Abstract
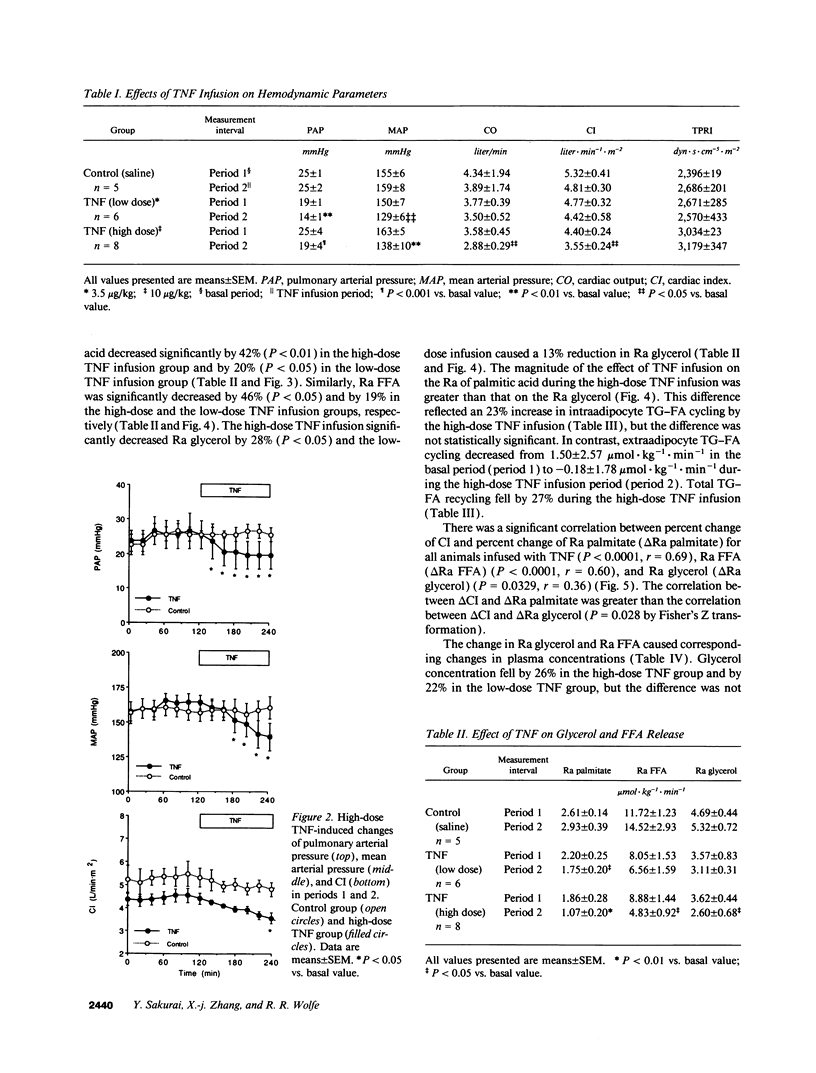
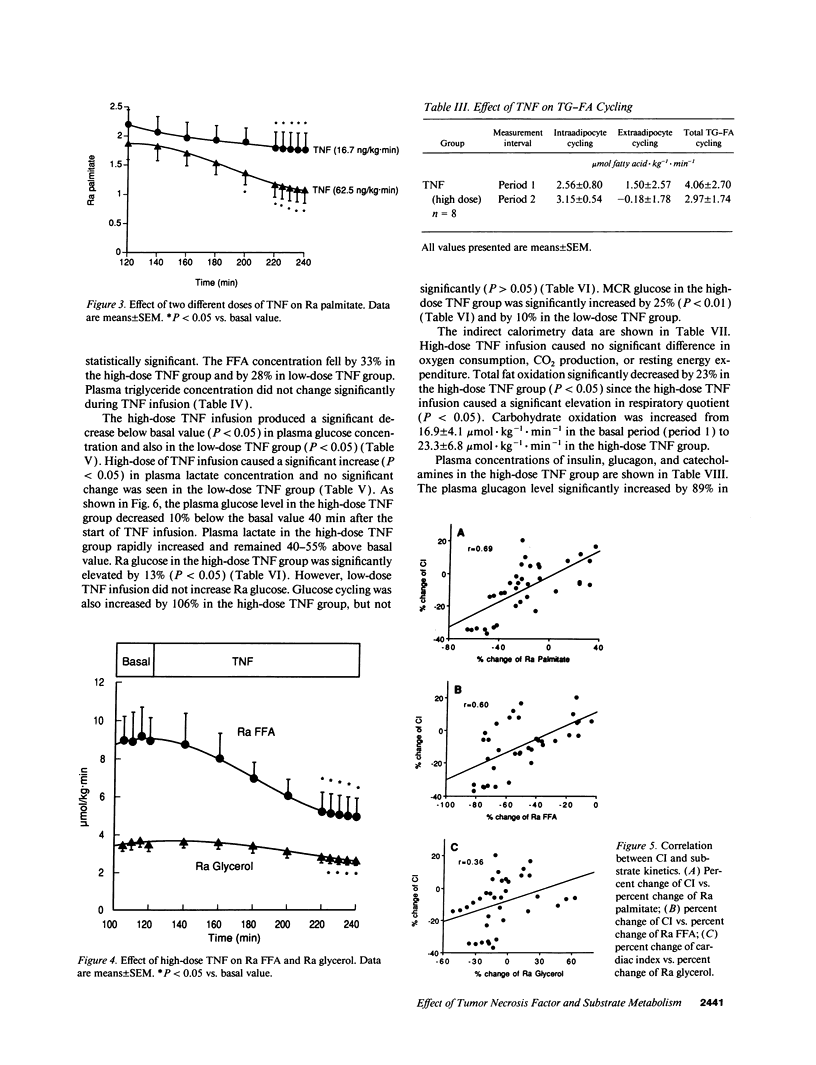
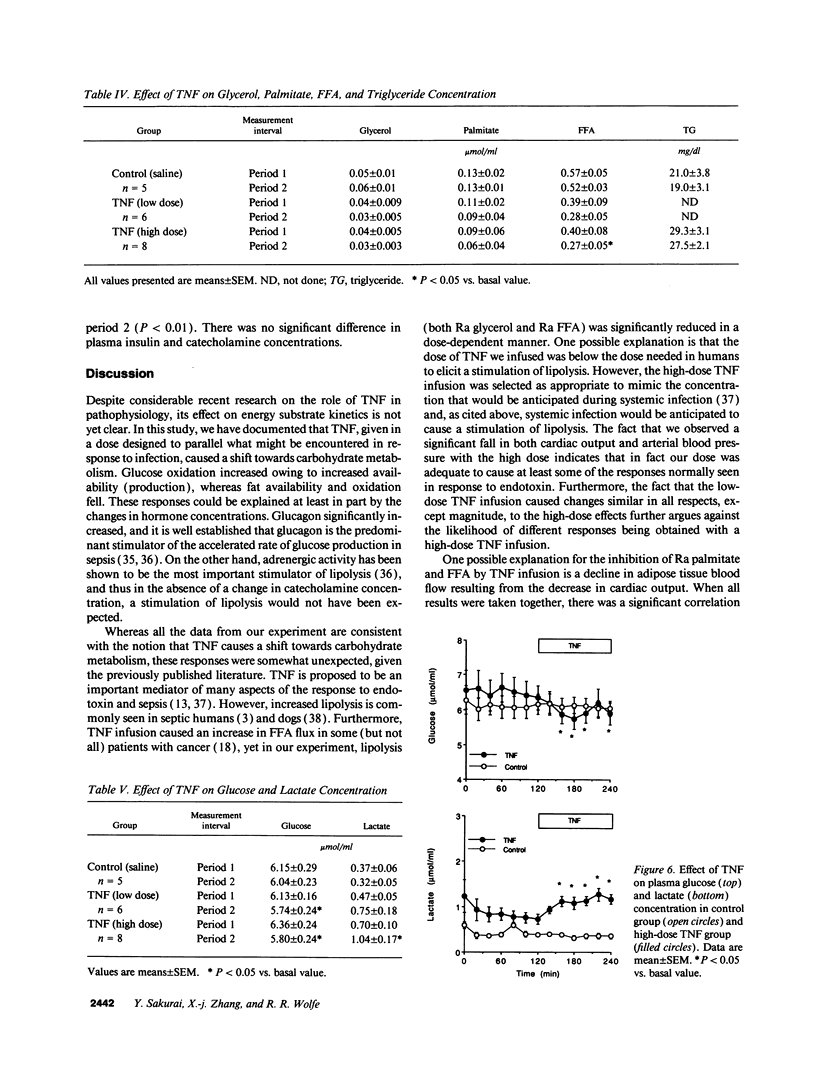
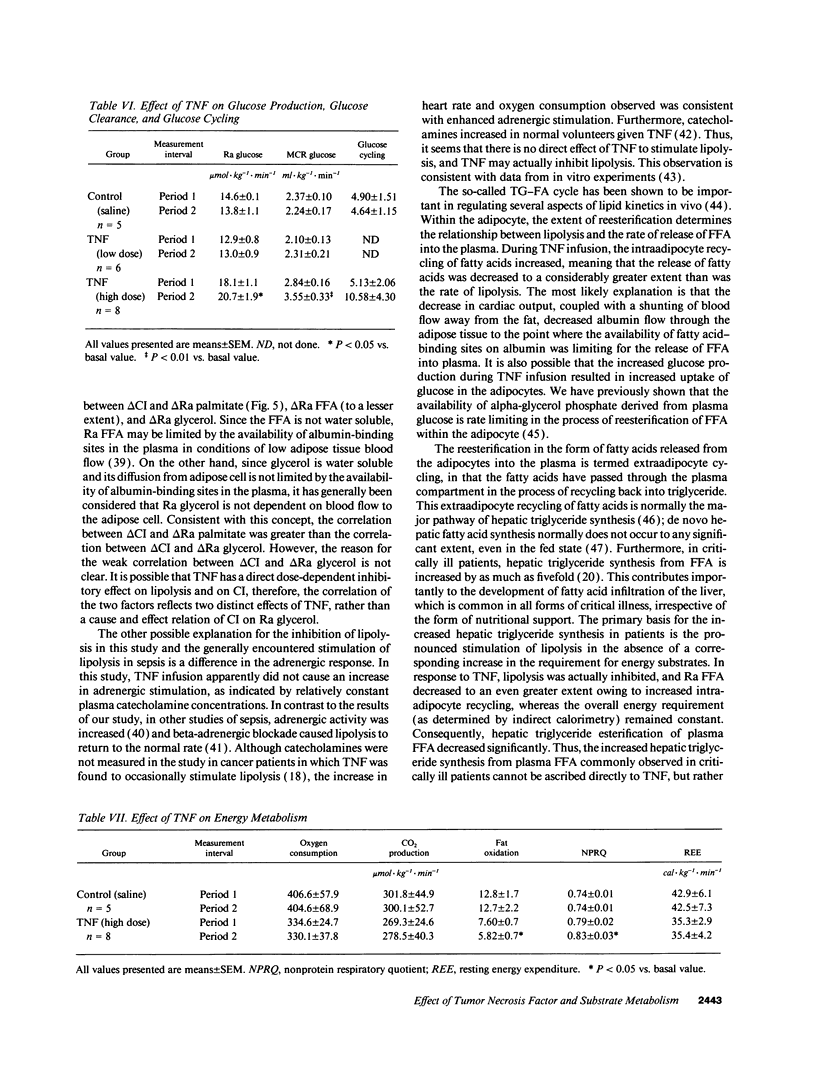
In vivo short-term effects of recombinant human TNF-alpha on lipolysis, FFA flux, fat oxidation, triglyceride-fatty acid cycling, and glucose kinetics were evaluated with stable isotopic tracers and indirect calorimetry along with monitoring of hemodynamic parameters in fasted dogs. High-dose TNF infusion (10 micrograms/kg) caused a fall in mean arterial pressure (P < 0.01), pulmonary arterial pressure (P < 0.001), and cardiac index (CI) (P < 0.05). The rate of appearance of glycerol (Ra glycerol) and the rate of appearance of FFA (Ra FFA) were decreased by 20% (P < 0.05) and by 42% (P < 0.01), respectively. Total fat oxidation fell by 23% (P < 0.05). In contrast, TNF infusion significantly increased glucose production by 13% (P < 0.05) and metabolic clearance rate of glucose by 25% (P < 0.01). However, TNF infusion did not change energy expenditure. Low-dose TNF infusion (3.5 micrograms/kg) caused changes similar in all respects, except magnitude, to the high-dose effects. There was a significant correlation between percent change of CI (delta CI) and percent change of rate of appearance of palmitate (Ra palmitate; delta Ra palmitate) (P < 0.0001, r = 0.69), Ra FFA (delta Ra FFA) (P < 0.0001, r = 0.60), and Ra glycerol (delta Ra glycerol) (P < 0.0329, r = 0.36). The correlation between delta CI and delta Ra palmitate was greater than the correlation between delta CI and delta Ra glycerol (P = 0.028). We conclude that the acute response to TNF causes a shift towards carbohydrate as an energy substrate in a dose-dependent manner by both decreasing the availability of FFAs and increasing glucose production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. J., Lang C. H., Hargrove D. M., Thompson J. J., Wilson L. A., Spitzer J. J. Glucose kinetics in rats infused with endotoxin-induced monokines or tumor necrosis factor. Circ Shock. 1988 Feb;24(2):111–121. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Carpentier Y. A., Askanazi J., Elwyn D. H., Jeevanandam M., Gump F. E., Hyman A. I., Burr R., Kinney J. M. Effects of hypercaloric glucose infusion on lipid metabolism in injury and sepsis. J Trauma. 1979 Sep;19(9):649–654. doi: 10.1097/00005373-197909000-00002. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger W. H., Miller L. D., Albers J. J., Smith T. K., Parks J. S. Lipopolysaccharide and tumor necrosis factor cause a fall in plasma concentration of lecithin: cholesterol acyltransferase in cynomolgus monkeys. J Lipid Res. 1990 Jun;31(6):1099–1107. [PubMed] [Google Scholar]
- Evans D. A., Jacobs D. O., Revhaug A., Wilmore D. W. The effects of tumor necrosis factor and their selective inhibition by ibuprofen. Ann Surg. 1989 Mar;209(3):312–321. doi: 10.1097/00000658-198903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins J. P., Buchanan B. J. In vivo vs in vitro effects of endotoxin on glycogenolysis, gluconeogenesis, and glucose utlization. Proc Soc Exp Biol Med. 1977 Jun;155(2):216–218. doi: 10.3181/00379727-155-39776. [DOI] [PubMed] [Google Scholar]
- Flores E. A., Istfan N., Pomposelli J. J., Blackburn G. L., Bistrian B. R. Effect of interleukin-1 and tumor necrosis factor/cachectin on glucose turnover in the rat. Metabolism. 1990 Jul;39(7):738–743. doi: 10.1016/0026-0495(90)90110-x. [DOI] [PubMed] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982 Oct;28(10):2077–2080. [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Guckian J. C. Role of metabolism in pathogenesis of bacteremia due to Diplococcus pneumoniae in rabbits. J Infect Dis. 1973 Jan;127(1):1–8. doi: 10.1093/infdis/127.1.1. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Christiansen M., Kaempfer S., Kletke C., Wu K., Reid J. S., Mulligan K., Hellerstein N. S., Shackleton C. H. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest. 1991 May;87(5):1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A. Recombinant human tumor necrosis factor does not inhibit lipoprotein lipase in primary cultures of isolated human adipocytes. J Lipid Res. 1988 Jul;29(7):909–914. [PubMed] [Google Scholar]
- Lee M. D., Zentella A., Vine W., Pekala P. H., Cerami A. Effect of endotoxin-induced monokines on glucose metabolism in the muscle cell line L6. Proc Natl Acad Sci U S A. 1987 May;84(9):2590–2594. doi: 10.1073/pnas.84.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. L., Spencer J. L., Kinney J. M., Geiger J. W. Carbohydrate metabolism in man: effect of elective operations and major injury. J Appl Physiol. 1971 Jul;31(1):110–116. doi: 10.1152/jappl.1971.31.1.110. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Shulman G. I., Peters E. J., Wolfe M. H., Elahi D., Wolfe R. R. Hormonal control of substrate cycling in humans. J Clin Invest. 1988 May;81(5):1545–1555. doi: 10.1172/JCI113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros K., Lang C. H., Bagby G. J., Spitzer J. J. Tumor necrosis factor increases in vivo glucose utilization of macrophage-rich tissues. Biochem Biophys Res Commun. 1987 Nov 30;149(1):1–6. doi: 10.1016/0006-291x(87)91596-8. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Nordenström J., Carpentier Y. A., Askanazi J., Robin A. P., Elwyn D. H., Hensle T. W., Kinney J. M. Free fatty acid mobilization and oxidation during total parenteral nutrition in trauma and infection. Ann Surg. 1983 Dec;198(6):725–735. doi: 10.1097/00000658-198312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Revhaug A., Michie H. R., Manson J. M., Watters J. M., Dinarello C. A., Wolff S. M., Wilmore D. W. Inhibition of cyclo-oxygenase attenuates the metabolic response to endotoxin in humans. Arch Surg. 1988 Feb;123(2):162–170. doi: 10.1001/archsurg.1988.01400260042004. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Conyers R. A., Bais R., Gamble J. R., Vadas M. A. The effects of recombinant tumour necrosis factor (cachectin) on metabolism in isolated rat adipocyte, hepatocyte and muscle preparations. Biochem J. 1987 Nov 1;247(3):789–792. doi: 10.1042/bj2470789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J., Chinkes D., Wolfe M., Wolfe R. R. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol. 1992 Sep;263(3 Pt 1):E584–E596. doi: 10.1152/ajpendo.1992.263.3.E584. [DOI] [PubMed] [Google Scholar]
- Ryan N. T. Metabolic adaptations for energy production during trauma and sepsis. Surg Clin North Am. 1976 Oct;56(5):1073–1090. doi: 10.1016/s0039-6109(16)41032-7. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. A conscious septic dog model with hemodynamic and metabolic responses similar to responses of humans. Surgery. 1984 May;95(5):553–561. [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Response to glucose and lipid infusions in sepsis: a kinetic analysis. Metabolism. 1985 May;34(5):442–449. doi: 10.1016/0026-0495(85)90210-0. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Ladenson P. W., Wolfe M. H., Ridgway E. C., Wolfe R. R. Substrate cycling between gluconeogenesis and glycolysis in euthyroid, hypothyroid, and hyperthyroid man. J Clin Invest. 1985 Aug;76(2):757–764. doi: 10.1172/JCI112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber D. L., Redl H., Schlag G., Herndon D. N., Kimura R., Prien T., Traber L. D. Cardiopulmonary responses to continuous administration of endotoxin. Am J Physiol. 1988 May;254(5 Pt 2):H833–H839. doi: 10.1152/ajpheart.1988.254.5.H833. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Tredget E. E., Yu Y. M., Zhong S., Burini R., Okusawa S., Gelfand J. A., Dinarello C. A., Young V. R., Burke J. F. Role of interleukin 1 and tumor necrosis factor on energy metabolism in rabbits. Am J Physiol. 1988 Dec;255(6 Pt 1):E760–E768. doi: 10.1152/ajpendo.1988.255.6.E760. [DOI] [PubMed] [Google Scholar]
- Van der Poll T., Romijn J. A., Endert E., Borm J. J., Büller H. R., Sauerwein H. P. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991 Oct;261(4 Pt 1):E457–E465. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Gerich J. E. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981 Jun;30(6):535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Aulick L. H., Mason A. D., Pruitt B. A., Jr Influence of the burn wound on local and systemic responses to injury. Ann Surg. 1977 Oct;186(4):444–458. doi: 10.1097/00000658-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W. Hormonal responses and their effect on metabolism. Surg Clin North Am. 1976 Oct;56(5):999–1018. doi: 10.1016/s0039-6109(16)41029-7. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Burke J. F. Somatostatin infusion inhibits glucose production in burn patients. Circ Shock. 1982;9(5):521–527. [PubMed] [Google Scholar]
- Wolfe R. R., Evans J. E., Mullany C. J., Burke J. F. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980 Apr;7(4):168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Herndon D. N., Jahoor F., Miyoshi H., Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987 Aug 13;317(7):403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Klein S., Carraro F., Weber J. M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990 Feb;258(2 Pt 1):E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Peters E. J. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987 Feb;252(2 Pt 1):E218–E223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H. Glucose and FFA kinetics in sepsis: role of glucagon and sympathetic nervous system activity. Am J Physiol. 1985 Feb;248(2 Pt 1):E236–E243. doi: 10.1152/ajpendo.1985.248.2.E236. [DOI] [PubMed] [Google Scholar]


