Abstract
Hexavalent chromium [Cr(VI)] is a well-known human carcinogen associated with the incidence of lung cancer. Inhibition of metal induced carcinogenesis by a dietary antioxidant is a novel approach. Luteolin, a natural dietary flavonoid found in fruits and vegetables, possesses potent antioxidant and anti-inflammatory activity. We found that short term exposure of human bronchial epithelial cells (BEAS-2B) to Cr(VI) (5 μM) showed a drastic increase in ROS generation, NADPH oxidase (NOX) activation, lipid peroxidation, and glutathione depletion, which were significantly inhibited by the treatment with luteolin in a dose dependent manner. Treatment with luteolin decreased AP-1, HIF-1α, COX-2, and iNOS promoter activity induced by Cr(VI) in BEAS-2B cells. In addition, luteolin protected BEAS-2B cells from malignant transformation induced by chronic Cr(VI) exposure. Moreover, luteolin also inhibited the production of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α) and VEGF in chronic Cr(VI) exposed BEAS-2B cells. Western blot analysis showed that luteolin inhibited multiple gene products linked to survival (Akt, Fak, Bcl-2, Bcl-xL), inflammation (MAPK, NF-κB, COX-2, STAT-3, iNOS, TNF-α) and angiogenesis (HIF-1α, VEGF, MMP-9) in chronic Cr(VI) exposed BEAS-2B cells. Nude mice injected with BEAS-2B cells chronically exposed to Cr(VI) in the presence of luteolin showed reduced tumor incidence compared to Cr(VI) alone treated group. Overexpression of catalase (CAT) or SOD2, eliminated Cr(VI)-induced malignant transformation. Overall, our results indicate that luteolin protects BEAS-2B cells from Cr(VI)-induced carcinogenesis by scavenging ROS and modulating multiple cell signaling mechanisms that are linked to ROS. Luteolin, therefore, serves as a potential chemopreventive agent against Cr(VI)-induced carcinogenesis.
Keywords: Hexavalent chromium, Luteolin, Carcinogenesis, Inflammation, Angiogenesis
Introduction
Hexavalent chromium [Cr(VI)] is recognized as a human carcinogen associated with an increased risk of lung cancer. Exposure to Cr(VI) induces DNA damage, cell morphological changes and malignant transformation in human lung epithelial cells (Ding et al., 2013). Epidemiological studies have reported that the lung cancer morbidity rate is 20 times higher in ex-chromate workers than for non-smokers (Nakagawa et al., 1984). Overproduction of reactive oxygen species (ROS) has been suggested to play a major role in Cr(VI) carcinogenesis (O’Brien et al., 2003; Wang et al., 2011). Reports suggested that ROS plays an important role in inflammation (Khodr and Khalil, 2001; Barbieri et al., 2003), angiogenesis (Ushio-Fukai and Alexander, 2004) and malignant cell survival (Fruehauf and Meyskens, 2007). NADPH oxidase (NOX) is one of the major sources of cellular ROS, and previous reports from our laboratory implicate the role of NOX in Cr(VI)-induced ROS generation and carcinogenesis (Wang et al., 2011). In addition, chronic Cr(VI) exposure has been shown to induce lipid peroxidation (LPO) and decrease glutathione (GSH) (Anand, 2005; Thompson et al., 2011) in in vivo.
Chronic inflammation is associated with lung carcinogenesis. Repetitive exposure to Cr(VI) results in persistent inflammation, and such an inflammatory microenvironment can further promote lung carcinogenesis (Beaver et al., 2009a; Beaver et al., 2009b). Cyclooxygenase-2 (COX-2) is a key enzyme in the conversion of arachidonic acid to prostanoids, and its activation is associated with inflammation and carcinogenesis (Hakozaki et al., 2014). Elevated COX-2 expression has been demonstrated in Cr(VI)-exposed cultured cells (Zuo et al., 2012; Son et al., 2013). Pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α) are involved in several pathological processes (Bouraoui et al., 2008). Upregulation of these cytokines was closely linked to chronic inflammation and lung cancer (Azad et al., 2008; Pratheesh Kumar and Kuttan, 2009; Pine et al., 2011; Pratheeshkumar and Kuttan, 2011a; Pratheeshkumar et al., 2011). STAT3 is linked to inflammation-associated oncogenic transformation, by promoting pro-oncogenic inflammatory pathways, including nuclear factor-κB (NF-κB) and interleukin-6 (IL-6) (Yu et al., 2009). Previous studies demonstrated a prolonged STAT3 activation and transactivation of IL-6, with Cr(VI) exposure in human epithelial cells (O’hara et al., 2007). Inducible nitric oxide synthase (iNOS), one of the three isoforms of nitric oxide synthase, catalyzes the oxidative deamination of l-arginine to produce citrulline and nitric oxide (Chen et al., 2005). Aberrant or excessive expression of iNOS leads to an accumulation of nitric oxide, which can participate in cancer development (Weiming et al., 2002; Chen et al., 2005). Cr(VI) exposure also leads to activation of mitogen-activated protein kinases (MAPKs), including c-Jun N-terminal kinase (JNK)1/2, p38, and extracellular-signal regulated kinase (ERK)1/2 (Gao et al., 2002; Wakeman et al., 2005; Chen et al., 2009).
The nuclear transcription factor NF-κB controls a number of genes involved in inflammatory responses, cell cycle progression, inhibition of apoptosis and cell adhesion, and thus plays a critical role in promoting carcinogenesis and cancer progression (Okamoto et al., 2007). Altered activation of NF-κB has been reported in many tumors (Perkins, 1997). Exposure to Cr(VI) caused NF-κB activation in Jurkat cells (Shi et al., 1999) and human bronchial epithelial cells (Zuo et al., 2012). Hypoxia inducible factor 1 α (HIF-1α) plays a crucial role in coordinating the cellular response to oxygen stress conditions (Semenza, 2003; Vaupel, 2004). During hypoxic stress, HIF-1α activates the transcription of a variety of genes involved in the process of promoting malignant cell survival and carcinogenesis (Simiantonaki et al., 2008b). Vascular endothelial growth factor (VEGF) plays a central role in tumor angiogenesis by increasing the vascular permeability and proliferation of vascular endothelial cells. In addition, there is a signi cant correlation between HIF-1α and VEGF expression (Blancher et al., 2000). HIF-1α is upregulated in response to hypoxia and accompanied by a significant increase in the production of VEGF (Simiantonaki et al., 2008b). Previous studies has shown that exposure to Cr(VI) induces HIF-1α and VEGF expression through the production of ROS in human prostate carcinoma cells (Gao et al., 2002).
Cancer prevention with naturally occurring dietary agents has gained immense interest because of their safety, low toxicity, and general availability (Pratheeshkumar et al., 2012c). Flavonoids are a group of important naturally occurring polyphenolic compounds with a wide range of biological effects (Zheng et al., 2014). Luteolin (3′,4′,5,7-tetrahydroxyflavone, Fig. 1A) is a common dietary flavonoid found in fruits, vegetables, and medicinal herbs with recognized anti-oxidant, anti-inflammatory and anticancer activities (Harris et al., 2006; Tang et al., 2011; Zhang et al., 2013; Zhang et al., 2014). Recently we have shown that luteolin could inhibit human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis (Pratheeshkumar et al., 2012b).
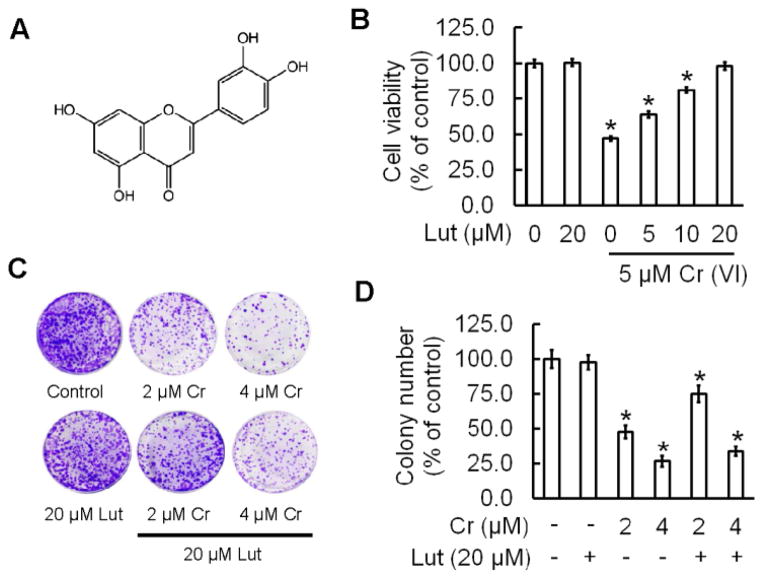
Figure 1.
Luteolin inhibits Cr (VI)-induced cytotoxicity. (A) Chemical structure of luteolin (B) Cell viability, determined by MTT, was assessed in BEAS-2B cells treated with Cr(VI) (5 μM) for 24 h in the presence of luteolin (0, 5, 10, 20 μM). (C–D) BEAS-2B cells were treated with 2 μM or 4 μM Cr(VI) with or without 20 μM luteolin for 48 h, reseeded and cultured in drug free medium for an additional 7 days and stained with crystal violet. The data are expressed as the mean ± SD of three independent experiments.*(p<0.05).
In this study, we investigated the protective effect of luteolin on Cr(VI)-induced malignant transformation of human bronchial epithelial cells (BEAS-2B) and with a focus on the key molecular events involved. We found that treatment of luteolin resulted in a significant inhibition of Cr(VI)-induced (i) ROS generation, NOX activation, lipid peroxidation, and glutathione depletion; (ii) malignant transformation; (iii) pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α); and multiple gene products linked to (iv) cell survival (Erk½, p38, JNK, Akt, Fak, Bcl-2, and Bcl-xL); (v) activation of NF-κB and IKKα, and degradation of IκBα; (vi) inflammation (COX-2, STAT-3, iNOS, TNF-α); and (vii) angiogenesis (HIF-1α, VEGF, MMP-9) in human bronchial epithelial cells.
Materials and methods
Antibodies and chemicals
Luteolin (>99% pure) was purchased from Sigma (St. Louis, MO, USA), dissolved in DMSO, aliquoted, and stored at −20°C. Potassium dichromate (K2Cr2O7) was obtained from Sigma-Aldrich (St. Louis, MO). Dichlorodihydrofluoresceine acetate (DCFDA) and dihydroethidium (DHE) were obtained from Molecular Probes (Eugene, OR). Lipofectamine 2000 was purchased from Invitrogen Corporation (Carlsbad, CA). Antibodies specific for p-P38, P38, p-AKT, AKT, p-FAK, FAK, p-STAT3, STAT3, Bcl-2, Bcl-xL, iNOS were obtained from cell signaling Technology (Beverly, MA). The primary antibodies specific for p-ERK, ERK, p-JNK, JNK, NF-κB/p65, IKKα, IκBα, COX-2, TNF-α, MMP-9, VEGF, HIF-1-α, β-actin and the secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Glutathione (GSH) assay kit was obtained from Cayman Chemical (Ann Arbor, MI). Assay kit for thiobarbituric acid reactive substances (TBARS) was purchased from BioAssay Systems (Hayward, CA, USA). Luciferase assay kit was obtained from Promega (Madison, WI).
Cell lines and cell culture
BEAS-2B (Human bronchial epithelial cell line), obtained from the American Type Culture Collection (Rockville, MD), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, and 5% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2 in air. K2Cr2O7 was used for Cr (VI) treatment. For short-term exposure, cells at 80–90% confluence, experienced an overnight incubation with DMEM containing 0.1% FBS and Cr(VI) indicated. In some experiments, cells were pretreated with inhibitors for 2 h and then exposed to Cr (VI). For chronic exposure to Cr (VI), the cells were continuously cultured in growth medium with Cr (VI) as indicated for 6 months.
ELISA kits
Highly specific quantitative ‘sand wich’ Elisa kits for human IL-1β, IL-6, IL-8, and TNF-α were purchased from BioLegend, Inc. (CA, USA) and the ELISA kit for VEGF was purchased from RayBiotech (GA, USA).
Plasmid constructs and transfection
CAT-Myc-DDK-tagged plasmid was purchased from Origene (Rockville, MD). The SOD2-EGFP-tagged plasmid was obtained from Addgene (Cambridge, MA). Transfections were performed using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, BEAS-2B cells were seeded in 6-well culture plates; when approximately 50% confluent, cells were transfected with 4 μg plasmid. Cell clones resistant to G418 were isolated and overexpression of SOD2 and CAT protein production were confirmed by immunoblot as described previously (Wang et al., 2011).
HIF-1α luciferase reporter (HIF-1α-Luc) was purchased from Panomics (Fremont, CA) and AP1–luciferase (AP1-Luc) plasmid was from Stratagene (Santa Clara, CA, USA). Other luciferase reporter plasmids (COX-2-Luc, and iNOS-Luc) were kindly provided by Dr. Chuanshu Huang (Li et al., 2006; Ouyang et al., 2007) from Nelson Institute of Environmental Medicine, New York University School of Medicine, NY. Transfection experiments were performed with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Cell viability assay
Cell viability was determined using 3-(4,5-dimethylthiazol-2yl-2,5-diphenyl tetrazolium bromide (MTT) assay. Active mitochondrial dehydrogenases in living cells metabolize MTT to a purple formazan dye, which is measured photometrically at 570 nm using a spectrophotometer as described previously (Pratheeshkumar and Kuttan, 2011c).
Clonogenic assay
BEAS-2B cells (105 cells) seeded into each well of a 6-well plate, were to attach overnight. After the indicated exposure, cells were collected by trypsinization, three hundred cells were then reseeded into each of three dishes (60 mm diameter), and grown for 10 days. The cells were fixed with 2% formalin for 10 min, stained with 0.5% crystal violet and counted.
Intracellular ROS determination
Cells were washed once with warm PBS and incubated with 10 μmol/L 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate ethyl ester (CM-H2DCFDA; Molecular Probes) or 5 μmol/L dihydroethidium (DHE; Molecular Probes), respectively, in warm PBS for 40 minutes. Cells were subsequently harvested with trypsin, washed twice with cold PBS, and analyzed by fluorescence-activated cell sorting (FACS Calibur, BD Biosciences). The fluorescence intensity of DCF was measured at an excitation wavelength of 492 nm and an emission wave length of 517 nm. The fluorescence intensity of DHE was measured at an excitation wavelength of 535 nm and an emission wavelength of 610 nm.
NOX activity assay
NOX activity was measured by the lucigenin enhanced chemiluminescence method as described previously (Wang et al., 2011). Briefly, cells were harvested and homogenized by sonication in cold lysis buffer (20 mM KH2PO4, pH 7.0, 1 mM EGTA, 1mM phenyl methyl sulfonyl fluoride, 10 μg/ml aprotinin, and 0.5 μg/ml leupeptin). Centrifugation (800 × g at 4°C for 10 min) removed unbroken cells and debris, and used immediately. To start the assay, 100-μl aliquots of homogenates were added to 900 μl of 50 mM phosphate buffer, pH 7.0, containing 1mM EGTA, 150 mM sucrose, 5 μM lucigenin, and 100 μM NADPH. Photon emission in terms of relative light units was measured in a luminometer every 30 s for 5 min. There was no measurable activity in the absence of NADPH. Superoxide anion production was expressed as relative chemiluminescence (light) units (RLU)/mg protein.
Glutathione assay
Harvested cells were sonicated in cold buffer (50 mM Phosphate, pH 6–7, containing 1 mM EDTA), then centrifuged at 10,000 g for 15 min at 4 °C. Total GSH content in the supernatants was determined by using the Cayman’s GSH assay kit (Cayman Chemical Co., Ann Arbor, MI) following the manufacturer’s instruction.
TBARS assay
Lipid peroxidation was determined using a QuantChromTM TBARS assay kit. Cells sonicated in ice cold PBS, were subjected to centrifugation at 12,000 g for 15 min at 4°C, and the supernatant assayed according to the manufacturer’s instructions. Reacted samples were added to wells of a 96 well plate and measured spectrophotometrically at 532 nm using MDA as a standard.
Luciferase reporter assay
BEAS-2B cells transfected with the luciferase reporter constructs were seeded into 24-well plates (5 × 104/well), maintained until 80–90% confluent, and then subjected to various treatments. Cellular lysates were subjected to a luciferase reporter assay (Promega) using a luminometer (Wallac 1420 Victor 2 multilabel counter system; PerkinElmer, Waltham, MA, USA) as described previously (Ding et al., 2006). The reported results are expressed as relative activity normalized to the luciferase activity in the control cells without treatment.
Anchorage-independent colony growth assay
Soft agar colony formation assay was performed as described previously (Wang et al., 2011). BEAS-2B cells were cultured in DMEM supplemented with 10% FBS containing Cr(VI) (0 or 0.5 μM) with or without luteolin (1 and 2 μM) for 6 months. After 6-month treatment, cells were harvested, and 1 × 104cells were suspended in 2 ml of medium containing 0.35% agar and seeded into six-well plates with 2 ml of a 0.5% agar base layer and maintained in a 37°C, 5% CO2 incubator for four weeks, and colonies greater than 0.1 mm in diameter were scored by microscopic examination.
ELISA assays for VEGF and pro-inflammatory cytokines
BEAS-2B cells were cultured in DMEM supplemented with 10% FBS containing Cr (VI) (0 or 0.5 μM) with or without luteolin (1 and 2 μM) for 6 months. Subsequently, the culture media was collected and used to estimate the levels of VEGF and pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α using commercially available ELISA kits according to the manufacturers’ recommendation.
ELISA for NF-KB/p65 and STAT3
NF-κB/p65 and STAT3 were quantitatively analyzed using the TransAM ELISA kit (Active Motif, Carlsbad, CA), following the manufacturer’s protocol. For this assay, the nuclear extracts of cell samples from various treatment groups were prepared using the Nuclear Extraction kit (Active Motif) according to the manufacturer’s direction. Absorbance was recorded at 450 nm with reference taken at 650 nm. The assay was done in duplicate and the results are expressed as the percentage absorbance of control group.
Western blot analyses
Cells lysates were prepared in ice-cold RIPA buffer (Sigma-Aldrich) with freshly added protease inhibitor cocktail. The lysate was then centrifuged at 12 000 g for 10 min at 4°C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80°C. Nuclear and cytoplasmic extracts were prepared using a extraction kit from Thermo Scientific (Rockford, IL) according to the manufacturer’s protocol. The protein concentration was determined using Coomassie Protein Assay Reagent (Thermo, Rockford, IL). Approximately 40 μg cellular proteins were separated through 6%–12% SDS-polyacrylamide gel, and then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). Nonspecific binding was blocked with 5% fat-free dry milk in 1X Tris-buffered saline (TBS) and the membrane incubated with antibody, as indicated. Protein bands, detected with horseradish peroxidase-conjugated antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD), were visualized with enhanced chemiluminescence reagent (Perkin Elmer, Boston, MA).
Tumorigenesis studies
Athymic nude mice (NU/NU, 6–8 weeks old; Charles River), housed in a pathogen-free room in the animal facilities at the Chandler Medical Center, University of Kentucky, were handled according to the Institutional Animal Care and Use (IACUC). Cells (2×106 cells per mouse) from different treatments were resuspended in serum-free medium with matrigel basement membrane matrix (BD Biosciences) at a 1:1 ratio (total volume=100 μl) and subcutaneously injected into the flanks of nude mice. Mice were monitored daily for tumor appearance, and tumor volume was measured every 3 days for 30 days. Tumor volume was determined using a Vernier caliper, following the formula of A×B2×0.52, where A is the longest diameter of tumor and B is the shortest diameter. At the end of the experiment, mice were sacrificed and the tumors excised and snap frozen.
Histology and Immunohistochemistry
Frozen tumor sections (5 μm) were hydrated in phosphate buffered saline (PBS), and non-specific binding sites blocked with 10% horse serum in PBS, then processed according to Vectastain ABC Kit protocol (Vector Laboratories, Burlingame, CA). Briefly, the sections were incubated with rabbit anti-CD31 (1:100; Novus Biologicals Inc, Littleton, CO), mouse anti-CD34 (1:100; BD Pharmingen Inc, San Diego, CA) and rabbit anti-Ki67 (1:100, Abcam, Cambridge, MA) antibodies for 2 h at room temperature, washed and then incubated with biotinylated secondary antibody for 45 min followed by incubation with ABC reagent. After washing in PBS, sections were developed in DAB solution until the desired staining intensity was achieved. Finally, the sections were counterstained with hematoxylin.
Statistical analysis
The values presented are means ± SD. One-way analysis of variance (ANOVA) was used for statistical analysis. p<0.05 was considered significantly different.
Results
Luteolin inhibits Cr (VI)-induced cell viability loss in culture
The effect of luteolin on Cr (VI)-induced cytotoxicity was determined by MTT assay (Fig 1B). In BEAS-2B cells, Cr(VI) at 5 μM showed a drastic decrease (53 %) in cell viability and treatment of luteolin (10 and 20 μM) significantly ameliorates the Cr(VI)-induced cell viability loss in a dose dependent manner. Luteolin (20 μM) alone was found to be non-toxic towards BEAS-2B cells (Fig 1B). The above result was further confirmed by clonogenic assay (Fig 1C). Cr(VI) at 2 and 4 μM significantly decreased the colony number, 52 % and 73 % respectively, whereas treatment with luteolin (20 μM) inhibited the adverse effect of Cr(VI) by increasing colony number (Fig 1D).
Luteolin inhibits Cr(VI)-induced ROS generation in BEAS-2B cells
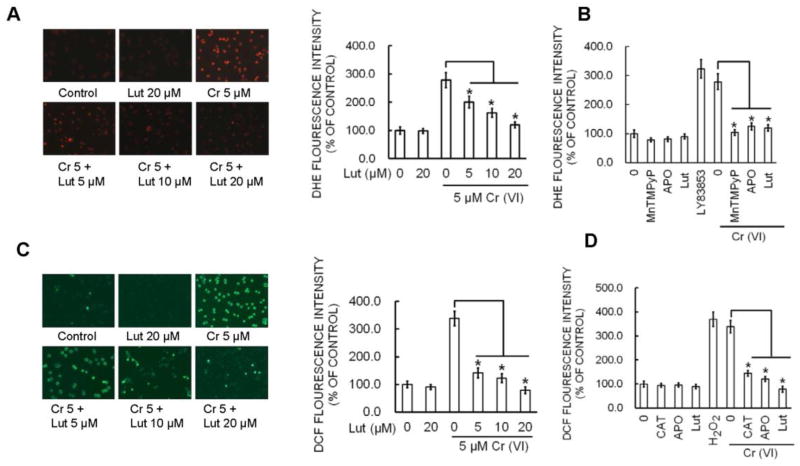
Cr(VI)-induced ROS production was quantified by flow cytometry using the fluorescent probes DCFDA and DHE. Cr(VI) exposure dramatically stimulated O2·− and H2O2 generation as indicated by an increase of DHE (Fig. 2A–B) and DCFDA (Fig. 2D–E) fluorescence intensity respectively in BEAS-2B cells compared to untreated control cells. DHE signal was increased by Cr(VI) and LY83853 (O2·− donor) and inhibited by MnTMPyP, cell-permeable SOD mimetic (O2·− scavenger) (Fig. 2C). Similarly, DCF signal was increased by Cr(VI) and H2O2 and inhibited by CAT (H2O2 scavenger) (Fig. 2F). The fluorescence intensity stimulated by Cr(VI) was also abolished by APO, a NOX inhibitor. Pretreatment with luteolin (5, 10 and 20 μM) significantly decreased the Cr(VI)-induced O2·− (Fig. 2A–B) and H2O2 generation (Fig. 2D–E). Taken together, the results suggested that luteolin was able to inhibit Cr(VI)-induced O2·− and H2O2 generation in BEAS-2B cells.
Figure 2.
Luteolin inhibits Cr(VI)-induced ROS generation. (A–B) BEAS-2B cells were exposed to Cr(VI) (0 or 5 μM) with or without luteolin (0, 5, 10, 20 μM) for 12 h and then were labeled with DHE (5 μM) or (D–E) DCFDA (10 μM). Images were taken with fluorescence microscopy and fluorescent intensity determined by flow cytometry. *(p<0.05). BEAS-2B cells were exposed to Cr(VI) (0 or 5 μM) or were pretreated with SOD (500 U/ml), CAT (1000 U/ml), or APO (50 μM) for 2 h followed by Cr(VI) (5 μM) treatment for 6 h and then were labeled with (C) DHE (5 μM) or (F) DCFDA (10 μM) as described previously. LY83853 (10 μM) and H2O2 (0.1 mM) were used as positive controls for DHE and DCF measurements, respectively. The data are expressed as mean ± SD of three independent experiments. *p<0.05, statistically significant difference from control cells.
Luteolin inhibits Cr(VI)-induced NADPH Oxidase activity in BEAS-2B cells
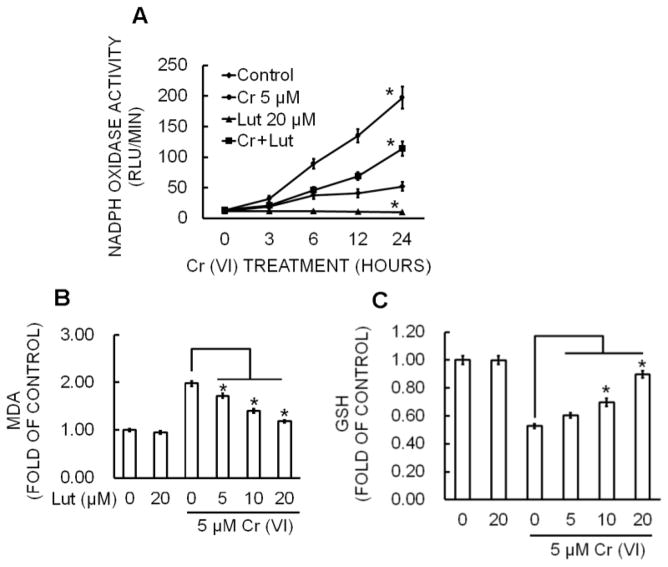
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is an important source of Cr(VI)-induced ROS production (Wang et al., 2011). To examine the effect of luteolin on Cr(VI)-induced NADPH oxidase, we measured NOX activity (Fig. 3A). BEAS-2B cells exposed to 5 μM Cr(VI) caused a time-dependent increase in NOX activation whereas co-treatment with luteolin significantly (p<0.05) reduced this activation.
Figure 3.
Luteolin inhibits Cr(VI)-induced NOX activation, Lipid Peroxidation, Glutathione depletion in BEAS-2B cells. (A) NOX activity was measured by the lucigenin chemiluminescence assay with Cr(VI) (0 or 5 μM) in the presence of luteolin (0, 5, 10, 20 μM) for indicated times. The data are expressed as the mean ± SD of three independent experiments. *p<0.05, statistically significant difference from control cells. BEAS-2B cells were treated with Cr(VI) (5 μM) for 24 h in the presence of luteolin (0, 5, 10, 20 μM). Cell lysates were prepared by sonication in ice cold PBS and was used for the determination of oxidative stress in terms of (B) lipid peroxidation and (C) glutathione depletion. The data are expressed as the mean ± SD of three independent experiments. *p<0.05, statistically significant difference from control cells.
Luteolin inhibits Cr(VI)-induced lipid peroxidation and glutathione depletion in BEAS-2B cells
Malondialdehyde (MDA) is a byproduct of lipid peroxidation (LPO) and is one of the biomarkers of increased oxidative stress (Bartsch and Nair, 2004). The effect of luteolin on Cr(VI)-induced lipid peroxidation is shown in Figure 3B. Lipid peroxidation was significantly higher in Cr(VI) (5 μM) exposed BEAS-2B cells compared with the control (p<0.05). Treatment with luteolin reduced Cr (VI)-induced accumulation of MDA in a concentration-dependent manner. Luteolin at 10 and 20 μM significantly attenuated lipid peroxidation in Cr(VI)-exposed BEAS-2B cells (p<0.05; Fig. 3B).
Glutathione (GSH) is the most abundant non-enzymatic antioxidant and plays a protective role in cells under oxidative stress (Reliene and Schiestl, 2006). Cr(VI) exposure drastically reduced the glutathione level in BEAS-2B cells compared to untreated control (Fig. 3C). However, treatment with luteolin significantly (p<0.05) attenuated the Cr(VI)-induced GSH depletion in BEAS-2B cells.
Luteolin inhibits Cr(VI)-induced AP-1, HIF-1α, Cox-2, and iNOS dependent transactivation in BEAS-2B cells
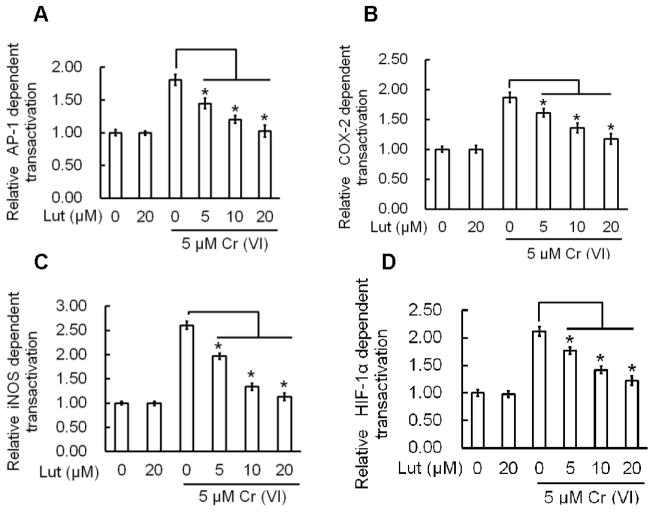
Previous studies have shown that AP-1, HIF-1α, Cox-2, and iNOS play an important role in carcinogenesis (Gao et al., 2002; Li et al., 2006; Farinati et al., 2010; Zuo et al., 2012). We investigated the effect of luteolin on Cr(VI)-induced transactivation of AP-1, HIF-1α, Cox-2, and iNOS using a luciferase reporter assay in BEAS-2B cells. Exposure to Cr(VI) at 5 μM showed a marked increase in AP-1, HIF-1α, Cox-2, and iNOS promoter activity. Treatment with luteolin at 5, 10 and 20 μM produced a dose-dependent decrease in AP-1, HIF-1α, Cox-2, and iNOS promoter activity induced by Cr(VI) in BEAS-2B cells (Fig 4, A–D).
Figure 4.
Luteolin inhibits Cr(VI)-induced AP-1, Cox-2, iNOS, HIF-1α, dependent transactivation in BEAS-2B cells. BEAS-2B cells transfected with the luciferase reporter constructs were treated with Cr(VI) (5 μM) for 24 h in the presence of luteolin (0, 5, 10, 20 μM). Cellular lysates were assessed for luciferase reporter activity as described previously. The results are expressed as relative activity normalized to the luciferase activity in the control cells without treatment. The data are expressed as the mean ± SD of three independent experiments. *p<0.05, statistically significant difference from control cells.
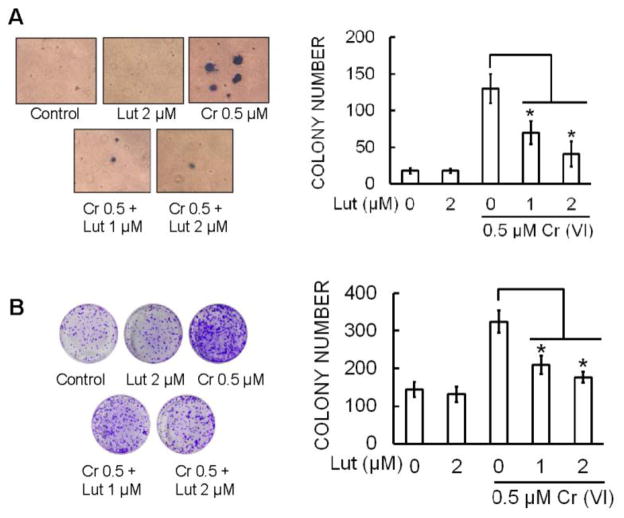
Luteolin inhibits malignant transformation induced by chronic Cr(VI) exposure in BEAS-2B cells
Previous reports demonstrate that chronic Cr(VI) exposure to BEAS-2B cells results in malignant transformation as assessed by increased cell proliferation, and anchorage independent growth in soft agar (Wang et al., 2011). Malignant transformation was assessed by anchorage-independent growth in soft agar (Carney et al., 1980). Continuous exposure of BEAS-2B cells to 0.5 μM Cr(VI) for 6 months induced marked increase in size and number of colonies compared to untreated control (Fig. 5A). However, co-treatment of luteolin (1 and 2 μM) with Cr(VI) significantly (p<0.05) decreased anchorage-independent growth in soft agar. Similar result were observed with the colony formation assay; Cr(VI) exposure dramatically increased the number of colonies and co-treatment of luteolin with Cr(VI) significantly (p<0.05) reduced the colony number in a dose-dependent manner (Fig. 5B).
Figure 5.
Luteolin inhibits chronic Cr(VI)-induced malignant transformation. BEAS-2B cells were maintained in a medium containing Cr(VI) (0 or 0.5 μM) with or without luteolin (1 and 2 μM) for 6 months. (A) Cells were cultured in 0.35% soft agar for 5 weeks. Colony numbers in the entire dish were counted. (B) Cells cultured in drug free medium for an additional 7 days and stained with crystal violet. Colony numbers in the entire dish were counted. The data are expressed as the mean ± SD of three independent experiments. *(p<0.05).
Luteolin inhibits VEGF and pro-inflammatory cytokine production induced by chronic Cr(VI) exposure in BEAS-2B cells
The effect of luteolin on production of pro-inflammatory cytokines after chronic Cr(VI) treatment was investigated and are shown in Figure 6(A–D). Chronic Cr(VI) exposure resulted in elevated secretion of the pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-8 in BEAS-2B cells. The co-treatment of luteolin with Cr(VI) caused a dose-dependent and statistically significant (p<0.05) decrease in the production of TNF-α (Fig. 6A), IL-6 (Fig. 6B), IL-1β (Fig. 6C), and IL-8 (Fig. 6D) in BEAS-2B cells in culture.
Figure 6.
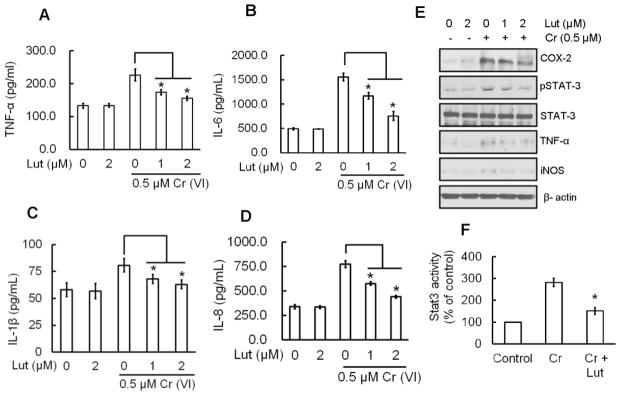
Luteolin inhibits mediators of inflammation induced by chronic Cr(VI) exposure in BEAS-2B cells. BEAS-2B cells were treated as described in Fig. 5. Culture medium was collected to estimate the (A) TNF-α, (B) IL-6, (C) IL-1β and (D) IL-8 levels using commercially available ELISA kits according to manufacturer’s recommendation.(E) Total cell lysates were prepared for Western blot analysis using specific antibodies against COX-2, STAT3, iNOS, TNF-α and β-actin. (F) STAT3 activity was measured in the nuclear fraction of cell lysates by ELISA following the manufacturer’s protocol. The data are expressed as the mean ± SD of three independent experiments. Significant difference compared to Cr(VI) alone, *p<0.05.
Luteolin inhibits mediators of inflammation that are induced by chronic Cr(VI) exposure in BEAS-2B cells
Inflammation is implicated in Cr(VI)-induced human lung cancer development (Zuo et al., 2012). Repetitive exposure to Cr(VI) increases the expression of mediators of inflammation as shown from the increased protein expressions of COX-2, STAT-3, TNF-α, and iNOS in Western blots (Fig. 6E). However, luteolin co-treatment with Cr(VI) drastically reduced the protein expressions of COX-2, STAT-3, TNF-α, and iNOS when compared to Cr(VI) treated cells. Luteolin alone did not produce any change in COX-2, STAT-3, TNF-α, and iNOS protein expression when compared to untreated BEAS-2B cells (Fig. 6E). Moreover, treatment of luteolin significantly (p<0.05) reduced the Cr(VI)-induced STAT-3 activation, which confirms the above results (Fig. 6F).
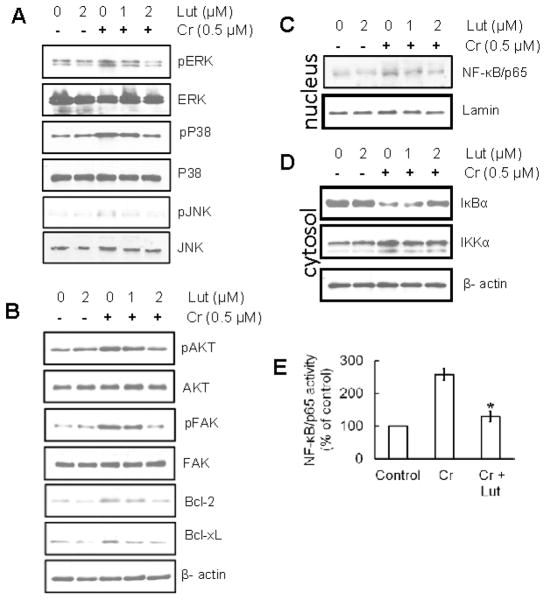
Luteolin inhibits phosphorylation of MAPK proteins induced by chronic Cr(VI) exposure in BEAS-2B cells
Previous studies have shown that Cr(VI)-induced oxidative stress is implicated in the activation of MAPK, a protein that is widely acknowledged as playing an important role in carcinogenesis (Samet et al., 1998; Gao et al., 2002; Wang Jr et al., 2010). Therefore, we studied the effect of luteolin on activation of the MAPK family proteins (ERK1/2, p38 and JNK1 2) that had been induced by chronic Cr(VI) exposure using Western blot analysis (Fig. 7A). The results indicate that chronic Cr(VI) exposure in BEAS-2B cells caused an increase in the phosphorylation of ERK1/2, p38 and JNK1 2 proteins. But co-treatment with luteolin markedly reduced the Cr(VI)-mediated phosphorylation of ERK1/2, p38 and JNK1 2 proteins when compared to Cr(VI) treatment alone. Treatment of luteolin alone did not produce any change in MAPK family proteins in BEAS-2B cells. Furthermore, the total amount of ERK1/2, JNK, and p38 proteins remained unchanged in each treatment group (Fig. 7A).
Figure 7.
Luteolin inhibits MAPK, AKT, FAK, Bcl-2, Bcl-xL and NF-κB induced by chronic Cr(VI) exposure in BEAS-2B cells. (A) Luteolin inhibited Cr(VI)-induced MAPK signaling in in BEAS-2B cells. Cells were treated as described in Fig. 5 and lysates were prepared to determine the phosphorylated and total protein levels of ERK1/2, p38, and JNK by Western blot analysis, as described previously. (B) Western blot analysis demonstrating that luteolin inhibited Cr(VI)-induced AKT, FAK, Bcl-2, and Bcl-xL in BEAS-2B cells. (C–E) Western blot analysis demonstrating that luteolin inhibited Cr(VI)-induced activation of NF-κB/p65 and IKKα, and degradation of IκBα in in BEAS-2B cells. (C) nuclear translocation of NF-κB/p65 (D) activation of IKKα, or degradation of IκBα in cytosol. The relative intensities of each band after normalization for the levels of lamin/β-actin are shown under each blot. A representative blots from three independent experiments with identical observations, and equivalent protein loading was confirmed by probing stripped blots for β-actin as shown. (E) The activity of NF-κB in nuclear fraction of cell lysates was measured using ELISA following the manufacturer’s protocol. The data are expressed as the mean ± SD of three independent experiments. Significant difference compared to Cr alone, *p<0.05.
Luteolin inhibits chronic Cr(VI)-induced AKT, FAK, Bcl-2, and Bcl-xL in BEAS-2B cells
Chronic Cr(VI) exposure elevated the expression levels of AKT, FAK and the anti-apoptotic proteins Bcl-2, Bcl-xL in BEAS-2B cells. Luteolin decreased the protein expression of AKT, FAK, Bcl-2, and Bcl-xL in a dose-dependent manner (Fig. 7B).
Luteolin inhibits chronic Cr(VI)-induced activation of the NF-κB pathway in BEAS-2B cells
NF-κB/p65 is a downstream target of the MAPK signal transduction pathways. Our Western blot analysis indicated that chronic Cr(VI) exposure in BEAS-2B cells stimulated the activation and translocation of NF-κB/p65 to the nucleus when levels were compared to the untreated control. Treatment of luteolin markedly inhibited the Cr(VI)-induced NF-κB activation and nuclear translocation in a dose-dependent manner (Fig. 7C). Investigations to understand the mechanisms involved in this process were pursued. Previous studies showed that Cr(VI) exposure resulted in degradation of the IκBα protein with subsequent activation and translocation of NF-κB/p65 to the nucleus (Zuo et al., 2012). IKK phosphorylates serine residues in IκBα and its degradation activates NF-κB. Chronic exposure to Cr(VI) also activates IKKα and has been shown to be essential for the degradation of IκBα. To study the inhibitory effect of luteolin on Cr(VI)-induced degradation of IκBα, we determined the cytoplasmic level of IκBα protein expression. Western blot analysis showed that treatment with luteolin suppressed the IκBα degradation (Fig. 7D). Western blot analysis also indicated that levels of activated IKKα were higher in cells chronically treated with Cr(VI); however, co-treatment of luteolin inhibited the levels of activated IKKα in cytosols (Fig. 7D). The inhibitory effect of luteolin on Cr(VI)-induced NF-κB activation was further confirmed using ELISA for NF-κB/p65 (Fig. 7E).
Luteolin inhibits angiogenic mediators induced by Cr(VI) in BEAS-2B cells
Expression of HIF-1α and VEGF are upregulated in many human cancers and are associated with treatment failure (Bos et al., 2003; Koukourakis et al., 2006; Goel and Mercurio, 2013). Previous studies demonstrated an elevation in HIF-1α and VEGF levels that was induced by Cr(VI) treatment (Gao et al., 2002). Therefore, we investigated the effect of luteolin on protein expressions of HIF-1α, HIF-1β, VEGF, and MMP-9 in BEAS-2B cells chronically exposed to Cr(VI) by Western blot, and VEGF level by ELISA (Fig. 8). The results show that protein expressions of HIF-1α, VEGF, and MMP-9 (Fig. 8A), and VEGF levels (Fig. 8B) were increased markedly with chronic Cr(VI) exposure, whereas the expression of HIF-1β protein was not altered. Treatment of luteolin markedly decreased the Cr(VI)-induced protein expressions of HIF-1α, VEGF, and MMP-9 and the VEGF level.
Figure 8.
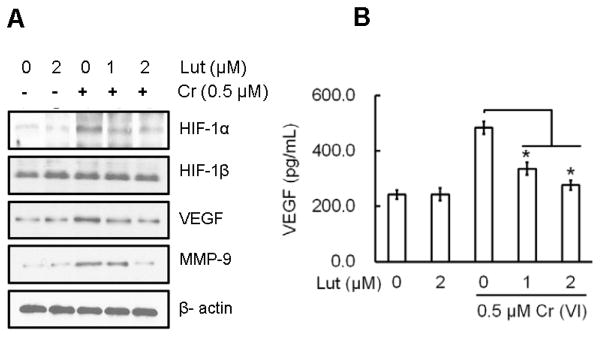
Luteolin inhibits chronic Cr(VI)-induced angiogenic mediators in BEAS-2B cells BEAS-2B cells were treated as described in Fig. 5 and lysates prepared to determine the protein levels of HIF-1α, HIF-1β, VEGF, and MMP-9 by Western blot analysis. (E) Culture medium was collected to estimate VEGF levels using a commercially available ELISA kit according to manufacturer’s recommendation. The data are expressed as the mean ± SD of three independent experiments. Significant difference compared to Cr alone, *p<0.05.
Luteolin inhibits growth of xenograft tumors in mice from cells chronically exposed to Cr(VI)
In this study, nude mice were injected subcutaneously with BEAS-2B cells that had been exposed to the indicated concentration of Cr(VI), with or without luteolin, for 6 months as displayed in Figure 9A. In mice injected with Cr(VI)-treated BEAS-2B cells we observed visible tumor formation that progressively increased over a 4-week period; no tumor growth was observed in animals injected with untreated cells (Fig. 9A). Mice injected with BEAS-2B cells exposed to luteolin along with Cr(VI) showed a reduced tumor incidence. BEAS-2B cells chronically exposed to luteolin alone did not produce any tumors in nude mice. Consistent with our in vitro findings above, luteolin markedly attenuated the growth of tumors produced by Cr(VI) exposure in a dose-dependent manner.
Figure 9.
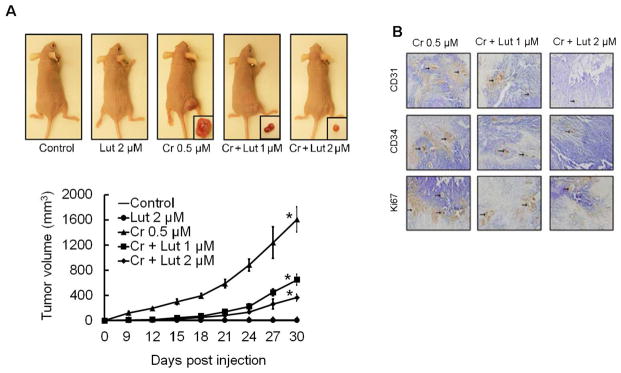
Luteolin inhibits growth of xenograft tumors in mice chronically exposed to Cr(VI). Cells treated as indicated from different treatments were injected into the flanks of 6-week old athymic nude mice (2×106 cells per mouse) and checked daily for tumor appearance; tumor volume was measured every 3 days for 30 days. Tumor volume was determined by Vernier caliper, following the formula of A×B2×0.52, where A is the longest diameter of tumor and B is the shortest diameter. (A) Mice injected with BEAS-2B cells exposed to luteolin along with Cr(VI) showed reduced tumor incidence. (B) Angiogenic (CD31, CD34) and proliferation (Ki67) markers were decreased in tumors treated with both Cr(VI) and luteolin as evident from immunohistochemistry. Frozen tumour sections (5 μM thick) were subjected to immunoperoxidase staining (dark brown) to detect CD31, CD34, and Ki67 expression.
To extend these investigations, we performed immunohistochemical analysis on these murine tumors. We observed a large number of CD31, CD34, and Ki67 (Fig. 9B) positive cells in the Cr(VI) treated group, and fewer stained cells from tumors co-treated with Cr(VI) and luteolin. All of these observations indicate the antiangiogenic efficacy of luteolin in vivo and strongly support the in vitro studies outline above.
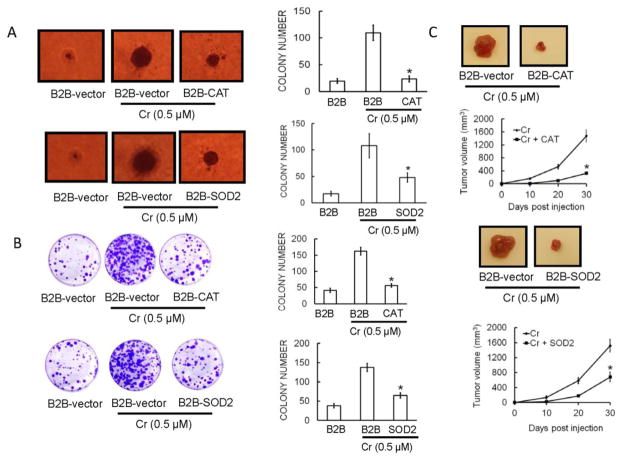
Overexpression of antioxidant enzymes attenuates Cr(VI)-induced carcinogenicity in BEAS-2B cells
To study the role of ROS in Cr(VI)-induced malignant transformation and tumorigenesis, BEAS-2B cells were generated that stably overexpress CAT, SOD2 or their corresponding vectors (Wang et al., 2011). These stable cells were continuously exposed to 0.5 μM Cr(VI) for six months. As shown in Figures 10(A–B), overexpression of SOD or CAT prominently decreased colony number and size in soft agar (Fig. 10A). Similar results were obtained with the colony formation assay, (Fig. 10B), indicating that ROS plays a key role in malignant transformation of BEAS-2B cells induced by chronic Cr(VI) exposure. To confirm these findings, we evaluated in vivo tumorigenicity in mice. After a 6-month exposure with or without Cr(VI), BEAS-2B cells stably expressing CAT, SOD2, or vector were injected to nude mice. Four weeks after injection, tumor development was assessed. Cr(VI)-exposed BEAS-2B-CAT, and BEAS-2B-SOD2 formed tumors that were smaller than those of Cr(VI)-exposed BEAS-2B-vector cells (Fig. 10C).
Figure 10.
Overexpression of antioxidant enzymes block Cr(VI)-induced malignant cell transformation and tumorigenesis in BEAS-2B cells. Inhibition of in vitro colony formation in BEAS-2B cells with overexpressed antioxidant enzymes is demonstrated in (A) soft agar and (B) clonogenic assay. BEAS-2B cells were stably transfected with CAT (BEAS-2B-CAT), SOD2 (BEAS-2B-SOD2), or their corresponding vectors (BEAS-2B-vectors) as controls. After exposure of above stable cell lines with Cr(VI) (0 or 0.5 μM) for 6 months, soft agar assay and clonogenic assay was performed as previously described. (C) Inhibition of in vivo tumor growth in nude mice with overexpressed antioxidant enzymes. After BEAS-2B-vector controls, BEAS-2B-CAT, and BEAS-2B-SOD2, cells were exposed to Cr(VI) (0 or 0.5 μM) for 6 months, xenograft growth of tumors in nude mice was performed as described previously. The data are expressed as the mean ± SD of three independent experiments. *p < 0.05, statistically significant difference from Cr(VI)-treated cells.
Discussion
Chromium is a potent human mutagen and carcinogen (Cancer and Cancer, 1990). Chromate Cr(VI) compounds, widely used in industries, such as leather tanning and wood treatment, cause environmental pollution and health concerns worldwide (Cohen et al., 1993; Costa, 1997). The capability of chromium to cause cancers has been known for more than a century, and numerous epidemiological studies have been performed on workers exposed to Cr(VI) to determine its carcinogenicity (Holmes et al., 2008; Xia et al., 2014). Occupational exposure to hexavalent chromium [Cr(VI)] has been associated with the development of several pathologies, notably lung cancer (Abreu et al., 2014). Phytomedicines have traditionally played a major role in the management of human health and are still important for health care in many countries (Kuttan et al., 2011). Chemoprevention by use of natural products has emerged as a promising medical approach to reduce the risk of cancer. Luteolin is a common dietary antioxidant flavonoid found in fruits, vegetables, and medicinal herbs (Pratheeshkumar et al., 2012b). Inhibition of metal induced carcinogenesis by a dietary antioxidant is a novel approach. Studies have demonstrated that co-treatment with Epigallocatechin-3-gallate (EGCG), the major polyphenol present in green tea, protected BEAS-2B cells from Cr(VI)-induced cell death in a dose-dependent manner (Wu et al., 2012).
Intracellular ROS are primarily generated through aerobic metabolism or through a specialized group of enzymes, known as the NADPH oxidases (Bedard and Krause, 2007). NADPH oxidase activity is associated with several characteristic features of cancer, including cellular transformation, cell proliferation, malignant cell survival, invasion, and metastasis (Maraldi et al., 2009; Block and Gorin, 2012; Liu et al., 2014). In particular, increases in NADPH oxidase activity are observed in human bronchial epithelial cells exposed to hexavalent chromium (Wang et al., 2011). Cr(VI) treatment also caused a significant increase in lipid peroxidation and decreases in total glutathione. Taken together these findings indicate that Cr(VI) causes oxidative stress in human bronchial epithelial cells. Similar results have been observed in previous studies involving Cr(VI) exposure (Ning and Grant, 2000; Ahmad et al., 2011). Our data demonstrate that treatment with luteolin significantly (p<0.05) attenuates acute Cr(VI)-induced ROS generation, NOX activation, lipid peroxidation, and glutathione depletion in BEAS-2B cells in a dose dependent manner. Furthermore, luteolin also decreased the chronic Cr(VI)-induced ROS generation (data not shown).
Inflammation is an immediate defensive mechanism or reaction to an injury, which may be caused by infection, chemical agents or physical trauma (Kuby, 1997). Inflammation can accelerate cancer and chronic inflammation and is regarded as an essential factor for the progression of the neoplastic process (Wiseman and Halliwell, 1996). Several cytokines such as Tumor necrosis factor α (TNF-α), Interleukin-6 (IL-6), Interlukin-1 (IL-1β), and Interleukin-8 (IL-8) play an important role in the inflammatory process (Pratheesh Kumar and Kuttan, 2009). High circulating levels of these pro-inflammatory cytokines were reported to be associated with lung cancer (Azad et al., 2008; Pine et al., 2011). As a part of this investigation, we studied the effect of luteolin on proinflammatory cytokine levels in BEAS-2B cells chronically exposed to Cr(VI). The results shows that luteolin significantly suppresses proinflammatory cytokine levels in BEAS-2B cells chronically exposed to Cr(VI).
In addition to inflammation, a variety of biological mediators have been implicated in cell transformation and/or tumor growth. COX-2 plays an important role in the development of various types of cancer, including lung cancer (Şahin et al., 2009); drugs targeting this enzyme have achieved widespread clinical use (Bertagnolli, 2007). Nitric oxide has been implicated in the induction of neoplastic cell transformation (Mordan et al., 1993; Robertson et al., 1996), and also with tumor angiogenesis by stimulating VEGF production (Konopka et al., 2001; Kisley et al., 2002). Expression of iNOS is regulated by transcription factors, including NF-κB (Stoner et al., 1989), AP-1 (Lee et al., 2003), and by the signal transducer and activator of transcription 1α (STAT1) (Tedeschi et al., 2003). In the present study, we observed that Cr(VI) exposure resulted in the activation of AP-1, COX-2, iNOS, and HIF-1 α, and is consistent with previously published studies showing that Cr(VI) exposure leads to the activation these proteins in an oxidative-stress–dependent manner (Gao et al., 2002; Li et al., 2006; Yao et al., 2008; Zuo et al., 2012). Treatment with luteolin markedly reduced the Cr(VI)-induced trans-activation of AP-1, COX-2, iNOS, and HIF-1 α in BEAS-2B cells.
The signal transducer and activator of transcription 3 (STAT3) is a transcription factor activated in response to cytokines and growth factors, and plays critical role in various biological activities including cell proliferation, migration, and carcinogenesis (Macias et al., 2013). STAT3 is constitutively activated in cells transformed by the oncogenes v-Src and v-Abl, and in a variety of human cancers, including hematologic, pancreas, breast, head and neck, and prostate cancer (Bowman et al., 2000; Macias et al., 2013). Moreover, STAT3 is upregulated in both premalignant tumors (papillomas) and squamous cell carcinomas of mouse skin induced by topical treatment with DMBA + TPA (Chan et al., 2004). Cr(VI)-induced transactivation of STAT3 has been reported in human airway epithelial cells (O’hara et al., 2007). Cr(VI) exposure in BEAS-2B cells increased Stat3 activation and phosphorylation whereas treatment of luteolin suppressed them. MAPKs are made up of three family members that include extracellular-signal-related protein kinases (ERKs), stress-activated c-JUN N-terminal (JNKs/SAPs) and p38 kinases (Einspahr et al., 2008). Previous studies have shown that Cr(VI) markedly induces MAP kinase pathways in CL3 cells and is positively correlated with oxidative stress (Chuang et al., 2000). Antioxidants have been shown to attenuate the activation of MAPK signaling, indicating that MAPK signaling pathway is an important target of oxidative stress (Ballif and Blenis, 2001; Katiyar et al., 2001; Sharma et al., 2007). Luteolin remarkably downregulated the Cr(VI) induced MAPK signaling in BEAS-2B cells. Chronic exposure to Cr(VI) increased anti-apoptotic proteins such as Bcl-2 and Bcl-XL, leading to apoptotic resistance and accumulation of genetically damaged cells (unpublished data). In our study luteolin dramatically suppressed the initiation of Cr(VI)-induced malignant transformation thereby attenuating the up regulation of anti-apoptotic proteins.
The transcription factor nuclear factor-κB (NF-κB) has been implicated in cancer development due to its ability to upregulate the expression of genes with pro-oncogenic functions (Pratheeshkumar et al., 2012a). NF-κB is inactive when cytoplasmic, and bound to the inhibitory proteins IκBs. Upon activation, IκB becomes phosphorylated, a process that targets it for ubiquitination and degradation by proteasomes. This activation results the rapid translocation of NF-κB to the nucleus, where it binds to κB binding sites in the promoter region of target genes, and induces the transcription of pro-inflammatory mediators, including iNOS, COX-2, TNF-α, IL-6 and others (Perkins, 1997; Pratheeshkumar and Kuttan, 2011c; Wang et al., 2012). Previous studies have shown that NF-κB activation occurs in responses to several environmental carcinogens (Ding et al., 2006; Ouyang et al., 2007). In addition, p65 was required for Cr(VI)-induced NF-κB activation and COX-2 expression (Zuo et al., 2012). Several chemopreventive phytochemicals have been shown to inhibit COX-2 and iNOS expression by blocking NF-κB activation (Singh and Aggarwal, 1995; Lyss et al., 1997; Surh et al., 2001). It has been documented that NF-κB is a downstream target of the MAPK signal transduction pathway, and that activation of NF-κB plays crucial roles in inflammation, cellular proliferation, and induction of cancers (Sharma et al., 2007). The present study shows that co-treatment of luteolin markedly decreased the Cr(VI)-induced degradation of IκBα and activation of NF-κB/p65 in BEAS-2B cells.
Increased expression of matrix metalloproteinases (MMPs) has been strongly implicated in tumor growth, invasion, and metastasis (Sounni et al., 2003; Pratheeshkumar and Kuttan, 2011a). Among diverse proteolytic enzymes, gelatinases such as MMP-2 and MMP-9 play a key role in degrading ECM components surrounding tumour tissue (Davidson et al., 2003). Accordingly, inhibition of angiogenesis is an attractive approach to treat cancer (Carmeliet and Jain, 2000). Hypoxia-inducible factor 1 alpha (HIF-1alpha) is involved in processes promoting carcinogenesis of many tumors (Simiantonaki et al., 2008a). In addition, HIF-1α is overexpressed in 70% of human cancers and their metastases. One of the genes up-regulated by HIF-1 is VEGF, a potent mediator of angiogenesis that enhances endothelial cell survival and invasion and also induces vasodilatation (Pratheeshkumar and Kuttan, 2011b). Previous studies have demonstrated the elevated expressions of HIF-1α, VEGF and MMP-9 by Cr(VI) exposure (Gao et al., 2002; Son et al., 2013). In our present study chronic Cr(VI) exposure elevated the expressions of HIF-1α, VEGF and MMP-9 and these were markedly suppressed by the treatment of luteolin in BEAS-2B cells.
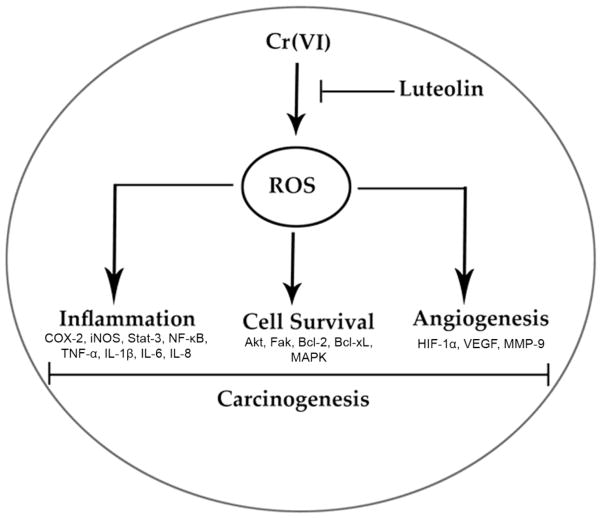
In summary, our findings show that luteolin inhibited Cr(VI)-induced ROS generation, NOX activation, lipid peroxidation, and glutathione depletion in a dose dependent manner. Moreover, treatment with luteolin decreased AP-1, HIF-1α, COX-2, and iNOS promoter activity induced by Cr(VI) in BEAS-2B cells. Luteolin protected normal human bronchial epithelium from malignant transformation, production of pro-inflammatory cytokines and VEGF induced by chronic Cr(VI) exposure. Stable overexpression of SOD2, or CAT in BEAS-2B cells attenuated the colony and tumor formation induced by chronic Cr(VI) exposure, suggesting an important role for ROS in Cr(VI) induced carcinogenesis. Western blot analysis showed that luteolin inhibited multiple gene products linked to malignant cell survival (Akt, Fak, Bcl-2, Bcl-xL), inflammation (MAPK, NF-κB, COX-2, STAT-3, iNOS, TNF-α) and angiogenesis (HIF-1α, VEGF, MMP-9) in BEAS-2B cells after chronic Cr(VI) exposure (Fig. 11). Nude mice injected with BEAS-2B cells chronically exposed to Cr(VI) in the presence of luteolin showed reduced tumor incidence compared to Cr(VI) treatment alone. Overall, our results indicate that luteolin protects BEAS-2B cells from Cr(VI)-induced carcinogenesis by scavenging ROS and modulating multiple cell signaling mechanisms that are linked to ROS. Luteolin, therefore, serves as a potential chemopreventive agent against Cr(VI) induced carcinogenesis.
Figure 11.
Highlights.
Luteolin inhibited Cr(VI)-induced oxidative stress.
Luteolin inhibited chronic Cr(VI)-induced malignant transformation.
Luteolin inhibited chronic Cr(VI)-induced inflammation.
Luteolin inhibited chronic Cr(VI)-induced angiogenesis.
Acknowledgments
This research was supported by National Institutes of Health (R01ES017244, R01ES015518, R01ES020870).
ABBREVIATIONS
- COX-2
cyclooxygenase-2
- IL
interleukin
- TNF-α
tumor necrosis factor-α
- iNOS
inducible nitric oxide synthase
- AP-1
activator protein 1
- HIF-1α
Hypoxia-inducible factor 1-alpha
- VEGF
Vascular endothelial growth factor
- MMP-9
Matrix metalloproteinase-9
- STAT-3
signal transducer and activator of transcription 3
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu P, Ferreira L, Alpoim M, Urbano A. Impact of hexavalent chromium on mammalian cell bioenergetics: phenotypic changes, molecular basis and potential relevance to chromate-induced lung cancer. BioMetals. 2014;27:409–443. doi: 10.1007/s10534-014-9726-7. [DOI] [PubMed] [Google Scholar]
- Ahmad MK, Syma S, Mahmood R. Cr (VI) induces lipid peroxidation, protein oxidation and alters the activities of antioxidant enzymes in human erythrocytes. Biological trace element research. 2011;144:426–435. doi: 10.1007/s12011-011-9119-5. [DOI] [PubMed] [Google Scholar]
- Anand SS. Protective effect of vitamin B6 in chromium-induced oxidative stress in liver. Journal of Applied Toxicology. 2005;25:440–443. doi: 10.1002/jat.1077. [DOI] [PubMed] [Google Scholar]
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. Journal of Toxicology and Environmental Health, Part B. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell growth and differentiation. 2001;12:397–408. [PubMed] [Google Scholar]
- Barbieri SS, Eligini S, Brambilla M, Tremoli E, Colli S. Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: critical role of NADPH oxidase. Cardiovascular research. 2003;60:187–197. doi: 10.1016/s0008-6363(03)00365-1. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer detection and prevention. 2004;28:385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicology and applied pharmacology. 2009a;235:47–56. doi: 10.1016/j.taap.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, Patierno SR. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environmental health perspectives, 1896–1902. 2009b doi: 10.1289/ehp.0900715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. The lancet oncology. 2007;8:439–443. doi: 10.1016/S1470-2045(07)70139-0. [DOI] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1α and HIF-2α expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Research. 2000;60:7106–7113. [PubMed] [Google Scholar]
- Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nature Reviews Cancer. 2012;12:627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- Bouraoui Y, Ricote M, García-Tuñón I, Rodriguez-Berriguete G, Touffehi M, Rais NB, Fraile B, Paniagua R, Oueslati R, Royuela M. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer detection and prevention. 2008;32:23–32. doi: 10.1016/j.cdp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Cancer, I.A.f.R.o., Cancer, I.A.f.R.o. Chromium, nickel and welding. IARC monographs on the evaluation of carcinogenic risks to humans. 1990:49. [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Minna JD. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer research. 1980;40:1820–1823. [PubMed] [Google Scholar]
- Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. The Journal of clinical investigation. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan Y, Li J, Ma Q, Stoner GD, Ye J, Huang C. Differential requirement of signal pathways for benzo [a] pyrene (B [a] P)-induced nitric oxide synthase (iNOS) in rat esophageal epithelial cells. Carcinogenesis. 2005;26:1035–1043. doi: 10.1093/carcin/bgi052. [DOI] [PubMed] [Google Scholar]
- Chen L, Ovesen JL, Puga A, Xia Y. Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ Health Perspect. 2009;117:1124–1130. doi: 10.1289/ehp.0800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium (VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. CRC Critical Reviews in Toxicology. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Costa M. Toxicity and carcinogenicity of Cr (VI) in animal models and humans. CRC Critical Reviews in Toxicology. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R. Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer and Metastasis Reviews. 2003;22:103–115. doi: 10.1023/a:1022272204045. [DOI] [PubMed] [Google Scholar]
- Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, Castranova V, Jiang BH, Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. Journal of Biological Chemistry. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- Ding SZ, Yang YX, Li XL, Michelli-Rivera A, Han SY, Wang L, Pratheeshkumar P, Wang X, Lu J, Yin YQ. Epithelial–mesenchymal transition during oncogenic transformation induced by hexavalent chromium involves reactive oxygen species-dependent mechanism in lung epithelial cells. Toxicology and applied pharmacology. 2013;269:61–71. doi: 10.1016/j.taap.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr JG, Timothy Bowden G, Alberts DS, McKenzie N, Saboda K, Warneke J, Salasche S, Ranger-Moore J, Curiel-Lewandrowski C, Nagle RB. Cross-validation of Murine UV Signal Transduction Pathways in Human Skin†. Photochemistry and photobiology. 2008;84:463–476. doi: 10.1111/j.1751-1097.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Digestive Diseases. 2010;28:579–584. doi: 10.1159/000320052. [DOI] [PubMed] [Google Scholar]
- Fruehauf JP, Meyskens FL. Reactive oxygen species: a breath of life or death? Clinical Cancer Research. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- Gao N, Jiang BH, Leonard SS, Corum L, Zhang Z, Roberts JR, Antonini J, Zheng JZ, Flynn DC, Castranova V. p38 Signaling-mediated hypoxia-inducible factor 1α and vascular endothelial growth factor induction by Cr (VI) in DU145 human prostate carcinoma cells. Journal of Biological Chemistry. 2002;277:45041–45048. doi: 10.1074/jbc.M202775200. [DOI] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nature Reviews Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakozaki M, Tajino T, Konno S, Kikuchi S, Yamada H, Yanagisawa M, Nishida J, Nagasawa H, Tsuchiya T, Ogose A. Overexpression of Cyclooxygenase-2 in Malignant Peripheral Nerve Sheath Tumor and Selective Cyclooxygenase-2 Inhibitor-Induced Apoptosis by Activating Caspases in Human Malignant Peripheral Nerve Sheath Tumor Cells. PloS one. 2014;9:e88035. doi: 10.1371/journal.pone.0088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. The Journal of nutrition. 2006;136:1517–1521. doi: 10.1093/jn/136.6.1517. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise S, Wise JP., Sr Carcinogenicity of hexavalent chromium. Indian Journal of Medical Research. 2008;128:353. [PubMed] [Google Scholar]
- Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicology and Applied Pharmacology. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- Khodr B, Khalil Z. Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radical Biology and Medicine. 2001;30:1–8. doi: 10.1016/s0891-5849(00)00378-6. [DOI] [PubMed] [Google Scholar]
- Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer research. 2002;62:6850–6856. [PubMed] [Google Scholar]
- Konopka TE, Barker JE, Bamford TL, Guida E, Anderson RL, Stewart AG. Nitric oxide synthase II gene disruption implications for tumor growth and vascular endothelial growth factor production. Cancer research. 2001;61:3182–3187. [PubMed] [Google Scholar]
- Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E, Harris AL. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. Journal of Clinical Oncology. 2006;24:727–735. doi: 10.1200/JCO.2005.02.7474. [DOI] [PubMed] [Google Scholar]
- Kuby J. Immunology, 1997. WH Freeman and Company; New York: 1997. [Google Scholar]
- Kuttan G, Pratheeshkumar P, Manu KA, Kuttan R. Inhibition of tumor progression by naturally occurring terpenoids. Pharmaceutical biology. 2011;49:995–1007. doi: 10.3109/13880209.2011.559476. [DOI] [PubMed] [Google Scholar]
- Lee JK, Choi SS, Won JS, Suh HW. The regulation of inducible nitric oxide synthase gene expression induced by lipopolysaccharide and tumor necrosis factor-α in C6 cells: involvement of AP-1 and NFκB. Life sciences. 2003;73:595–609. doi: 10.1016/s0024-3205(03)00317-5. [DOI] [PubMed] [Google Scholar]
- Li J, Song L, Zhang D, Wei L, Huang C. Knockdown of NFAT3 blocked TPA-induced COX-2 and iNOS expression, and enhanced cell transformation in Cl41 cells. Journal of cellular biochemistry. 2006;99:1010–1020. doi: 10.1002/jcb.20834. [DOI] [PubMed] [Google Scholar]
- Liu L, Rezvani HR, Back JH, Hosseini M, Tang X, Zhu Y, Mahfouf W, Raad H, Raji G, Athar M. Inhibition of p38 MAPK Signaling Augments Skin Tumorigenesis via NOX2 Driven ROS Generation. PloS one. 2014;9:e97245. doi: 10.1371/journal.pone.0097245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyss G, Schmidt TJ, Merfort I, Pahl HL. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. Biological chemistry. 1997;378:951–962. doi: 10.1515/bchm.1997.378.9.951. [DOI] [PubMed] [Google Scholar]
- Macias E, Rao D, DiGiovanni J. Role of STAT3 in skin carcinogenesis: insights gained from relevant mouse models. Journal of skin cancer. 2013;2013 doi: 10.1155/2013/684050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi T, Prata C, Vieceli Dalla Sega F, Caliceti C, Zambonin L, Fiorentini D, Hakim G. NAD (P) H oxidase isoform Nox2 plays a prosurvival role in human leukaemia cells. Free radical research. 2009;43:1111–1121. doi: 10.1080/10715760903186132. [DOI] [PubMed] [Google Scholar]
- Mordan LJ, Burnett TS, Zhang LX, Tom J, Cooney RV. Inhibitors of endogenous nitrogen oxide formation block the promotion of neoplastic transformation in C3H 10T1/2 fibroblasts. Carcinogenesis. 1993;14:1555–1559. doi: 10.1093/carcin/14.8.1555. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Matsubara T, Kinoshita I, Tsuchiya E, Sugano H, Hirano T. Surveillance study of a group of chromate workers—early detection and high incidence of lung cancer. Lung Cancer. 1984;24:301–310. [Google Scholar]
- Ning J, Grant M. The role of reduced glutathione and glutathione reductase in the cytotoxicity of chromium (VI) in osteoblasts. Toxicology in vitro. 2000;14:329–335. doi: 10.1016/s0887-2333(00)00024-2. [DOI] [PubMed] [Google Scholar]
- O’hara K, Vaghjiani R, Nemec A, Klei L, Barchowsky A. Cr (VI)-stimulated STAT3 tyrosine phosphorylation and nuclear translocation in human airway epithelial cells requires Lck. Biochem J. 2007;402:261–269. doi: 10.1042/BJ20061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Sanda T, Asamitsu K. NF-κB signaling and carcinogenesis. Current pharmaceutical design. 2007;13:447–462. doi: 10.2174/138161207780162944. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Ma Q, Li J, Zhang D, Ding J, Huang Y, Xing M, Huang C. Benzo [a] pyrene diol-epoxide (B [a] PDE) upregulates COX-2 expression through MAPKs/AP-1 and IKKβ/NF-κB in mouse epidermal Cl41 cells. Molecular carcinogenesis. 2007;46:32–41. doi: 10.1002/mc.20260. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Achieving transcriptional specificity with NF-κB. The international journal of biochemistry & cell biology. 1997;29:1433–1448. doi: 10.1016/s1357-2725(97)00088-5. [DOI] [PubMed] [Google Scholar]
- Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer Institute. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheesh Kumar P, Kuttan G. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model. Immunopharmacology and immunotoxicology. 2009;31:94–102. doi: 10.1080/08923970802438391. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. European journal of pharmacology. 2011a;668:450–458. doi: 10.1016/j.ejphar.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Vernolide-A inhibits radiation-induced hypoxia-mediated tumor angiogenesis by regulating HIF-1α, MMP-2, MMP-9, and VEGF. Journal of Environmental Pathology, Toxicology and Oncology. 2011b;30 doi: 10.1615/jenvironpatholtoxicoloncol.v30.i2.50. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Kuttan G. Vernolide-A, a sesquiterpene lactone from Vernonia cinerea, induces apoptosis in B16F-10 melanoma cells by modulating p53 and caspase-3 gene expressions and regulating NF-κB-mediated bcl-2 activation. Drug and chemical toxicology. 2011c;34:261–270. doi: 10.3109/01480545.2010.520017. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Raphael TJ, Kuttan G. Nomilin inhibits metastasis via induction of apoptosis and regulates the activation of transcription factors and the cytokine profile in B16F-10 cells. Integrative cancer therapies. 2011 doi: 10.1177/1534735411403307. 1534735411403307. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Sheeja K, Kuttan G. Andrographolide induces apoptosis in B16F-10 melanoma cells by inhibiting NF-κB-mediated bcl-2 activation and modulating p53-induced caspase-3 gene expression. Immunopharmacology and immunotoxicology. 2012a;34:143–151. doi: 10.3109/08923973.2011.588233. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Son YO, Budhraja A, Wang X, Ding S, Wang L, Hitron A, Lee JC, Kim D, Divya SP. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PloS one. 2012b;7:e52279. doi: 10.1371/journal.pone.0052279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheeshkumar P, Sreekala C, Zhang Z, Budhraja A, Ding S, Son YO, Wang X, Hitron A, Hyun-Jung K, Wang L. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2012c;12:1159–1184. doi: 10.2174/187152012803833035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reliene R, Schiestl RH. Glutathione depletion by buthionine sulfoximine induces DNA deletions in mice. Carcinogenesis. 2006;27:240–244. doi: 10.1093/carcin/bgi222. [DOI] [PubMed] [Google Scholar]
- Robertson FM, Long BW, Tober KL, Ross MS, Oberyszyn TM. Gene expression and cellular sources of inducible nitric oxide synthase during tumor promotion. Carcinogenesis. 1996;17:2053–2059. doi: 10.1093/carcin/17.9.2053. [DOI] [PubMed] [Google Scholar]
- Şahin M, Şahin E, Gümüşlü S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology. 2009;60:242–253. doi: 10.1177/0003319708318378. [DOI] [PubMed] [Google Scholar]
- Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Molecular cancer therapeutics. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- Shi X, Ding M, Ye J, Wang S, Leonard SS, Zang L, Castranova V, Vallyathan V, Chiu A, Dalal N. Cr (IV) causes activation of nuclear transcription factor-κB, DNA strand breaks and dG hydroxylation via free radical reactions. Journal of inorganic biochemistry. 1999;75:37–44. doi: 10.1016/S0162-0134(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Simiantonaki N, Jayasinghe C, Michel-Schmidt R, Peters K, Hermanns MI, Kirkpatrick CJ. Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines. International journal of oncology. 2008a;32:585–592. [PubMed] [Google Scholar]
- Simiantonaki N, Taxeidis M, Jayasinghe C, Kurzik-Dumke U, Kirkpatrick CJ. Hypoxia-inducible factor 1 alpha expression increases during colorectal carcinogenesis and tumor progression. BMC cancer. 2008b;8:320. doi: 10.1186/1471-2407-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane) Journal of Biological Chemistry. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Son YO, Pratheeshkumar P, Wang L, Wang X, Fan J, Kim DH, Lee JY, Zhang Z, Lee JC, Shi X. Reactive oxygen species mediate Cr (VI)-induced carcinogenesis through PI3K/AKT-dependent activation of GSK-3β/β-catenin signaling. Toxicology and applied pharmacology. 2013;271:239–248. doi: 10.1016/j.taap.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix biology. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Babcock MS, Mc Corquodale MM, Gunning WT, III, Jamasbi R, Budd N, Hukku B. Comparative properties of untreated andN-nitrosobenzylmethyl-amine-transformed rat esophageal epithelial cell lines. In vitro cellular & developmental biology. 1989;25:899–908. doi: 10.1007/BF02624002. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2001;480:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radical Biology and Medicine. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Tedeschi E, Menegazzi M, Margotto D, Suzuki H, Förstermann U, Kleinert H. Anti-inflammatory actions of St. John’s wort: inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1α (STAT-1α) activation. Journal of pharmacology and experimental therapeutics. 2003;307:254–261. doi: 10.1124/jpet.103.054460. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Proctor DM, Haws LC, Hébert CD, Grimes SD, Shertzer HG, Kopec AK, Hixon JG, Zacharewski TR, Harris MA. Investigation of the mode of action underlying the tumorigenic response induced in B6C3F1 mice exposed orally to hexavalent chromium. Toxicological Sciences. 2011 doi: 10.1093/toxsci/kfr164. kfr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD (P) H oxidase. Molecular and cellular biochemistry. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- Vaupel P. The role of hypoxia-induced factors in tumor progression. The oncologist. 2004;9:10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- Wakeman TP, Wyczechowska D, Xu B. Involvement of the p38 MAP kinase in Cr (VI)-induced growth arrest and apoptosis. Molecular and cellular biochemistry. 2005;279:69–73. doi: 10.1007/s11010-005-8216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jr, Sheu HM, Guo YL, Lee YH, Lai CS, Pan MH, Wang YJ. Hexavalent chromium induced ROS formation, Akt, NF-κB, and MAPK activation, and TNF-α and IL-1α production in keratinocytes. Toxicology letters. 2010;198:216–224. doi: 10.1016/j.toxlet.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Wang QS, Xiang Y, Cui YL, Lin KM, Zhang XF. Dietary blue pigments derived from genipin, attenuate inflammation by inhibiting LPS-induced iNOS and COX-2 expression via the NF-κB inactivation. PloS one. 2012;7:e34122. doi: 10.1371/journal.pone.0034122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z, Chen F, Luo J. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicological Sciences. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiming X, Li Zhi L, Marilena LOIZIDOU MA, Ian GC. The role of nitric oxide in cancer. Cell research. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Sun H, Kluz T, Clancy HA, Kiok K, Costa M. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity< i> in vitro. Toxicology and applied pharmacology. 2012;258:166–175. doi: 10.1016/j.taap.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Ren X-h, Zhuang Z-x, Yang L-q, Huang H-y, Pang L, Wu D-s, Luo J, Tan Y-l, Liu J-j. Effect of hexavalent chromium on histone biotinylation in human bronchial epithelial cells. Toxicology letters. 2014;228:241–247. doi: 10.1016/j.toxlet.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Yao H, Guo L, Jiang B-H, Luo J, Shi X. Oxidative stress and chromium (VI) carcinogenesis. Journal of Environmental Pathology. Toxicology and Oncology. 2008;27 doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wu W, Li D, Xu T, Zhu H, Pan D, Zhu S, Liu Y. Anti-oxidant and anti-apoptotic effects of luteolin on mice peritoneal macrophages stimulated by angiotensin II. International immunopharmacology. 2014;20:346–351. doi: 10.1016/j.intimp.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Gan FF, Shelar SB, Ng KY, Chew EH. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in< i> Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food and Chemical Toxicology. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
- Zheng S, Ma Z, Han H, Ye J, Wang R, Cai S, Zhou H, Yu L, Zeng S, Jiang H. Post-column mobile phase adjustment: A strategy to eliminate the contradiction between liquid chromatography and mass spectrometry in the determination of flavonoids in rat plasma. Journal of pharmaceutical and biomedical analysis. 2014;95:176–183. doi: 10.1016/j.jpba.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Cai T, Li J, Zhang D, Yu Y, Huang C. Hexavalent chromium Cr (VI) up-regulates COX-2 expression through an NF kappa B/c-Jun/AP-1-dependent pathway. Environmental health perspectives. 2012;120:547–553. doi: 10.1289/ehp.1104179. [DOI] [PMC free article] [PubMed] [Google Scholar]