neovascularization leading to increased ROP.
Although our ability to detect differences between infant groups in the overall time interval between events using the proposed mathematical models was limited, we were able to isolate a window of time between IH of 1–20 minutes that was associated with severe ROP. Chronic intermittent hypoxia has been shown to increase oxidative stress in adults (11) and oxidative stress has been linked to ROP through mechanisms related to oxygenation of retinal tissue (12). In a rodent model, superoxide anion concentration, a marker of oxidative stress, has been shown to increase during the re-oxygenation phase following hypoxic exposure (13) with a delay in the response of a few minutes. This time span corresponds to the differences between groups seen in this study at a time interval between events of 1–20 minutes. Although there were a substantial number of events occurring <1min apart, the incidence was comparable between groups indicating that this time interval may not be long enough for an oxidative stress response, relating to the initiation of the ROP cascade, to occur. This may also suggest that periodic breathing, associated with short intervals between IH, may not play a role in the relationship between IH and ROP.
In this study, quantification of possible patterns of oxygen saturation associated with ROP was limited by the arbitrarily chosen definition of IH. Therefore, SamEn and Wavelet analyses were employed to evaluate unbiased patterns of the entire SpO2 waveform. Both spectral and SamEn measurements have been shown to be useful tools in revealing respiratory patterns associated with morbidity (14) and maturation (15). Sample Entropy has been used by our group and others in animal (16) and human models (15,17) to assess respiratory and EEG arousal pattern variability. After minimizing the influence of autocorrelation on the Sample Entropy calculations (18) and defining a constant tolerance (SD=16) within and between infant groups, we have shown a trend towards a higher SamEn in the severe ROP group, however, this was not statistically significant. A larger sample size may be needed to detect these subtle pattern differences between groups.
The number of infants with severe ROP may be a limitation of this study as significant differences were found between infant groups in demographics and clinical course. After controlling for gestational age, race, gender, and multiple birth in the statistical model we were still able to detect differences in patterns of IH between infant groups. However, with respiratory support and oxygen changing on a day by day basis we were unable to control for these variables in the analyses. Therefore, the results of this study reveal an association and not a causal relationship between IH and severe ROP. To distinguish intermittent hypoxia from prolonged changes in baseline oxygenation, we prospectively defined our IH events by a duration range of 10 seconds and 3 minutes, therefore, events > 3 minutes were not included in the analysis. Subsequent analysis of the data looking at IH events >3 minutes shows that these events would account for a median of 5% and 3% of all events in the ROP and No or Mild ROP groups, respectively. Therefore, we feel that inclusion of these events would have minimal impact on our findings. Lastly, inclusion of infants with mild ROP in the control group may have affected our results. As there is inter-examiner variability in this area we chose what we thought to be the clearest distinction between groups.
Wavelet analysis is the most promising tool, revealing distinguishing patterns between the infant groups that were frequency- and age-dependent. Infants with severe ROP have lower power in the D1–D3 frequency range associated with short SpO2 oscillations of 4–30 s, such as those occurring during periodic breathing or short apnea. In contrast, severe ROP infants had increased power in the D6–D7 frequency range associated with longer SpO2 oscillations of 2–8 minutes. These frequency-dependent differences between groups were detected as early as 14 days of life. In contrast to adult rats, neonatal rats have been shown to have an enhanced carotid body response to acute hypoxia following chronic intermittent hypoxic exposure (19). This sensitized carotid body activity may lead to a change in gain of the CNS (20) with resultant overcompensation of ventilation. The ventilatory gain mismatch may drive CO2 down leading to ventilatory depression with accompanying oscillations in oxygen saturation and may explain the increased power seen in the SpO2 oscillations of long duration. The Wavelet analysis methodology uses the entire SpO2 waveform without the restriction of arbitrarily defined thresholds or durations of IH events, can identify early distinguishable patterns in the oxygen saturation waveform, and may prove useful in the NICU setting to identify infants at risk for severe ROP.
Application of the mathematical models presented in this study requires higher sample rates and shorter averaging times than conventionally used in clinical practice and previous studies. Prior infant data have suggested a relationship between fluctuations in oxygenation and ROP with interpretation of results limited by intermittent blood gas monitoring (21–23). This study included minimally processed (24) and highest resolution data available from the Masimo pulse oximeter with data continuously recorded over 8 weeks of life (7) allowing for identification of subtle SpO2 waveform patterns associated with ROP. Storage of high-resolution data is currently limited by the pulse oximeter data storage capacity. Further development of long-term data storage at the bedside along with automated application of mathematical signal analysis algorithms may lead to identification of these early morbidity markers in the clinical setting.
Analysis for at risk IH patterns was analyzed by both post natal and post menstrual age. Palmer et al (25) found an association between both postnatal and post menstrual age and onset of prethreshold ROP with less variability when data were analyzed by postmenstrual age. Although we also detected group differences in IH patterns by post menstrual age, these differences corresponded to 28–35 days of life in our youngest infants of 24 weeks gestation. In contrast, the post natal age model comparisons revealed group differences as early as 14 days of life. Therefore, we believe post natal age to be the superior model as it may lead to earlier intervention in this infant cohort with an increased chance of preventing this morbidity.
In conclusion, infants with severe ROP have a higher incidence of IH of longer, more variable and less predictable duration. These events are more likely to occur with a time interval between events of 1–20 minutes. Infants who go on to have severe ROP have a slightly lower baseline SpO2 which may destabilize respiratory control and/or lead to more complex patterns of oxygen saturation that may be identified by more comprehensive data analysis models such as Sample Entropy or Wavelet analysis. The results of this study may elucidate early patterns of IH that put infants at risk for future development of severe ROP.
Methods
The study population was our prior cohort in which we characterized the incidence of IH events in preterm infants. It comprised 79 infants, (gestational age, 24–27 6/7 weeks) admitted to Rainbow Babies & Children’s Hospital between 2005 and 2009. Infants with no congenital malformations were eligible for enrollment regardless of their need for ventilatory support or supplemental oxygen. As this study included previously acquired data, Rainbow Babies & Children’s Hospital Institutional Review Board approval and waiver of consent was obtained for the study.
Patients received normal clinical care and were kept in an oxygen saturation target range of 85–95%. To minimize disparities in diagnosis of less severe forms of ROP, infants were stratified into two groups; 1) those requiring laser therapy for severe ROP and 2) those having no or mild ROP. The guidelines of the Early Treatment for Retinopathy of Prematurity Cooperative Trial were used to determine the need for laser treatment (26).
IH events were documented from continuous, high-resolution (2 second averaging and 0.5 Hz sampling rate) recordings of oxygen saturation (Radical, Masimo Corp, Irvine, California) over the first 8 weeks of life. To distinguish IH from prolonged changes in baseline oxygenation, IH was defined as a fall in oxygen saturation (SpO2) to ≤80% for ≥10 seconds and ≤3 minutes in duration. Customized software was developed to identify the incidence, duration, severity (nadir) and the time interval between IH. A suite of linear and non-linear analysis software algorithms was developed to quantify patterns of IH including mean, standard deviation (SD), and coefficient of variation (CoV) to quantify changes in frequency and variability of IH, and algorithms derived from time-frequency signal analysis (Wavelets), information theory (Histogram Entropy) and statistical signal processing (Sample Entropy), were used to quantify different aspects of IH pattern variability. During the monitoring periods there were intermittent times when signal loss occurred due to infants not being monitored, corrupt files, and noise or motion artifact. Periods with 0% SpO2 or 0 bpm for heart rate were considered artifact and removed from the data stream with the remaining data analyzed in 24 hour (mean, SD, CoV and Histogram Entropy) or 1 hour (Sample Entropy, Wavelet analysis) sliding windows. Large areas of missing data, i.e. 24 hours, were treated as missing cells in the statistical analysis.
Histogram Entropy was used to further understand how the patterning of the duration and time interval between hypoxic events influences the development of ROP. This measure can be used to quantify the degree of randomness in the data. For example, a signal with higher Histogram Entropy has a wider distribution of values, meaning the data are more variable and, thus, less predictable. The uniform distribution would have maximal Histogram EntropyAccurate computation of Histogram Entropy requires a reliable distribution of the dataset, therefore days with <10 events were excluded from this particular analysis.
While Histogram Entropy is used for analyses where variables can be sorted, such as IH durations or time intervals between events, Sample Entropy is used for continuous waveform data. Similar to Histogram Entropy, Sample Entropy quantifies predictability in time series data based on the likelihood that a given window of SpO2 data will, over time, remain close to the last window of SpO2 data—for example, given one hour of SpO2 data, will the next one hour window of data be similar in extent and variability? Examining this measure across groups offers a relative comparison of the “complexity” of the time series with higher values of Sample Entropy (SamEn) associated with less predictability or higher system complexity. Sample Entropy was computed using the formula:
where m=pattern length, r= the tolerance parameter, n= number of samples, τ= time delay, Ai= the number of points in the time series within tolerance r for the m+1-dimensional pattern and Bi= the number of points in the time series within tolerance r for the m-dimensional pattern. Sample Entropy was calculated using m=2, r=0.2 x SD, and τ=20 samples. A non-unity time delay (τ) was chosen to reduce the contribution of the linear statistical autocorrelation properties of the signal (18). The value of τ=20 samples was chosen based on pilot data in 10 infants identifying a time delay where the linear (auto)correlation was minimized. Because SamEn detects SpO2 patterns falling within a given tolerance (r=0.2 xSD), the same tolerance criteria was used to compare within and between infants. A constant standard deviation of 16 was chosen based on the maximum standard deviation of SpO2 values for all days in all infants.
When viewing an overall SpO2 waveform over time, it can be seen that the waveform consists of oscillations throughout both “normal” and “hypoxic” ranges of SpO2. These oscillations can be decomposed into separate frequency components, using time-frequency (Wavelet) analysis, allowing for quantifiable comparisons of the power of individual frequency ranges between the two infant groups. A seven-level 1-D stationary Wavelet decomposition was performed using a Daubechies (4th in the series) orthogonal wavelet decomposition filter over the entire waveform time series. The total power in each frequency range (D1: 0.125–0.250Hz, D2: 0.063–0.125Hz, D3: 0.031–0.063Hz, D4: 0.016–0.031Hz, D5: 0.008–0.016Hz, D6: 0.004–0.008Hz, D7: 0.002–0.004Hz, and A7: 0–0.002Hz) was calculated for each 1h window. Power in the D1–D3 frequency range corresponds to SpO2 waveform oscillations of 4–30 seconds in duration, such as those associated with periodic breathing or short apnea, while power in the D6–D7 frequency range corresponds to longer SpO2 waveform oscillations of 2–8 minutes. Data were analyzed, using both Wavelet and Sample Entropy algorithms, in one hour sliding windows with no overlap and windows with missing data were excluded from the analyses.
Statistical analysis of all measures was performed on daily averages computed for each subject from the hourly data. A logarithmic or square root transformation was applied when the original data were skewed. A t-test was used for demographic comparisons between infant groups. Data are presented as mean±SEM. Longitudinal profiles of severe ROP and no or mild ROP groups were estimated and compared using linear mixed models, modeling the nonlinear relationship of each outcome with age using cubic splines with knots at 10, 14, and 30 days (27). Simpler quadratic or cubic models were used for five variables (entropy of the time intervals, mean SpO2, nadir, IH with time interval of 1–20 minutes, Wavelet D1, and Wavelet D2). Models included terms for gestational age, race, gender, multiple birth, and their interactions with terms involving day. A likelihood ratio test was used to compare overall mean curves of severe ROP versus no or mild ROP with post comparisons at days 1, 7, 14, ..., 56.. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Acknowledgments
Statement of Financial Support: Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) 1RO3HD064830-01
References
- 1.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Eng J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow LC, Wright KW, Sola A CSMC Oxygen Administration Study Group. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 3.Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–518. doi: 10.1016/j.ophtha.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RJ, Beharry KD, Brock RS, Abad-Santos P, Abad-Santos M, Modanlou HD. Effects of brief, clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy. Pediatr Res. 2008;64:50–55. doi: 10.1203/PDR.0b013e31817307ac. [DOI] [PubMed] [Google Scholar]
- 5.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: Correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004;79:623–630. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shannon CE. A mathematical theory of communication. The bell system technical journal. 1948;27:379–423. 623–656. [Google Scholar]
- 9.Sands SA, Edwards BA, Kelly VJ, Davidson MR, Wilkinson MH, Berger PJ. A model analysis of arterial oxygen desaturation during apnea in preterm infants. PLoS Comput Biol. 2009;5(12):e1000588. doi: 10.1371/journal.pcbi.1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nock ML, Di Fiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr. 2004;144:291–5. doi: 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Pialoux V, Hanly PJ, Foster G, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 12.Hartnett ME. The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: Knowledge from the 50/10 OIR model. Doc Ophthalmol. 2010;120:25–39. doi: 10.1007/s10633-009-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabian RH, Perez-Polo JR, Kent TA. Extracellular superoxide concentration increases following cerebral hypoxia but does not affect cerebral blood flow. Int J Dev Neurosci. 2004;22:225–230. doi: 10.1016/j.ijdevneu.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Marchenko V, Rogers RF. Time-frequency coherence analysis of phrenic and hypoglossal activity in the decerebrate rat during eupnea, hyperpnea, and gasping. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1430–R1442. doi: 10.1152/ajpregu.00218.2006. [DOI] [PubMed] [Google Scholar]
- 15.Engoren M, Courtney SE, Habib RH. Effect of weight and age on respiratory complexity in premature neonates. J Appl Physiol. 2009;106:766–773. doi: 10.1152/japplphysiol.90575.2008. [DOI] [PubMed] [Google Scholar]
- 16.Dragomir A, Akay Y, Curran AK, Akay M. Investigating the complexity of respiratory patterns during the laryngeal chemoreflex. J Neuroeng Rehab. 2008;5:17. doi: 10.1186/1743-0003-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamasebi R, Redline S, Patel SR, Loparo KA. Entropy-based measures of EEG arousals as biomarkers for sleep dynamics: Applications to hypertension. Sleep. 2008;31:935–943. [PMC free article] [PubMed] [Google Scholar]
- 18.Kaffashi F, Fogylano R, Wilson CG, Loparo KA. The effect of time on approximate and sample entropy calculations. Physica D. 2008;237:3069–3074. [Google Scholar]
- 19.Pawar A, Peng Y, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body response to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CA, Forster HC, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: Evidence and implications of ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–87. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 22.Saito Y, Omoto T, Cho Y, Hatsukawa Y, Fujimura M, Takeuchi T. The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefe’s Arch Clin Exp Ophthalmol. 1993;231:151–156. doi: 10.1007/BF00920938. [DOI] [PubMed] [Google Scholar]
- 23.Hausburg AK, Allred AN, Vanderveen DK, et al. Blood gases and retinopathy of prematurity: The ELGAN study. Neonatol. 2010;99:104–111. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farre R, Montserrat JM, Ballester E, Hernandez L, Rotger M, Navajas D. Importance of the pulse oximeter averaging time when measuring oxygen desaturation in sleep apnea. Sleep. 1998;21:386–390. doi: 10.1093/sleep/21.4.386. [DOI] [PubMed] [Google Scholar]
- 25.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 26.Early Treatment For Retinopathy Of Prematurity Cooperative. Group Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]