Abstract
We reviewed bone marrow studies from 351 multiple myeloma (MM) cases, selecting 12 cases (3.4%) with predominantly small lymphocyte–like morphologic features resembling B-cell lymphoma, and correlated their genetic and clinical features. All exhibited a diffuse interstitial pattern of marrow involvement. Small lymphocyte–like plasma cells were all CD45− with bright CD38 and CD138 expression and CD20 expression in 5 cases. No case had an increase in bone marrow B lymphocytes by flow cytometry. Of 12 cases, 9 were classified as the CD-2 molecular class by gene expression profiling (GEP). The 29 CD-2 class cases with (n = 9) and without (n = 20) small lymphocyte–like features could not be discerned from one another using global GEP. Event-free, but not overall, survival was significantly better in cases with small lymphocyte–like features among those sharing the CD-2 subtype. Small lymphocyte–like MM is a rare, morphologically challenging variant distinguished from B-cell lymphoma by lack of CD45 and presence of CD138 and the clinical presentation of MM. Most cases share a common GEP signature dominated by hyperexpression of cyclin D1 or cyclin D3 genes, with increased expression of the B-cell genes CD20 and VPREB3.
Keywords: Plasma cell myeloma, Gene expression profiling, Morphology, Cyclin D1, B-cell lymphoma
Plasma cell multiple myeloma can exhibit a spectrum of morphologic features. Rarely, we have encountered cases with predominantly small lymphocyte–like morphologic features mimicking mature small B-cell lymphoma and causing initial diagnostic confusion. Small plasma cell or lymphoplasmacytic variants of plasma cell myeloma have been well described.1 In series by Bartl et al1 including 674 patients, 11% had small cell morphologic features. Since then, studies of cases with the t(11;14)(q13;q32) translocation revealed frequent small mature plasma cell morphologic features, or more than 25% to 30% lymphoplasmacytoid cells.2-6 We reviewed 351 cases of plasma cell myeloma to assess for cases with a predominance of small lymphocyte–like plasma cells (>50%) that could morphologically mimic mature B-cell lymphoma with or without plasmacytic differentiation. We then correlated the clinical characteristics of the patients, flow cytometry immunophenotype, cytogenetics, and gene expression profile of the plasma cells with this distinct morphologic pattern.
Materials and Methods
Archival material was obtained for 351 patients with newly diagnosed MM who were seen at the University of Arkansas for Medical Sciences, Little Rock, between 2000 and 2004. All patients were enrolled in Total Therapy 2, a high-dose, melphalan-based tandem transplant trial at the University of Arkansas for Medical Sciences.7 All patients provided informed consent in accordance with the Helsinki doctrine.
Bone marrow aspirate and core biopsy specimens obtained before any therapy were reviewed by 3 authors (A.H.-M., J.W., and S.H.) to identify cases with predominantly small lymphocyte–like morphologic features, defined as having a nuclear/cytoplasmic ratio of 0.6 or greater in at least 50% of the plasma cells, with lymphocyte-like dense and uniform chromatin.8 The authors reviewed the cases as 2 independent observers (J.W. and A.H.-M. with S.H.). During both reviews, cases were designated as definite, probable, or negative for small lymphocyte–like morphologic features based on an initial screen. Cases deemed “probable” had a 200 plasma cell count performed to see if they met inclusion criteria. A consensus opinion was reached on all cases.
Cases with small lymphocyte–like morphologic features were then further evaluated for the following: (1) percentage of nucleated cells composed of plasma cells on the core biopsy specimen; (2) histologic pattern of plasma cell infiltration in the core; (3) presence of mast cells and their association with lymphoid aggregates, if present; and (4) the presence of circulating plasma cells or plasmacytoid lymphocytes in the peripheral blood smear. The pattern of plasma cell infiltration on the core biopsy specimen was based on the low-power architectural appearance, using the terminology proposed by Bartl et al.1 The patterns were defined as follows: (1) interstitial, scattered infiltrates of plasma cells without significant architectural distortion of the marrow; (2) paratrabecular, paratrabecular accumulation of plasma cells; (3) nodular, rounded masses of plasma cells; and (4) obliterative, a packed marrow with sheeting infiltrates of plasma cells effacing the marrow architecture.
Cases with small lymphocyte–like morphologic features were then correlated with clinical disease features and gene expression profiling (GEP)-defined molecular subgroups as previously described.9 The 8 subgroups are briefly described as follows: (1) PR, the proliferation subgroup characterized by overexpression of numerous cell cycle–and proliferation-related genes; (2) LB, the low bone disease group lacking a clear genetic signature, but with fewer focal bony lesions on magnetic resonance imaging; (3) MS, characterized by spiked FGFR3 and MMSET expression; (4) HY, characterized by a gene expression pattern common to cases with hyperdiploidy; (5) CD-1, characterized by a spike in cyclin D1 expression; (6) CD-2, characterized by a spike in cyclin D3 expression; (7) MF, characterized by spikes in MAF and MAFB proto-oncogenes; and (8) MY, a “myeloid signature” characteristic of myeloid lineage cells, often found in cases with low plasma cell burdens despite CD138-magnetic bead selection. The clinical records were reviewed for the occurrence of relapse or death.
Results
Of 351 cases, 12 (3.4%) had a predominance of small lymphocyte–like plasma cells Image 1. The bone marrow aspirate and core biopsy specimens were available for review in every case. In addition, results at initial diagnosis of bone marrow cytogenetics, serum electrophoresis, skeletal imaging studies, CD138+ cell GEP, and clinical follow-up data were available for all 12 cases. Of 12 cases, 11 had initial diagnostic flow cytometric immunophenotyping studies available, and for 10, the peripheral blood smear from diagnosis was available for review. Bone marrow, serologic, and staging parameters for the 12 cases are given in Table 1. Of the 12 cases, 5 (42%) had an exclusively small lymphocyte–like morphologic pattern (>90% of the plasma cells) and caused initial diagnostic confusion with mature B-cell lymphoma for hematopathologists. All cases (100%) displayed an interstitial pattern of marrow involvement. In addition, 8 cases had an obliterative pattern (67%), 2 cases had a nodular pattern (17%), and 1 case had a paratrabecular pattern of involvement (8%). None of the cases had lymphoid aggregates or were associated with mast cells. Plasma cell leukemia was identified in 2 (18%) of 11 cases with circulating morphologically similar-appearing small plasma cells.
Image 1.
Small lymphocyte–like plasma cell myeloma morphologic features. A, Bone marrow aspirate. The plasma cells are small with a scant rim of cytoplasm with an appearance similar to that of a mature lymphocyte (Wright, ×1,500). B, Bone marrow core biopsy specimen. Small lymphocyte–like plasma cells infiltrate the marrow in an interstitial and nodular pattern (H&E, ×300). C, Appearance of nodular focus at higher power. Small lymphocyte–like plasma cells demonstrate mature condensed chromatin and minimal cytoplasm (H&E, ×600). D, CD138 immunohistochemical staining (B-A38, Serotec, Oxford, England, 1:20 dilution, Benchmark automatic stainer with standard antigen retrieval). Diffuse CD138 immunohistochemical labeling of small lymphocyte–like plasma cells is present (×600).
Table 1.
Clinical Features and Bone Marrow Findings in Patients With Small Lymphocyte–Like Plasma Cell Myeloma
| Plasma Cells (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Case No./ Sex/Age (y) |
Salinon- Durie Stage |
M Component (g/dL) |
Secretory Component |
Lytic Lesions |
in Bone Marrow |
in Peripheral Blood |
With Lymphocyte- Like Morphology |
Pattern of Marrow Involvement |
| 1/M/69 | 2A | 5.3 | IgG κ | Solitary | >80 | NA | 50-90 | Interstitial |
| 2/F/43 | 3A | 0.7 | IgA κ | Multiple | >80 | 3 | >90 | Interstitial, obliterative |
| 3/F/51 | NA | Trace | Free κ | Multiple | 50 | 0 | 50-90 | Interstitial, obliterative |
| 4/F/59 | 2B | 0.4 | IgG λ | Solitary | >80 | 40 | 50-90 | Interstitial, obliterative |
| 5/M/63 | 2A | Trace | Free κ | Multiple | 60 | NA | 50-90 | Interstitial, nodular |
| 6/F/64 | 3A | Trace | Free λ | Multiple | 60 | 2 | >90 | Interstitial, obliterative |
| 7/M/74 | 3B | 3 | IgG λ | Multiple | >80 | 52 | >90 | Interstitial, obliterative |
| 8/M/79 | 2A | 5.7 | IgG κ | Focal (2) | 70 | 1 | 50-90 | Interstitial |
| 9/M/45 | 3A | 4.8 | IgA κ | Multiple | >80 | 2 | 50-90 | Interstitial, obliterative |
| 10/M/60 | 3A | Trace | Free κ | Multiple | >80 | 2 | 50-90 | Interstitial, nodular, obliterative |
| 11/F/60 | 2A | 3.5 | IgA κ | Focal (2) | >80 | 1 | >90 | Interstitial, obliterative |
| 12/M/61 | 1A | 1.9 | IgG κ | * | 10 | 0 | >90 | Interstitial, paratrabecular |
NA, not available.
Diffuse involvement of the skull base and nonfocal lesions of the spine.
Clinical parameters are given in Table 1. Patients had a median age of 60.5 years (range, 43-79 years), and 7 were men. The M-component was significantly elevated (≥0.4 mg/dL) in 8 cases and was a trace in 4 (33%) of 12 cases. All patients had a secretory component composed of IgA (3 [25%]), IgG (5 [42%]), or free light chain only (4 [33%]). All cases had at least 1 focal lytic bony lesion. The t(11;14) (q13;q32) was identified by cytogenetics or fluorescence in situ hybridization (FISH) in 5 cases Table 2. Additional cytogenetic abnormalities included t(6;10)(p12;p13) in 1 case and 3 cases (25%) with a 13q14 deletion. Five cases had an uninformative study with normal results (Table 2). In 11 cases, flow cytometry was performed at diagnosis. The plasma cells were CD45−, CD38 bright, and CD138 bright in all cases (100%). CD20 was expressed on plasma cells in 5 cases (42%), including 2 with near exclusive small lymphocyte–like morphologic features (Table 2, example in Image 2). No case had increased numbers of bone marrow B lymphocytes by flow cytometry. CD117/c-kit expression was found in 4 cases. CD56 expression was uncommon (1 case). At 3 years’ follow-up after diagnosis, 6 patients (50%) had relapse of disease and 4 died (33%). Of the 4 patients who died, only 1 died of progressive MM disease.
Table 2.
Immunophenotypic and Molecular Characteristics of Small Lymphocyte–Like Plasma Cell Myeloma
| Flow Cytometric Plasma Cell Immunophenotype |
Microarray Signal* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | CD45 | CD19 | CD20 | CD56 | CD38 | CD138 | CD117 | Cytogenetics | Group | MS4A1 | PAX5 | VPREB3 |
| 1 | − | − | − | ND | + | + | − | 46,XY[cp20] | CD-2 | 466.30 | 332.70 | 109.70 |
| 2 | − | − | + | − | + | + | − | 46,XY,t(6;10)(p12;p13)[20] | CD-2 | 15,335.70 | 266.00 | 91.60 |
| 3 | − | ND | − | − | + | + | + | 46,XX[20] | CD-2 | 3,842.00 | 559.40 | 15.10 |
| 4 | − | ND | − | − | + | + | − | 46,XX[191/45,XX,−20[11] | MF† | 968.20 | 204.20 | 100.70 |
| 5 | − | ND | − | + | + | + | + | 46,XY[20] | CD-2 | 2,267.00 | 315.50 | 113.70 |
| 6 | − | ND | − | − | + | + | + | 46,XX[20], t(11;14)(q13;q32) (positive by FISH) |
CD-2 | 372.10 | 256.70 | 39.00 |
| 7 | ND | + | + | 46,XY,t(11;14)(q12~13.1;q32), del(13)(q14q22),?del(17)(p12) [cp2]/46,XY[18] |
CD-1 | 35.50 | 138.10 | 82.40 | ||||
| 8 | − | ND | + | − | + | + | + | 45,XY,t(11;14)(q12~13.1;q32), −13[11/46,XY[cp19] |
CD-2 | 17,436.40 | 188.40 | 40.30 |
| 9 | − | ND | + | − | + | + | − | 46,XY,der(14)t(11;14)(q13;q32) [41/46,XY[81] |
CD-2 | 7,569.80 | 199.00 | 56.90 |
| 10 | − | ND | + | − | + | + | − | 46,XY[20] | CD-2 | 5,697.40 | 181.30 | 29.60 |
| 11 | − | ND | + | − | + | + | − | 46,XX[20],t(11;14)(q13;q32) (positive by FISH) |
CD-2 | 6,569.70 | 104.10 | 89.40 |
| 12 | ND | ND | ND | ND | ND | ND | ND | 46,XY[20] | MY‡ | 9,123.90 | 360.60 | 67.50 |
FISH, fluorescence in situ hybridization; MY, a “myeloid signature” characteristic of myeloid lineage cells; ND, not done.
The minimum, mean, median, and maximum signals in the CD-2 subgroup for all 351 cases for MS4A1 expression were 254.00, 7,712.94, 6,659.70, and 24,309.50, respectively; for PAX5 expression, 28.20, 224.33, 190.90, and 571.70, respectively; and for VPREB3 expression, 82.30, 7,379.15, 4,252.70, and 38,039.50, respectively.
MAFB (MAFB proto-oncogene) spike; high CCND2 (24,454).
High CCND2 (14,790).
Image 2.
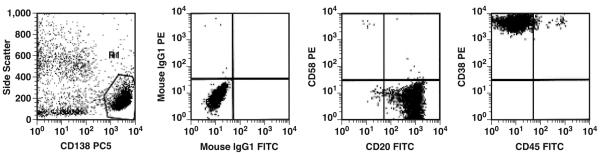
Flow cytometry of small lymphocyte–like plasma cell myeloma. The plasma cells are CD45−with bright CD38 and CD138 expression and are positive for CD20. FITC, fluorescein isothiocyanate; PC5, PE-Cy5; PE, phycoerythrin.
Of the 12 cases, 9 shared the previously described CD-2 gene expression signature consistent with disease harboring a cyclin D1 activating t(11;14) or cyclin D3 activating t(6;14) with MS4A1/CD20, PAX5, and VPREB3 expression (Table 2).9 Cases with the small lymphocyte–like morphologic features composed 9 (31%) of the 29 cases with the CD-2 gene expression signature reviewed. Of the 3 remaining cases with small lymphocyte–like morphologic features, 1 had the CD-1 signature, 1 had an MF signature, and 1 had a so-called myeloid contamination signature (MY). The MF case and the MY case also had strong cyclin D2/CCND2 expression detected. The MF signature case was morphologically unique compared with the rest of the 12 cases, with a greater degree of plasma cell pleomorphism in the larger cells, possibly reflecting clonal evolution.
While there is a strong relationship between the small lymphocyte–like plasma cell morphologic type and the CD-2 class, not all CD-2–type diseases share this morphologic pattern. We next compared survival in cases with and without small lymphocyte–like morphologic features within the CD-2 class of disease Figure 1. The Kaplan-Meier survival estimate of the median event-free survival for the cases with the small lymphocyte–like morphologic pattern was 56 months compared with 39 months for cases without this pattern (P = .0133). The 5-year overall survival estimates were 68% for cases with the small lymphocyte–like morphologic pattern and 52% for cases without it and was not statistically significant (P = .4998).
Figure 1.

Kaplan-Meier analysis of event-free survival (A) and overall survival (B) of small lymphocyte–like plasma cell myeloma (SLM) and non-SLM with the CD-2 class treated with Total Therapy 2.7 While event-free survival in the SLM subtype is significantly longer, this does not correspond to longer overall survival. A, P = .0133. B, P = .4998.
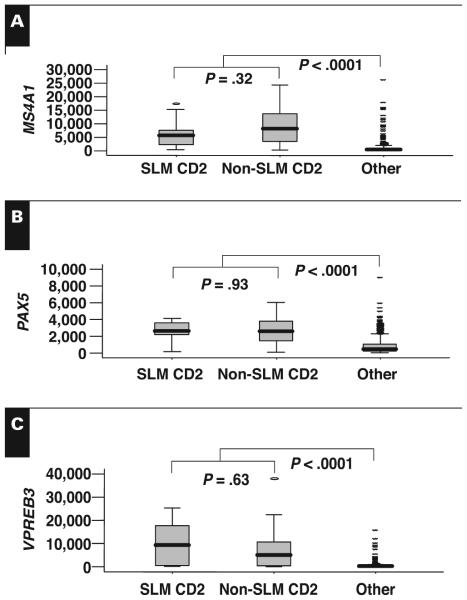
To determine if molecular signatures might distinguish cases with and without the small lymphocyte–like morphologic features within the CD-2 class, we compared gene expression profiles of the 2 groups. Our group has previously shown that CD-2 disease can be characterized by the elevated expression of MS4A1/CD20, which is also correlated with CD20 protein levels, PAX5, and VPREB3.10,11 There was no difference in the expression of these 3 genes between cases with and without the small lymphocyte–like morphologic features Figure 2. Consistent with these data, we were unable to identify any significant difference in the global gene expression profiles of cases with and without the small lymphocyte–like morphologic features within the CD-2 class (data not shown). Thus, the dramatic morphologic differences are not evident at the genomic level.
Figure 2.
Box plots comparing the messenger RNA expression of MS4A1/CD20 (A), PAX5 (B), and VPREB3 (C), as determined by microarray, in small lymphocyte–like plasma cell myeloma (SLM) CD-2 subtype (n = 9), non-SLM CD-2 (n = 20), and others (n = 322). Note that there is no significant difference in the expression of these genes within the CD-2 class but that expression is significantly lower in the other classes relative to the SLM and non-SLM CD-2 classes.
Discussion
Small lymphocyte–like morphologic features are rare in multiple myeloma (3.4% in this series). While this unusual morphologic pattern may cause diagnostic difficulty for hematopathologists, clinically, patients have typical MM disease. This morphologic pattern is likely a subset of the small cell type of plasma cell myeloma described by Bartl et al1 and the lymphoplasmacytoid morphologic features described in cases with t(11;14).3,4,6 Consistent with previous observations, our subset shares an association with CD20 expression and the presence of the t(11;14) translocation.5 The t(11;14) was detected by cytogenetics and/or FISH in 5 of 12 cases, compared with the reported frequency in the literature of 5% of MM by cytogenetics and 15% to 20% when assessed by FISH.2 Unlike previous studies describing small mature plasma cells, we defined our variant as having a predominance of small lymphocyte–like plasma cells to better elucidate characteristics of this rare morphologic pattern that can so closely mimic mature B-cell lymphoma.
We provide additional biologic insight from GEP.9 Most cases of small lymphocyte–like myeloma share a common gene expression pattern dominated by cyclin D1/CCND1 or cyclin D3/CCND3 overexpression, with overexpression of B-cell genes CD20, PAX5, and VPREB3. Of note, only approximately 31% of the CD-2 GEP group demonstrated the small lymphocyte–like morphologic features. This is similar to the observation of a lymphoplasmacytoid morphologic pattern in only 42% of cases with the t(11;14)(q13;q32).4 We were unable to identify genomic signatures that distinguish these 2 morphologically distinct disease entities within the CD-2 GEP group. Why some cases within the CD-2 class display this morphologic pattern and others do not is unknown.
The morphologic features of small lymphocyte-like MM can be strikingly similar to those of a mature B-cell lymphoma. Expression of the B-cell antigen CD20 or a paratrabecular growth pattern can reinforce the mistaken impression of mature B-cell lymphoma. The presence of a t(11;14)(q13;q32) could lead some who are not familiar with this translocation in MM to suspect the diagnosis of mantle cell lymphoma. Small lymphocyte–like MM is distinguished from mature B-cell lymphoma by its lack of CD45 expression with the presence of CD38 and CD138 and the presence of lytic bony lesions. Although all cases had a secretory component, in one third of cases the M-component was only a trace. No case had IgM secretion as seen in lymphoplasmacytic lymphoma.
Small lymphocyte–like MM is a rare subtype of MM, with high expression of B-cell genes, possibly contributing to the morphologic mimicry of B-cell lymphoma. The CD-2 gene expression signature common to most cases of small lymphocyte–like MM has a more favorable prognosis when treated with standard myeloma therapy. Despite the morphologic and genetic similarities to mature B-cell lymphoma, we have no evidence to suggest that this myeloma subtype might benefit from lymphoma-based therapy.
Upon completion of this activity you will be able to:
recognize the morphologic features of small lymphocyte–like plasma cell myeloma on bone marrow aspirates and histologic sections.
analyze flow cytometry and immunohistochemistry results allowing distinction of small lymphocyte–like plasma cell myeloma from mature small B-cell lymphoma.
discuss the common genetic pathways associated with the small lymphocyte–like plasma cell myeloma phenotype.
describe the key clinical differences between patients with small lymphocyte–like plasma cell myeloma and patients with mature small B-cell lymphoma.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 338. Exam is located at www.ascp.org/ajcpcme.
References
- 1.Bartl R, Frisch B, Fateh-Moghadam A, et al. Histologic classification and staging of multiple myeloma: a retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–355. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32): evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735–3741. doi: 10.1182/blood.v99.10.3735. [DOI] [PubMed] [Google Scholar]
- 3.Garand R, Avet-Loiseau H, Accard F, et al. t(11;14) and t(4;14) translocations correlated with mature lymphoplasmacytoid and immature morphology, respectively, in multiple myeloma. Leukemia. 2003;17:2032–2035. doi: 10.1038/sj.leu.2403091. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer JD, Hanson CA, Fonseca R, et al. The (11;14) (q13;q32) translocation in multiple myeloma: a morphologic and immunohistochemical study. Am J Clin Pathol. 2000;113:831–837. doi: 10.1309/4W8E-8F4K-BHUP-UBE7. [DOI] [PubMed] [Google Scholar]
- 5.Robillard N, Avet-Loiseau H, Garand R, et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 2003;102:1070–1071. doi: 10.1182/blood-2002-11-3333. [DOI] [PubMed] [Google Scholar]
- 6.Weh HJ, Bartl R, Seeger D, et al. Correlations between karyotype and cytologic findings in multiple myeloma. Leukemia. 1995;9:2119–2122. [PubMed] [Google Scholar]
- 7.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 8.Goasguen JE, Zandecki M, Mathiot C, et al. Mature plasma cells as indicator of better prognosis in multiple myeloma: new methodology for the assessment of plasma cell morphology. Leuk Res. 1999;23:1133–1140. doi: 10.1016/s0145-2126(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin P, Mahdavy M, Zhan F, et al. Expression of PAX5 in CD20-positive multiple myeloma assessed by immunohistochemistry and oligonucleotide microarray. Mod Pathol. 2004;17:1217–1222. doi: 10.1038/modpathol.3800169. [DOI] [PubMed] [Google Scholar]
- 11.Zhan F, Hardin J, Kordsmeier B, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]