Abstract
Background
The short (S) allele of the serotonin transporter gene (5-HTTLPR) is associated with reduced serotonin turnover compared to the long (L) allele in Caucasians. Few studies have examined its impact on memory and brain structure in healthy young adults.
Methods
Participants included 51 healthy young adults (25 female; ages 18-25). Multiple regressions examined the independent contribution of 5-HTTLPR biomarker genotype and its interactions with gender and sub-clinical depressive symptoms on hippocampal volumes and memory.
Results
The 5-HTTLPR genotype significantly interacted with gender in predicting larger left hippocampal volumes in S-carrying females and smaller hippocampal volumes in males (p<.03). Gender also moderated the impact of the 5-HTTLPR on neurocognition. In females, S allele carriers had poorer visual recall compared to L carriers (p<.05). A three-way interaction between 5-HTTLPR, gender, and depressive symptoms was also observed (p<.04). In females, larger left hippocampal volumes were associated with increased depressive symptoms while the opposite was seen in males. Finally, in male and female S carriers, increased depressive symptoms were marginally associated with poorer verbal memory (p<.09).
Conclusions
In females, the 5-HTTLPR S allele was associated with poorer memory performance, increased depressive symptoms and larger hippocampal volumes. In males, the S allele predicted smaller hippocampal volumes and increased depressive symptoms. The opposite morphometric patterns likely reflect gender differences in adolescent hippocampal development. Larger longitudinal studies are needed to examine whether the impact of 5-HTTLPR genotype on neurocognition across development differs according to extent of mood symptoms and gender.
Keywords: genetics, 5-HTTLPR, hippocampus, memory, depression, gender
1. INTRODUCTION
A potential biomarker of serotonin expression that may be related to individual variability in neurocognition and brain structure is the serotonin (5-HT) transporter gene (SLC6A4). A deletion/insertion polymorphism (5-HTTLPR) exists within the 5′ flanking regulatory region of the SLC6A4 and is associated with levels of 5-HT transporters (SERT) and reuptake, ultimately affecting levels of 5-HT transmission. Specifically, the short (S) allele has been shown to have 40% fewer SERT binding sites and decreased reuptake compared to the long (L) allele (Lesch et al., 1996). Animals and humans with at least one S allele demonstrate fewer or inefficient presynaptic 5-HT transporters, resulting in more 5-HT availability (Lesch & Mossner, 1998; Hensch et al., 2006; Holmes et al., 2003). However, greater synaptic 5-HT may be related to lower postsynaptic 5-HT signaling, likely due to receptor downregulation (Williams et al., 2003). Indeed, literature supports that S allele carriers have 5-HT-related disorders such as anxiety, depression, negative affect, and bipolar disorder (Lasky-Su et al., 2005; Lotrich et al., 2004; Neumeister et al., 2004; Schinka et al., 2004, Sen et al., 2004) and increased limbic responding to an affective processing task (Hariri et al., 2006). However, there is evidence of no association among those with psychosis (Dutt et al., 2009), and some prospective studies have reported the discrepant finding that young adults with the LL genotype who also experienced high amounts of family discord were more likely to experience depression and anxiety that those with other genotypes (Chipman et al., 2007; Laucht et al., 2009).
In addition to down-stream behavioral symptoms associated with 5-HT signaling, there is additional research examining 5-HTTLPR’s impact on biological endophenotypes, including hippocampal structure or memory functioning. Studies on SLC6A4-knockout mice have demonstrated a link between the genotype and 5-HT concentration and neurodegeneration in the hippocampus (Olivier, 2008). Gallinat and colleagues (2005) found lower hippocampal N-acetylaspartate (NAA, considered a mitochondrial marker and a reflection of neuronal density) levels among S allele carriers compared to those homozygous for the L allele (Gallinat et al., 2005). Taylor et al. (2005) reported a significant interaction between depression and 5-HTTLPR genotype in older adults, such that S/S predicted smaller hippocampal volumes for those with early-onset depression whereas L/L predicted smaller volumes for those with late-onset depression. In a relatively younger sample (mean age 33.6 years), Eker et al. (2011) found a gene by depression interaction, with smaller hippocampi among depressed S/S individuals. However, little research exists examining the 5-HTTLPR genotype and hippocampal morphometry in humans without serotonin-related disorders, especially among emerging adults. Frodl et al. (2008) examined the impact of the 5-HTTLPR genotype and its rs25531 variant on hippocampal volumes in healthy adults with and without depression. Control subjects with risk allele (S) profiles had reduced gray matter volumes in the right dorsolateral prefrontal cortex, left anterior cingulate cortex, left amygdala, and right hippocampus. The difference was less robust and in the opposite direction for depressed patients, in that the non-risk allele (L) was associated with smaller hippocampal volumes. A large study (n=357) examining interactions between genotype, gender, and childhood adversity (CA) found a main effect of genotype for smaller hippocampal volumes in S/S women, but only in the presence of CA did S/S men demonstrate smaller volumes (Everaerd et al., 2012). These findings suggest a link between hippocampal structure and 5-HTTLPR genotype in healthy adults, although this relationship may be moderated by the presence of a serotonin stressor (i.e., depressive symptoms, exposure to trauma).
5-HTTLPR genotype has been associated with attention (Enge et al., 2011a) and efficiency in working memory ability (Enge et al., 2011b). However, despite the role of 5-HT in memory, the association between 5-HTTLPR genotype and memory functioning also remains unclear, with some reporting association with verbal memory (Zilles et al., 2012) and others reporting null findings (Reneman et al., 2006; Mannie et al., 2009). Acute tryptophan depletion (ATD), which reduces 5-HT function, is a methodology that has been utilized in research to model serotonin-related neurocognitive functions and disorders. ATD has been shown to temporarily impair episodic memory (McAllister-Williams et al., 2002; Rubinsztein et al., 2001) and increase depressive symptoms (Neumeister et al., 2004; Leyton et al., 1997). For example, Roiser et al. (2008) demonstrated bias towards positive verbal stimuli and subcortical processing of emotion-related words associated with ATD. A separate study found that healthy S allele carriers who underwent acute tryptophan depletion (ATD) displayed poorer verbal recall than those administered a placebo, with no treatment effect for L allele carriers (Roiser et al., 2007). Such results suggest that the S allele confers verbal memory risk only when coupled with a serotonin-related stressor, like ATD. Even less is known about visual memory. SLC6A4-knockout rats performed worse on an object recognition task (Olivier et al., 2008), demonstrating a role for serotonin in visual memory. In humans, Roiser et al. (2007) found that S carriers had marginally better accuracy than L carriers on a pattern recognition task; this finding was replicated by Anderson, Bell, and Awh (2012), who reported better visual working memory ability among S/S and S/L genotypes compared to L/L genotypes.
Different gender and ethnicity composition across samples may help explain these inconsistent findings. The frequency of alleles differs among ethnic groups (Verona et al., 2006; Mannelli et al., 2006; Gelernter et al., 1997) and gender and ethnicity may moderate the functional impact of 5-HTTLPR on serotonin functioning. For example, Williams (2003) found increased serotonin turnover among African-American S/S females. Gender has also moderated the effects of 5-HTTLPR on down-stream behaviors. In adolescents, only female S allele carriers developed depressive symptoms when exposed to stressful environmental factors Sjoberg et al., 2006).
Given the paucity of research examining the relationship between 5-HTTLPR genotype and hippocampal morphometry and memory in youth, the primary goal of the current study was to examine whether the 5-HTTLPR genotype predicted hippocampal volume and verbal and visual memory functioning in a healthy sample of young adults. The secondary aim was to examine whether gender and sub-clinical depressive symptoms, a marker of potential serotonin stress (Everaerd et al., 2012; Frodl et al., 2008; Roiser et al., 2007), moderated the impact of 5-HTTLPR on neurocognition.
2. METHODS AND MATERIALS
2.1 PARTICIPANTS
Participants included 51 healthy young adults (25 female, 26 male). All participants were between the ages of 18-25, fluent in English, and right-handed. Exclusion criteria included MRI contraindications (pregnancy, claustrophobia, weight over 250 lbs., metal in body); history of chronic medical or neurologic illness or injury (meningitis, HIV, epilepsy, brain tumor, traumatic brain injury, injury resulting in greater than two minutes of unconsciousness, stroke, cerebral palsy, Parkinson’s disease, high blood pressure, diabetes, migraines, recent and/or multiple concussions); history of a learning disability; substantial complications during birth or premature birth; known prenatal exposure to alcohol (>4 drinks/day or >7 drinks/week), illicit drugs (>10), or nicotine (>10 cigarettes/day for >4 months); use of psychoactive medication; preexisting DSM-IV Axis I disorders not independent of substance use (including major depressive disorder, bipolar disorder, attention deficit hyperactivity disorder, conduct disorder, social phobia, agoraphobia, panic disorder, generalized anxiety disorder, obsessive compulsive disorder, anorexia, and bulimia); being considered a very heavy binge drinker; using marijuana >75 times in their lifetime; using other illicit drugs >50 times in their lifetime; and refusal to remain abstinent from all drugs and alcohol for at least 7 days.
2.2 PROCEDURES
The Institutional Review Board at the University of Cincinnati approved all aspects of this study. Participants were recruited through flyers and advertisements in community periodicals and college campuses. After individuals provided oral informed consent, trained research assistants screened prospective participants over the phone a semi-structured interview for Axis I psychotic, anxiety, attentional, and mood disorders. If participants screened positive, then additional diagnostic questions based on the SCID I/P were administered (determined by Dr. Medina; First et al., 2001). Eligible participants provided written informed consent and underwent urine toxicology, cotinine (measures recent nicotine use) and breathalyzer testing to rule out recent substance use and females took a pregnancy test (due to unknown fetal effects). Subjects provided a saliva sample for DNA analysis, were administered neuropsychological and psychological questionnaires, and underwent a magnetic resonance imaging (MRI) scan. All were paid $110 for successful completion of the study.
2.3 SCREENING INVENTORIES AND QUESTIONNAIRES
2.3.1 Demographic Information
Participants completed a Background Questionnaire outlining demographic variables including age, gender, ethnicity, self and biological parents’ educations, incomes, and employments, marital status, history of medical or neurologic illness, psychological disorders or use of psychiatric medication, and learning disability. Height and weight were also assessed to calculate body mass index (BMI), which has been associated with neurocognition (Gunstad, et al., 2007).
2.3.2 Drug Use
Drug use frequency was recorded to exclude for very heavy users as well as to control for possible variance in cognition based drug exposure. Urine cotinine levels (a nicotine metabolite and a proxy for recent nicotine exposure) were also collected. A modified version (Medina et al., 2005) of the Time-Line Follow-Back (Sobell et al., 1979) interview was conducted to measure past year and lifetime drug use, using memory cues and average use patterns. Drug categories assessed in standard units were as follows: nicotine cigarettes, chewing tobacco/snuff/pipe, cigars/hookah, alcohol, marijuana, ecstasy, sedatives, stimulants, hallucinogens, opioids, and inhalants.
2.3.3 Mood Inventories
Participants completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). The BDI-II is a well-validated self-report measure that contains 21 items measuring levels of recent depressive symptoms on a 0-3 point scale.
2.3.4 General Intellectual Functioning
The Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary subtest (Wechsler, 1999) was used as estimates of intelligence and quality of education for group comparison purposes (see Manly, 2002).
2.3.5 Memory Testing
The California Verbal Learning Test-2nd Edition (CVLT-2) was administered to assess verbal learning and memory (Delis et al., 2001). Subjects are exposed to a list of words over five trials. They are then exposed to a second distracter list and must recall this list then the original list. After a delay, free and cued recall and recognition ability are measured. The Rey Osterrieth Complex Figure Test (RCFT; Meyers & Meyers, 1995) and Wechsler Memory Scale-3rd Edition (WMS-III) Faces subtest (Wechsler, 1997) were administered to assess visual memory. For the RCFT, subjects are presented with a stimulus of a complex figure and asked to copy the figure onto a sheet of paper. After a short and long delay they are asked to draw the figure again from memory. Recognition of the visual material is then assessed. For the WMS-III Faces, subjects are presented with pictures of faces. Recognition memory for these faces is then assessed after a short and long delay.
A data reduction technique was used to create composite scores from individual variables (see Delis et al., 2003; Medina et al., 2007b). Individual raw scores were converted to z-scores for the entire sample and averaged to create each composite z-score. Standardized Cronbach’s alpha test were used to measure the internal consistency (all >.50). Final composites included: CVLT-2 immediate memory (total recall and Trial 1), delayed memory (short delay free recall, short delay cued recall, long delay free recall, long delay cued recall), retention (retention), and recognition memory (total recognition discrimination); RCFT recall (immediate and delayed) and recognition (recognition); and WMS-III Faces memory (immediate and delayed).
2.4 MRI DATA ACQUISITION
High-resolution anatomical images were optimized on a 4T Varian MRI scanner at the Center for Imaging Research. A T1-weighted, 3-D anatomical brain scan was obtained using a modified driven equilibrium Fourier transform sequence (TMD=1.1 s, TR=13 ms, TE=6 ms, FOV=25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96 pixels, flip angle=20 degrees; 15 min; Lee, et al., 1995).
2.5 IMAGE PROCESSING
3D anatomical datasets underwent AC-PC alignment and removal of non-brain materials before being processed with a combination of a hybrid watershed and deformable surface semi-automated skull-stripping program (Segonne et al., 2004) followed by manual editing. Trained researchers, blind to 5-HTTLPR and gender status, manually edited the anatomical masks in AFNI (Cox, 1996) to obtain intracranial volume (ICV). Direct manual tracing in AFNI (Cox, 1996) was utilized to obtain measurements for two hippocampal ROIs (see Nagel et al., 2004; Medina et al., 2007 and 2007a for stereotactic boundaries). Intra-rater reliability was achieved for the left [ICC=.95] and right [ICC =.96] hippocampus and volumes were each analyzed as proportions to ICV to control for individual variability in brain size (Giedd et al., 1996).
2.6 SLC6A4 GENOTYPING
Genotyping was performed for the L/S promoter polymorphism (5-HTTLPR) (Kaiser et al., 2002). Participants’ DNA underwent polymerase chain reaction (PCR), and the products were purified and digested with restriction enzyme HpaII. Undigested (L, 512 bp, and S, 469 bp) and digested products were run side-by-side on an agarose gel. Details of the amplification method and primers used were as in Thompson et al. (2010).
2.7 DATA ANALYSIS
ANOVAs and Chi-square tests were run to examine potential demographic differences between the genotype groups. In order to test whether 5-HTTLPR genotype, gender, and depressive symptoms were independently and interactively associated with hippocampal volumes and memory functioning, the analysis included a series of multiple regressions in which the 5-HTTLPR genotype (dummy coded for 1=S/S or S/L vs. 0=L/L) was entered to predict (1) verbal memory (CVLT-2 immediate memory, delayed memory, retention and recognition memory), (2) visual memory (RCFT recall, RCFT recognition memory, and WMS-III Faces memory), and (3) ICV-corrected right and left hippocampal volumes (RH/ICV; LH/ICV). In the first block, main effects of 5-HTTLPR, gender, depressive symptoms, and covariates associated with neurocognition (WASI Vocabulary, BMI, cotinine levels) were entered. In the second block, the interaction terms (genotype-by-gender and genotype-by-depressive symptoms) were entered. In the third block, the three-way interaction term (genotype-by-gender-by-depressive symptoms) was entered. Interpretations about statistical significance were made if p< .05.
A series of correlations were run to examine the bivariate relationships between memory and ICV-corrected hippocampal volumes, separately by genotype group and gender. Fisher Z tests were calculated to examine whether brain-behavior relationships were significantly different according to gender.
3. RESULTS
3.1 GENOTYPE FREQUENCIES
For 51 subjects, 5-HTTLPR genotype frequencies were: 17 L/L (9 female) and 34 S/S and S/L (16 female). The risk allele (S) group was comprised of 27 S/L and 6 S/S individuals. This genotype breakdown (approximately 12% S/S, 54% S/L, and 33% L/L) is consistent with frequencies gathered from several largely Caucasian, U.S. samples of adults (Goldman et al., 2010).
3.2 DEMOGRAPHIC & MOOD INFORMATION
ANOVA and chi-square tests were conducted to determine whether the 5-HTTLPR genotypes differed demographically (see Table A.1). There were no demographic differences between genotype groups by age, gender, WASI Vocabulary T-score, years of education, depressive symptoms, BMI, and cotinine levels. However, while genotype groups did not differ significantly for number of Caucasians included in the sample (L/L: 22; S/S and S/L: 13), there were discrepancies with respect to breakdown for other ethnicities, particularly African-Americans (L/L: Asian =1, African-American = 10, multiple races = 2; S/S and S/L: Asian = 0, African-American = 2, multiple races = 1). There were, however, differences by gender noted for genotype groups. For the S/S and S/L group, females had significantly lower WASI Vocabulary total scores than males (F (1, 51) = 5.55; p <.05); there was also evidence of marginal significance for BDI-II symptoms, with females endorsing on average two points higher than males (F (1, 51) = 3.69; p <.10). For the L/L group, females had significantly higher BMI (F (1, 51) = 4.67; p <.05). While WASI Vocabulary total score, BDI-II, and BMI were not significantly different between genotype groups, their differences between gender by genotype group (as well as relationships with neurocognition) led to their inclusion as covariates in the multivariate analyses.
Table A.1. Demographic Information According to Group.
| 5-HTTLPR | ||
|---|---|---|
|
S/S or S/L (n = 34) |
L/L (n = 17) |
|
|
| ||
|
M (SD)
[range] |
M (SD)
[range] |
|
|
| ||
| Age | 21.32 (2.51) [18 – 25] |
21.06 (2.22) [18 – 25] |
|
| ||
| % Female | 47.06% | 53.94% |
|
| ||
| % Caucasian | 64.71% | 76.47% |
|
| ||
| WASI Vocabulary T-score | 57.06 (9.25) [38-74] |
60.35 (7.32) [49 – 72] |
|
| ||
| Years of Education | 13.77 (1.67) [12 – 18] |
13.94 (2.19) [11 – 19] |
|
| ||
| BDI-II total | 6.09 (6.40) [0 – 26] |
4.29 (4.15) [0 – 16] |
|
| ||
| Body Mass Index | 24.86 (6.90) [13.63 – 40.35] |
25.44 (4.50) [20.16 – 36.58] |
|
| ||
| Cotinine levels | 2.15 (2.56) [0 – 6] |
3.00 (2.96) [0 – 6] |
Notes: M = mean; SD = standard deviation. There were no significant group differences.
3.3 MEMORY FUNCTIONING, HIPPOCAMPAL AND INTRACRANIAL VOLUMES
See Table A.2 for mean composite z-scores on the verbal and visual memory tests, left and right hippocampal volumes, and intracranial volumes.
Table A.2. Descriptive Statistics for Neuropsychological Composite Variables and Hippocampal Volumes by Genotype.
| 5-HTTLPR | ||||
|---|---|---|---|---|
|
S/S or S/L (n = 34) |
L/L (n = 17) |
|||
|
| ||||
| Composite Variables | M (SD) | Range | M (SD) | Range |
|
| ||||
| CVLT-2 immediate | −0.10 (0.88) | −1.61 – 1.71 | 0.20 (0.99) | −1.11 – 2.15 |
|
| ||||
| CVLT-2 delay | −0.07 (1.06) | −2.59 – 1.40 | 0.14 (0.58) | −0.83 – 1.04 |
|
| ||||
| CVLT-2 retention | −0.11 (1.12) | −4.82 – 0.98 | 0.21 (0.69) | −0.55 – 2.14 |
|
| ||||
| CVLT-2 recognition | 0.00 (1.12) | −3.61 – 0.91 | −0.01 (0.74) | −1.63 – 0.91 |
|
| ||||
| RCFT recall | −0.12 (1.00) | −1.95 – 1.64 | 0.26 (0.91) | −1.96 – 1.68 |
|
| ||||
| RCFT recognition | −0.05 (1.10) | −2.28 – 1.86 | 0.10 (0.79) | −1.69 – 1.88 |
|
| ||||
| WMS-III Faces | 0.09 (0.82) | −2.27 – 1.86 | −0.18 (1.06) | −2.13 – 1.33 |
|
| ||||
| Right Hippocampus (cc3) | 2.46 (0.33) | 1.78-3.14 | 2.43 (0.43) | 1.94-3.70 |
|
| ||||
| Left Hippocampus (cc3) | 2.42 (0.32) | 1.75-3.00 | 2.52 (0.45) | 1.78-3.72 |
|
| ||||
| ICV (cc3) | 1572.71 (146.23) |
1316.48- 1845.26 |
1634.46 (142.02) |
1395.19- 1895.41 |
Notes: All scores are sample z-scores. CVLT-2=California Verbal Learning Test – Second Edition; RCFT= Rey Complex Figure Test; WMS-III=Wechsler Memory Scale- 3rd Edition. ICV=intracranial volume.
3.4 MULTIVARIATE RELATIONSHIPS
Primary Regression Analysis: 5-HTTLPR Group
5-HTTLPR genotype did not independently predict memory or hippocampal volume variables (p=.28-.86).
5-HTTLPR*Gender
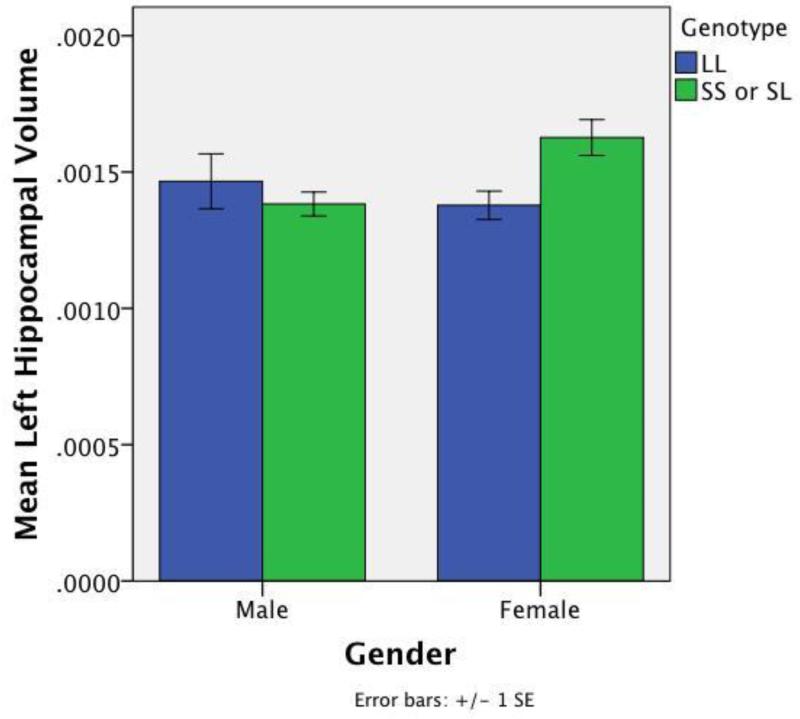
5-HTTLPR genotype interacted with gender in significantly predicting LH/ICV [t (51) = 2.33, beta=.36, p<.03] and marginally predicting RH/ICV [t (51) = 1.61, beta=.26, p<.10]. Visual inspection revealed larger volumes among S females vs. L/L females with the opposite pattern in males (Figure B.1). 5-HTTLPR genotype interacted with gender in significantly predicting RCFT recall performance [t (51) = −2.05, beta=−.27, p<.05]; poorer performance was primarily seen in S-carrying females compared to other subgroups.
Figure B.1. Mean Left Hippocampus/Intracranial Volume According to Gender and Genotype.
5-HTTLPR*Depressive Symptoms
5-HTTLPR genotype interacted with depressive symptoms in marginally predicting CVLT-2 immediate memory [t (51) = 1.80, beta=.27, p<.09]. Visual inspection revealed that in the male and female S allele carriers, increased depressive symptoms were associated with poorer immediate memory, while there was a very slight positive relationship in the L/L group.
5-HTTLPR *Gender*Depressive Symptoms
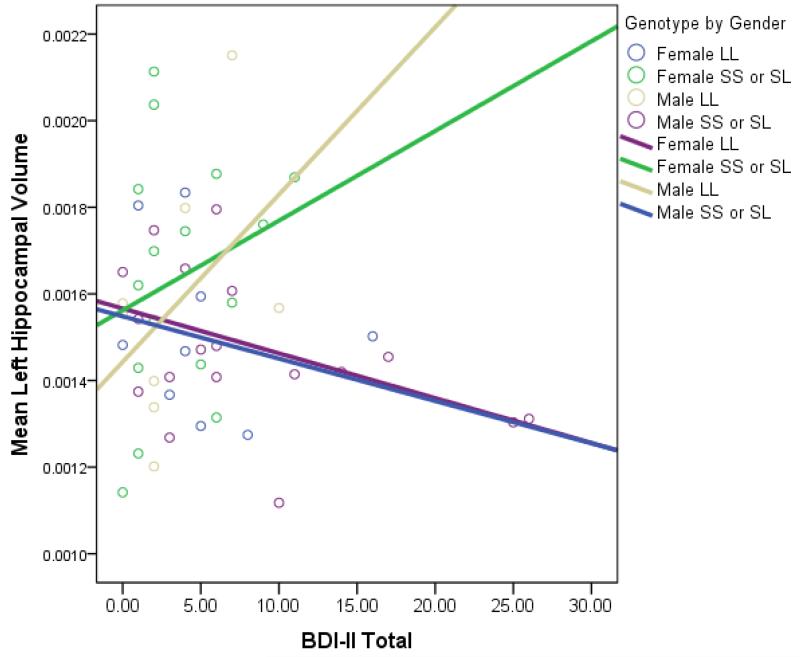
The three-way interaction between gender, depressive symptoms, and 5-HTTLPR genotype significantly predicted LH/ICV [t (51) = 2.08, beta=.36, p<.05]. Visual inspection revealed that in S-carrying females, increased depressive symptoms were associated with larger LH/ICV volumes while in S-carrying males, increased depressive symptoms were associated with smaller LH/ICV volumes (see Figure B.2). It should be noted that there was a restriction in range of depressive symptoms in L/L carrying males, so only the S-carrier results were interpreted here.
Figure B.2. Scatterplot Depicting Relationships Between Left Hippocampus/Intracranial Volume and Depressive Symptoms Separately by Gender and Genotype.
3.5 POST-HOC BRAIN-BEHAVIOR RELATIONSHIPS
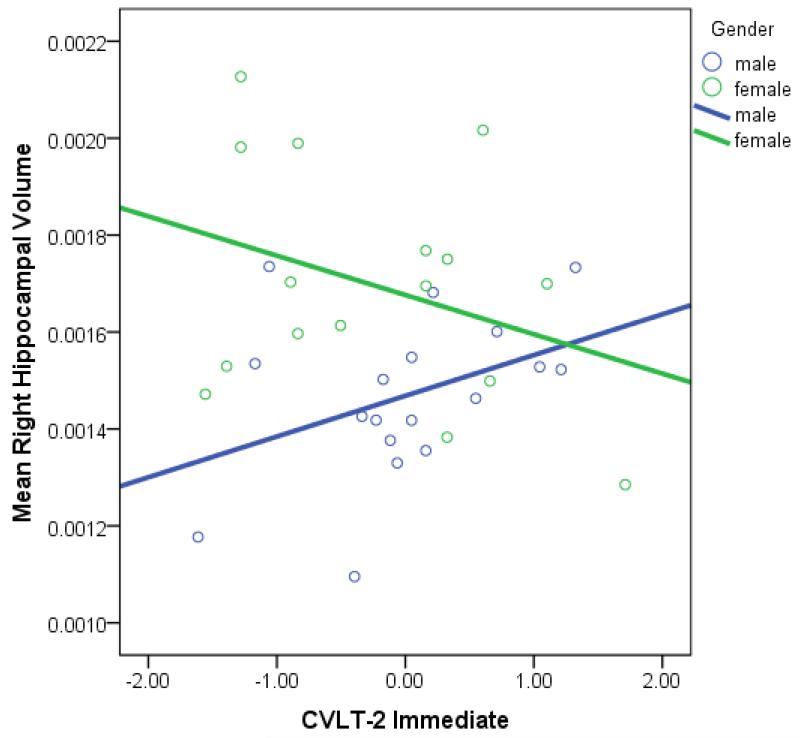
In order to examine whether memory-hippocampal relationships differed by gender, bivariate relationships between CVLT-2 immediate memory, RCFT recall and RH/ICV and LH/ICV were conducted separately by 5-HTTLPR genotype and gender (see Table A.3). Fisher’s Z tests were then conducted to determine whether brain-behavior correlations significantly differed by gender. In S carriers, brain-behavior relationships significantly differed by gender; poorer CVLT-2 immediate memory performance was associated with larger RH/ICV for the women and smaller volumes in the men (z=−1.99; see Figure B.3).
Table A.3. Bivariate Correlations between Behavioral Variables and Hippocampal Volumes by Genotype and Gender.
| Right Hippocampus/ICV | Left Hippocampus /ICV | |||
|---|---|---|---|---|
| Females |
S (n=16) |
L /L (n=9) |
S (n=16) |
L /L (n=9) |
| CVLT-2 immediate (z-score) | − .33 | .17 | −.10 | .12 |
| RCFT recall (z-score) | −.10 | −.38 | .08 | .24 |
| Males |
S (n=18) |
L /L (n=8) |
S (n=18) |
L /L (n=8) |
| CVLT-2 immediate (z-score) | .39 | .51 | −.26 | .15 |
| RCFT recall (z-score) | .01 | .03 | .10 | .10 |
Note: Correlations are Pearson Product Moment Correlations; none were statistically significant. Correlations in bold signify significantly different brain behavior relationships between the genders.
Figure B.3. Relationship between Mean Right Hippocampus/ICV and CVLT-2 Immediate Memory among S Carriers by Gender.
4. DISCUSSION
This study found that the 5-HTTLPR genotype status alone did not significantly predict left or right hippocampal volume or memory performance for the eight composite variables examined. Instead, there were significant interactions between 5-HTTLPR genotype and gender and depressive symptoms that predicted hippocampal volumes and two memory composite variables. In the healthy young adult females, the 5-HTTLPR S allele was associated with larger left (and marginally larger right) hippocampal volumes compared to L/L carriers, and larger left hippocampal volumes were in-turn associated with increased depressive symptoms. S-carrying females also demonstrated significantly poorer visual recall compared to other groups, and there was a significant correlation between poorer verbal memory performance and larger right hippocampal volumes in the females (the direction differed from males). Overall, this suggests that in young adult women, the S allele may be associated with reduced 5-HT signaling, thus increasing the risk for subtle hippocampal and memory abnormalities. In the males, the S allele was associated with marginally smaller left and right hippocampal volumes compared to the L/L genotype. Further, among male S carriers, increased depressive symptoms were associated with smaller left hippocampal volumes. There was a non-significant correlation between poorer verbal memory performance and smaller right hippocampal volumes in the males (opposite of females). Taken together, the S allele also appeared to be associated with a subtle disadvantage in the males, especially in the presence of greater depressive symptoms.
Animal research has demonstrated an association between the S allele and lower 5-HT levels and poorer neuronal health in the hippocampus (Olivier, 2008; Gallinat et al., 2005). Further, human imaging research has indicated reduced hippocampal volumes among S carriers in a sample of adult non-depressed controls (Frodl et al., 2008). As stated above, it is our interpretation that the S allele is disadvantageous and lends further evidence that it may be associated with poorer 5-HT signaling for both men and women, but gender differences in hippocampal development may alter the direction of the relationship between genotype and hippocampal volume. Sowell et al. (2002) found that despite total brain gray matter volume reduction from ages 7 to 16, females had larger temporal lobe volumes compared to males. Still, the literature is lacking in information regarding hippocampal developmental trajectories for men and women during early adolescence into young adulthood. A small number of studies have noted a slight increase in volume from childhood through adulthood especially in males, but less is known about female development (Giedd et al., 1996; Ostby et al., 2009; Suzuki et al., 2005). Two studies have reported smaller left hippocampal volumes in association with improved verbal memory functioning in samples with female adolescents (Foster et al., 1999; Medina et al., 2007a), suggesting larger volumes in females may reflect impaired pruning. Therefore, our observed gender differences in hippocampal-behavior correlations may be very specific to this developmental period, and larger longitudinal studies following healthy boys and girls from early adolescence through middle adulthood are needed to clarify these divergent brain-behavior relationships.
It is also possible that these findings are related to gender differences in the functional consequences of 5-HTTLPR on serotonin turnover and the serotonin system more globally. Low cerebral spinal fluid (CSF) 5HIAA (5-hydroxyindoleacetic acid; a serotonin metabolite considered a measurement of serotonin turnover) has been associated with increased risk for serotonin-related diseases (Doudet et al., 1995; Stanley et al., 1985). Some studies have reported that levels of the serotonin metabolite differ as a function of gender as well as with the 5-HTTLPR genotype (Jonsson et al., 2000; Williams et al., 2003). Converging lines of evidence suggest that gender may moderate the functional effects of 5-HTTLPR on upstream indicators of serotonin function, as well as downstream indicators like memory, personality, and negative affect, even in adult samples (Brummett et al., 2008). This may be due to several mechanisms, but one candidate is the impact of estrogen on signal transduction and gene expression affecting serotonin functioning (Pecins-Thompson et al., 1996; 1999).
In the current study we found that, in the S carriers, increased depressive symptoms were associated with poorer CVLT-2 immediate memory, larger left hippocampal volumes in women, and smaller volumes in men (see Table A.3). Sub-clinical depressive symptoms, a potential marker of subtle serotonin stress, in combination with S allele status may lead to early hippocampal changes, serving as an early biomarker of depression risk. This is consistent with studies finding lower SERT binding potential in the amygdala and midbrain in participants with major depression (Parsey et al., 2006), but serotonin binding in the presence of subclinical symptoms is relatively unknown. The current results suggest a possible disruption of neural development in the hippocampus either due to or leading to increased depressive symptoms. In males with depressive symptoms, reduced 5-HT signaling may lead to lower neurogenesis and smaller hippocampal volumes due to abnormal gene expression of growth factors, especially brain-derived neurotrophic factor (BDNF) (Martinowhich and Lu, 2008; Mattson et al., 2004). Longitudinal studies are needed to disentangle whether abnormal hippocampal volumes or depressive symptoms came first.
However, there were also several null results to report. In addition to no significant independent predictions of 5-HTTLPR status, there were also no interactions associated with other variables measured, including CVLT-2 delayed memory, CVLT-2 retention, CVLT-2 recognition memory, RCFT recognition, WMS-III Faces immediate memory, and WMS-III Faces delayed memory. Indeed, for the majority of our analyses we did not see a relationship between genotype and neurocognition. Nonetheless, in a samples of relatively healthy emerging adults without presence of Axis I disorders, the subtle, significant findings suggest a possible atrophic effect or developmental aberration in the hippocampus related to depressive symptoms, suggesting lower-functioning serotonin systems. Further, our ability to assess differential serotonergic function by genotype through the measurement of endophenotypes (like hippocampal volume and memory) may not accurately reflect the true complexity of gene/environment interactions.
Potential weaknesses in the present study need to be considered. This study only included young adults (18-25), a population that has been relatively understudied when trying to understand the functional consequences of 5-HTTLPR genotype. Due to continued hippocampal development, the direction of our hippocampal volume differences (e.g., larger volumes in S females and smaller in S males) may be specific to this developmental age range so results may not necessarily generalize to substantially older or younger samples. This highlights the need for further imaging genetics research across the developmental spectrum. In addition, this would be considered a relatively small sample for a candidate gene study, and thus issues related to power must be taken into account. For example, while not statistically different between genotype groups, there is a significant range in BMI among participants. Ideally, those with BMIs outside the normal range would be excluded, as there is potential for confound related to associations with the serotonergic system and cognition (Lam et al., 2010; Bruce-Keller et al., 2009; Ho et al., 2010; Volkow et al., 2009). This may have contributed to the ability to detect other associations as well as to the conclusions drawn from current results. Nonetheless, the genotype frequencies in our sample are consistent with largely Caucasian samples from other U.S. samples (Goldman et al., 2010), and BMI was controlled for in multivariate analyses. Finally, it is important to consider the heterogeneity in ethnicity included in the sample, particularly the differences in breakdown among African-Americans between genotype groups. As the primary findings were driven by the Caucasians in the sample, results cannot necessarily generalize to other ethnic groups (Williams et al., 2003; Goldman et al., 2010). Further research examining relationships between the 5-HTTLPR genotype and neurocognition in larger samples is indicated, such that separate analyses by ethnicity (homogenous subgroups) will allow for greater characterization and generalizability.
In conclusion, these results demonstrate that complex but subtle relationships exist between the 5-HTTLPR genotype and neurocognition in a group of young, healthy individuals with no history of psychiatric or neurologic illness. Additional studies will need to consider how several moderating factors (gender, ethnicity, stress exposure, and depressive symptoms) impact the functional consequence of 5-HTTLPR genotype on neurocognition. This important body of research can aid in early detection of disease states and identify at-risk groups across the course of development. This may help individualize clinical interventions including pharmacotherapy, psychotherapy, or lifestyle modifications.
We examined relationships between 5-HTTLPR genotype and memory and hippocampal volumes.
Participants were 51 healthy, emerging adults without Axis I disorders.
There were significant interactions with gender and BDI-II scores on neurocognitive variables.
Risk females had increased left volumes and deficits; the opposite pattern was seen in males.
In a healthy, maturing population, moderating factors may impact genotype function.
ACKNOWLEDGEMENTS
This research was supported by NIDA (R03DA027457-01; PI: Medina), the Center for Environmental Genetics (CEG) pilot grant (#P30 ES006096; PI: Medina), and URC Summer Graduate Student Research Fellowship Program (PI: Price, Mentor: Medina).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors reported no financial interests or potential conflicts of interest.
REFERENCES
- Anderson DE, Bell TA, Awh E. Polymorphisms in the 5-HTTLPR gene mediate storage capacity of visual working memory. Journal of Cognitive Neuroscience. 2012;24:1069–76. doi: 10.1162/jocn_a_00207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochimica et Biophysica Acta. 2008;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Muller CL, Collins AL, Boyle SH, Kuhn CM, Siegler IC, et al. 5-HTTLPR and gender moderate changes in negative affect responses to tryptophan infusion. Behavioral Genetics. 2008;38:476–83. doi: 10.1007/s10519-008-9219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition. The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- Doudet D, Hommer D, Higley JD, Andreason PJ, Moneman R, Suomi S, et al. Cerebral glucose metabolism, CSF 5-HIAA levels, and aggressive behavior in rhesus monkeys. American Journal of Psychiatry. 1995;152:1782–1787. doi: 10.1176/ajp.152.12.1782. [DOI] [PubMed] [Google Scholar]
- Dutt A, McDonald C, Dempster E, Prata D, Shaikh M, Williams I, et al. The effect of COMT, BDNF, 5-HTT, NRG1, and DTNBP1 genes on hippocampal and lateral ventricular volume in psychosis. Psychological Medicine. 2009;39:1783–97. doi: 10.1017/S0033291709990316. [DOI] [PubMed] [Google Scholar]
- Eker MC, Kitis O, Okur H, Eker OD, Ozan E, Isikli S, et al. Smaller hippocampus volume is associated with short variant of 5-HTTLPR polymorphism in medication-free major depressive disorder patients. Neuropsychobiology. 2011;63:22–8. doi: 10.1159/000321834. [DOI] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Strobel A. On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behavioural Brain Research. 2011a;216:122–8. doi: 10.1016/j.bbr.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Reif A, Strobel A. Serotonergic modulation in executive functioning: linking genetic variations to working memory performance. Neuropsychologia. 2011b;49:3776–85. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Everaerd D, Gerritsen L, Rijpkema M, Frodl T, van Oostrom I, Franke B, et al. Sex modulates the interactive effect of the serotonin transporter gene polymorphism and childhood adversity on hippocampal volume. Neuropsychopharmacology. 2012;37:1848–55. doi: 10.1038/npp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI, et al. The hippocampus and delayed recall: bigger is not necessarily better? Memory. 1999;7:715–732. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, Scheuerecker J, Zill P, Baghai T, Schüle C, Rupprecht R, Bondy B, Reiser M, Möller HJ, Meisenzahl EM. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry. 2008;13:1093–1101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Strohle A, Lang UE, Bajbouj M, Kalus P, Montag C, Seifert F, Wernicke C, Rommelspacher H, Rinneberg H, Schubert F. Association of human hippocampal neurochemistry, serotonin transporter genetic variation, and anxiety. Neuroimage. 2005;26:123–131. doi: 10.1016/j.neuroimage.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–6. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin YH, Weinstein M. The serotonin transporter polymorphism (5-HTTLPR): allelic variation and links with depressive symptoms. Depression and Anxiety. 2010;27:260–9. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychicatry. 2006;15:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hensch T, Wargelius HL, Herold U, Lesch KP, Oreland L, Brocke B. Further evidence for an association of 5-HTTLPR with intensity dependence of auditory-evoked potentials. Neuropsychopharmacology. 2006;31:2047–2054. doi: 10.1038/sj.npp.1301020. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. The effects of physical activity, education, and body mass index on the aging brain. Human Brain Mapping. 2010;32:371–82. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Norton N, Gustavsson P, Oreland L, Owen MJ, Sedvall GC. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Journal of Psychiatric Research. 2000;34:239–244. doi: 10.1016/s0022-3956(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Tremblay PB, Roots I, Brockmoller J. Validity of PCR with emphasis on variable number of tandem repeat analysis. Clin Biochem. 2002;35:49–56. doi: 10.1016/s0009-9120(02)00273-4. [DOI] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacology Biochemistry and Behavior. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, Zimmermann US, Schmidt MH, Esser G, Rietschel M, Banaschewski T. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults. Int J Neuropsychopharmacol. 2009;2:737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magnetic Resonance Medicine. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch K, Mossner R. Genetically driven variation in serotonin uptake: Is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14(3):121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindle K, Tharwani H, Gopalakrishnan R, Hill KP, Berrettini WH. Polymorphism in the serotonin transporter gene and moderators of prolactin response to meta-chlorophenylpiperazine in African-American cocaine abusers and controls. Psychiatry Research. 2006;144:99–108. doi: 10.1016/j.psychres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Barnes J, Bristow GC, Harmer CJ, Cowen PJ. Memory impairment in young women at increased risk of depression: Influence of cortisol and 5-HTT genotype. PsychologicalMedicine. 2009;39:757–762. doi: 10.1017/S0033291708004248. [DOI] [PubMed] [Google Scholar]
- Mattson MP, et al. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in Neuroscience. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Corcoran K. Ecstasy (MDMA) exposure and neuropsychological functioning: A polydrug perspective. Journal of the International Neuropsychological Society. 2005;11:1–13. doi: 10.1017/S1355617705050915. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, McQueeny T, Park A, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal asymmetry. Neurotoxicology and Teratology. 2007a;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007b;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. Rey complex figure test and recognition trial. The Psychological Corporation; San Antonio, TX: 1995. [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Archives of General Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Jans LA, Korte-Bouws GA, Korte SM, Deen PM, Cools AR, Ellenbroek BA, Blokland A. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology. 2008;200:243–254. doi: 10.1007/s00213-008-1201-0. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang Y, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. American Journal of Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Journal of Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. Journal of Neuroscience. 1996;16:7021–7029. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Schilt T, de Win MM, Booij J, Schmand B, van den Brink W, Bakker O. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. Journal of Psychopharmacology. 2006;20:389–399. doi: 10.1177/0269881106063266. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. International Journal of Neuropsychopharmacology. 2007;10:1–13. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Wang H, Hasler G, Sahakian BJ, et al. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33:1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9(2):197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127(1):85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research & Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine & Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stanley M, Traskman-Benz L, Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sciences. 1985;37:1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Heinrich C, Boehrer A, Mitsuya K, Kurokawa K, Matsuda M, et al. Male-specific volume expansion of the human hippocampus during adolescence. Cerebral Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, Payne ME, MacFall JR, Marchuk DA, Svenson IK, et al. Influence of serotonin transporter promote region polymorphisms on hippocampal volumes in late-life depression. Archives of General Psychiatry. 2005;62:537–44. doi: 10.1001/archpsyc.62.5.537. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Heffner JL, Strong JA, Blom TJ, Anthenelli RM. Relationship between the serotonin transporter polymorphism and obsessive-compulsive alcohol craving in alcohol-dependent adults: a pilot study. Alcohol. 2010;44:401–406. doi: 10.1016/j.alcohol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Joiner TE, Johnson F, Bender TW. Gender specific gene-environment interactions on laboratory-assessed aggression. Biological Psychology. 2006;71:33–41. doi: 10.1016/j.biopsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 1996;17:60–5. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psych Corp; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-3rd Edition. Psychological Corporation; New York: 1997. [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Zilles D, Meyer J, Schneider-Axmann T, Ekkawardhani S, Gruber E, Falkai P, et al. Genetic polymorphisms of 5-HTT and DAT but not COMT differentially affect verbal and visuospatial working memory function. 2012 doi: 10.1007/s00406-012-0312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]