Abstract
Due to the frequent occurrence of cyclopentane subunits in bioactive compounds, the development of efficient catalytic asymmetric methods for their synthesis is an important objective. In this report, we introduce a new family of chiral nucleophilic catalysts, biphenyl-derived phosphepines, and we apply them to an enantioselective variant of a useful [4+1] annulation first described by Tong. A range of one-carbon coupling partners can be employed, thereby generating cyclopentenes that bear a fully substituted stereocenter (either all-carbon or heteroatom-substituted (sulfur and phosphorus)). Stereocenters at the other four positions of the cyclopentane ring can also be introduced with good stereoselectivity. An initial mechanistic study indicates that phosphine addition to the electrophilic four-carbon coupling partner is not the turnover-limiting step of the catalytic cycle.
Keywords: annulation, asymmetric catalysis, atropisomerism, organocatalysis, phosphane
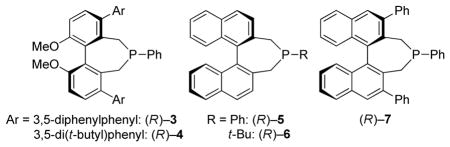
During the past decade, chiral tertiary phosphines have increasingly been employed not only as ligands in transition-metal-catalyzed reactions,[1] but also as enantioselective nucleophilic catalysts for a broad spectrum of useful transformations.[2,3] Studies by our lab and by others have established that 1,1′-binaphthyl-derived phosphepines (e.g., 1) are effective for a diverse array of interesting processes;[4,5,6] in contrast, to the best of our knowledge, there have been no applications of axially chiral biphenyl-derived phosphepines (e.g., 2) as asymmetric nucleophilic catalysts.[7]
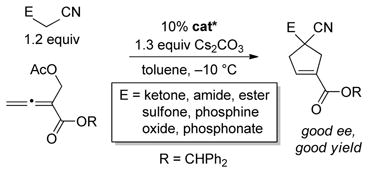
The use of chiral phosphines as nucleophilic catalysts for the synthesis of five-membered rings has been a focus of particular interest,[8] due in part to the occurrence of cyclopentane subunits in a wide range of bioactive molecules,[9] and Lu’s powerful methods for convergent [3+2] annulation have been especially well-explored.[10,11] With regard to the enantioselective construction of quaternary stereocenters,[12] nearly all reports have described the generation of spirocyclic structures.[8,13,14]
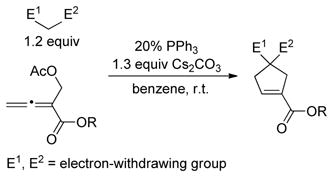
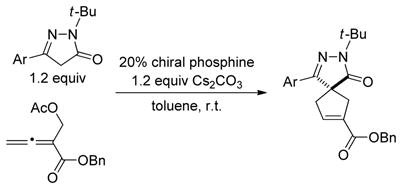
One complication in some of these [3+2] reactions is the formation of regioisomeric products.[10] In 2010, Tong described a complementary [4+1] annulation, which combines structural features of the three-carbon partners in Lu’s [3+2] reactions (allenoates and Morita–Baylis–Hillman derivatives) in a four-carbon partner [(Eq. 1)].[15] Such a [4+1] approach obviates the formation of regioisomeric products.[16,17]
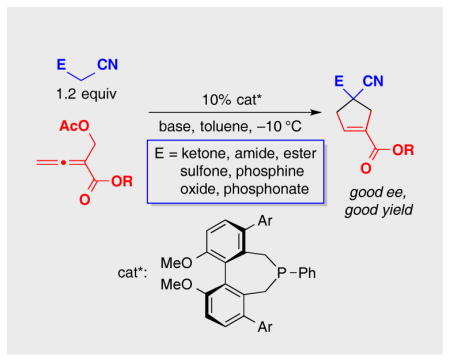
As part of an ongoing program to demonstrate the utility and the versatility of asymmetric nucleophilic catalysis,[18] we embarked on the development of an enantioselective variant of this [4+1] annulation. At the point that we completed our study,[19] we were not aware of any progress by others toward this objective. However, this year Lu has described asymmetric reactions that furnish spirocyclic products in good ee [(Eq. 2)],[20] which has prompted us to disclose our independent investigation. Herein, we report the first use of biphenyl-derived phosphepines (3 and 4) as enantioselective nucleophilic catalysts, specifically, their application in [4+1] annulations that generate cyclopentenes that bear non-spirocyclic, fully substituted stereocenters (either all-carbon or heteroatom-substituted; Eq. 3).[21,22]
 |
(1) |
 |
(2) |
 |
(3) |
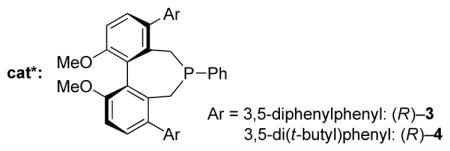
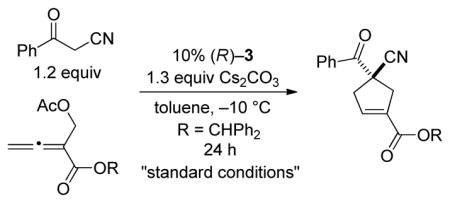
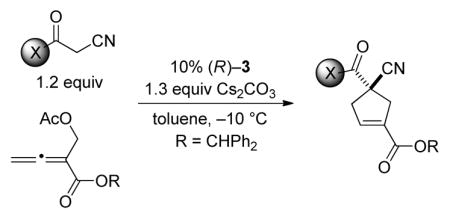
Upon determining that an array of chiral 1,1′-binaphthyl-derived phosphepines that we have reported to be useful in other nucleophile-catalyzed processes[5] are not sufficiently effective in a model [4+1] annulation (the coupling of benzoylacetonitrile with an allenoate; Table 1, entries 1–3), we chose to synthesize and examine the utility of biphenyl-derived phosphepines. We were pleased to determine that new chiral phosphines 3 and 4 afford the desired cyclopentene bearing a quaternary stereocenter in high ee and yield (entries 4 and 5).[23] If the catalyst loading is decreased to 5%, the reaction does not proceed to completion after 24 hours (entry 6). The annulation is also slower in the absence of Cs2CO3 and when Cs2CO3 is replaced with 2,6-lutidine (entries 7 and 8). Slightly lower enantioselectivity and/or yield are observed at room temperature or when a different ester is attached to the allene (benzyl rather than benzhydryl; entries 9 and 10). The presence of two equivalents of water is deleterious for the coupling (entry 11), whereas adventitious moisture and oxygen have a relatively small impact (entry 12).[24]
Table 1.
Phosphepine-Catalyzed Enantioselective [4+1] Annulation: Effect of Reaction Parametersa
| |||
|---|---|---|---|
| entry | change from the “standard conditions” | ee (%)b | yield (%)c |
| 1 | (R)–5, instead of (R)–3 | −38 | 70 |
| 2 | (R)–6, instead of (R)–3 | 6 | 26 |
| 3 | (R)–7, instead of (R)–3 | 76 | >95 |
| 4 | none | 94 | >95 |
| 5 | (R)–4, instead of (R)–3 | 91 | 92 |
| 6 | 5% (R)–3 | 93 | 64 |
| 7 | no Cs2CO3 | 92 | 32 |
| 8 | 2,6-lutidine, instead of Cs2CO3 | 92 | 56 |
| 9 | r.t. | 89 | >95 |
| 10 | R = Bn, instead of CHPh2 | 89 | 88 |
| 11 | 2.0 equiv of added water | 82 | 40 |
| 12 | under air, instead of nitrogen | 93 | 90 |
All data are the average of two experiments.
A negative ee value signifies that the major product of the reaction is the (R) enantiomer.
The yield was determined through the use of 1H NMR spectroscopy, with CH2Br2 as an internal standard.
Next, we investigated the scope of this new method, catalyzed by phosphepine 3, for the enantioselective synthesis of functionalized cyclopentenes that bear an all-carbon quaternary stereocenter (Table 2).[25] A variety of α-cyano ketones are suitable nucleophilic coupling partners, furnishing the annulation product in good ee and yield. In the case of aromatic ketones, the aryl ring can be electron-rich or electron-poor (entries 2 and 3). A thienyl ketone can be employed as a reactant (entry 4), as can alkyl ketones with alkyl groups that range from primary to tertiary (entries 5–7). The scope of the process is not limited to α-cyano ketones–both α-cyano amides (including a diphenyl amide and a Weinreb amide) and an α-cyano ester serve as useful substrates (entries 8–11).[26]
Table 2.
α-Cyano Ketones, Amides, and Esters as Nucleophiles in Phosphepine-Catalyzed Enantioselective [4+1] Annulationsa
| ||||
|---|---|---|---|---|
| entry |

|
ee (%) | yield (%)b | |
| 1 |
|
R1 = H | 94 | 85 |
| 2 | OMe | 94 | 97 | |
| 3 | CO2Me | 94 | 83 | |
| 4c |
|
90 | 88 | |
| 5 |
|
93 | 90 | |
| 6 |
|
93 | 96 | |
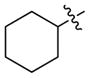
| 7 | t-Bu | 94 | 75 | |
| 8 | NMe2 | 83 | 73 | |
| 9 | NPh2 | 91 | 93 | |
| 10 |
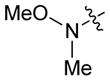
|
86 | 73 | |
| 11 |
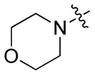
|
88 | 86 | |
| 12d | OMe | 82 | 61 | |
All data are the average of two experiments.
Yield of purified product.
Reaction was run at 0 °C.
Reaction was run with 20% (R)–3.
However, when we applied this method with either an α-cyano sulfone or an α-cyano phosphonate as the nucleophilic partner, the target cyclopentene was produced with moderate enantioselectivity (<80% ee). On the other hand, by employing a related biphenyl-derived phosphepine (4) as the chiral catalyst, we were able to achieve asymmetric [4+1] annulations with improved ee, thereby enabling the efficient synthesis of enantioenriched α-cyano sulfones,[27] phosphine oxides, and phosphonates (Table 3).
Table 3.
α-Cyano Sulfones, Phosphine Oxides, and Phosphonates as Nucleophiles in Phosphepine-Catalyzed Enantioselective [4+1] Annulationsa
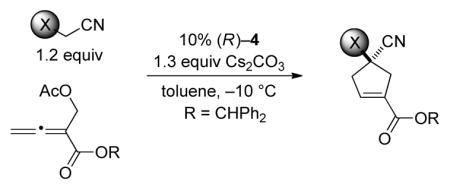
| ||||
|---|---|---|---|---|
| entry |

|
ee (%) | yield (%)b | |
| 1 |
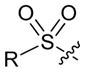
|
R = Ph | 89 | 96 |
| 2 | Cy | 86 | 89 | |
| 3 | t-Bu | 94 | 97 | |
| 4 |
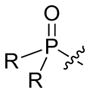
|
R = Ph | 84 | 86 |
| 5 | Oph | 87 | 88 | |
All data are the average of two experiments.
Yield of purified product.
The cyclopentene products of these catalytic asymmetric [4+1] annulations are well-poised for the introduction of additional stereocenters on the five-membered ring. For example, dihydroxylation and epoxidation of the illustrated α-cyano sulfone affords two new stereocenters with high diastereoselectivity [(Eqs. 4 and 5)].
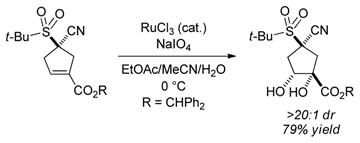 |
(4) |
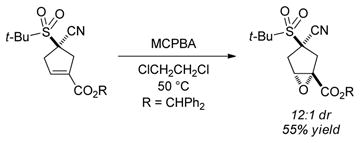 |
(5) |
The ability to achieve enantioselective [4+1] annulations of allenoates that include a substituent in the β′ position would add an important dimension to the scope of this method, as the product would bear contiguous quaternary and tertiary stereocenters. In his original report, Tong provided one example of a PPh3-catalyzed reaction of a β′-substituted allenoate (with benzoylacetonitrile; 2:1 dr).[15] In a preliminary study, we have obtained a promising lead with an α-cyano sulfone as the nucleophile [(Eq. 6)].[28]
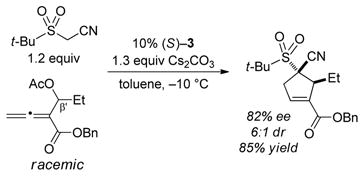 |
(6) |
To the best our knowledge, Tong [4+1] annulations have not previously been described in the case of allenes that bear a γ substituent. We were therefore pleased to observe that phosphepine 3 not only catalyzes the reaction of such a partner, it also furnishes the desired cyclopentene in good ee, diastereoselectivity, and yield [(Eq. 7)].
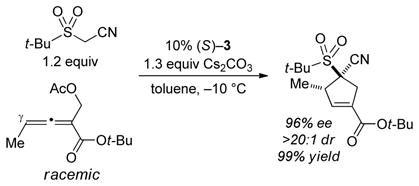 |
(7) |
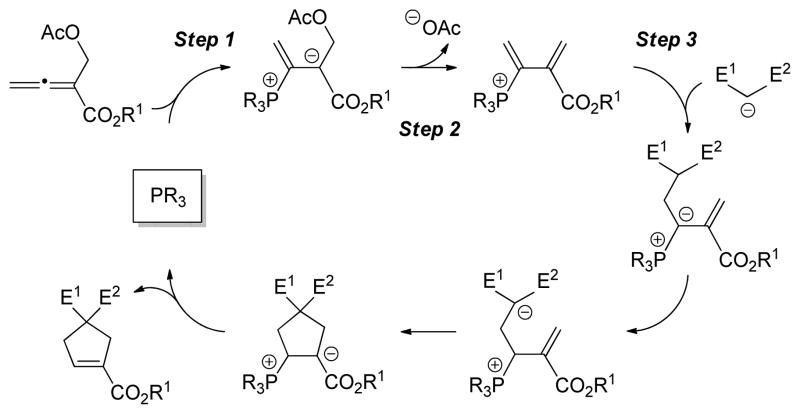
Tong has suggested that phosphine-catalyzed [4+1] annulations may proceed through the pathway outlined in Scheme 1.[15] In order to gain insight into the mechanism of the reaction catalyzed by (R)–3, we determined the rate law for the coupling illustrated in Table 1; this study was conducted at room temperature without Cs2CO3 (under these conditions, the annulation proceeds in 89% ee and >95% yield), due to the poor solubility of Cs2CO3 in toluene. The rate law for this process is first order in catalyst and zeroth order in the nucleophile and the allenoate. Furthermore, according to 31P NMR spectroscopy, the primary resting state of the phosphine during a catalytic asymmetric annulation is not the free phosphine, but instead a phosphonium salt. Taken together, these data are consistent with the suggestion that elementary steps such as Steps 1 and 3 (Scheme 1) are unlikely to be turnover-limiting.
Scheme 1.
Outline of a Possible Pathway for Phosphine-Catalyzed [4+1] Annulations (Tong)
In summary, we have established for the first time that biphenyl-derived axially chiral phosphepines can serve as useful enantioselective nucleophilic catalysts. Specifically, we have applied these phosphines to asymmetric [4+1] annulations that provide functionalized cyclopentenes in good ee and yield. An array of either all-carbon quaternary stereocenters or fully substituted heteroatom-bearing stereocenters can be generated in this process from readily available nucleophilic partners. The synthesis of even more highly functionalized/stereochemically rich products is possible through the use of substituted allenoates or through diastereoselective reactions of the product cyclopentenes. Determination of the rate law for a [4+1] annulation catalyzed by phosphepine 3 suggests that the turnover-limiting step occurs after the addition of the chiral phosphine to the allenoate, and likely after the addition of the second coupling partner as well. Additional studies directed at expanding the scope of enantioselective nucleophilic catalysis by chiral phosphines are underway.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM57034), EMD Serono (fellowship support for D.T.Z.), the Spanish MICINN (fellowship support for L.R.), Daiichi Sankyo Co., Ltd (fellowship support for T.I.), and Dainippon Sumitomo Pharma Co., Ltd. (fellowship support for Y.F.). We thank Trixia Buscagan, Dr. Søren Kramer, Dr. Allen Oliver (University of Notre Dame), Dr. Nathan D. Schley, Dr. Michael K. Takase, Dr. David VanderVelde, Dr. Scott C. Virgil, and Dr. Ashraf Wilsily for assistance and for helpful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.For example, see: Börner A, editor. Phosphorus Ligands in Asymmetric Catalysis. Wiley–VCH; New York: 2008.
- 2.For pioneering studies, see: Vedejs E, Daugulis O, Diver ST. J Org Chem. 1996;61:430–431. doi: 10.1021/jo951661v.Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. J Am Chem Soc. 1997;119:3836–3837.
- 3.For reviews, see: Fan YC, Kwon O. Science of Synthesis. Asymmetric Organocatalysis. 2012;1:723–782.Zhou Z, Wang Y, Tang C. Curr Org Chem. 2011;15:4083–4107.Marinetti A, Voituriez A. Synlett. 2010:174–194.Gröger H, Burda E. In: In Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 3. Wiley–VCH; New York: 2008. pp. 1175–1197.
- 4.These phosphepines were originally developed as ligands for transition-metal-catalyzed processes: Gladiali S, Dore A, Fabbri D, De Lucchi O, Manassero M. Tetrahedron: Asymmetry. 1994;5:511–514.Junge K, Hagemann B, Enthaler S, Oehme G, Michalik M, Monsees A, Riermeier T, Dingerdissen U, Beller M. Angew Chem Int Ed. 2004;43:5066–5069. doi: 10.1002/anie.200460190.Gladiali S, Alberico E, Junge K, Beller M. Chem Soc Rev. 2011;40:3744–3763. doi: 10.1039/c0cs00164c.
- 5.For initial reports of the use of these phosphepines as chiral nucleophilic catalysts, see: Wurz RP, Fu GC. J Am Chem Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d.Wilson JE, Fu GC. Angew Chem Int Ed. 2006;45:1426–1429. doi: 10.1002/anie.200503312.
- 6.For an overview of subsequent work by us and by others, see Reference 4c.
- 7.We are aware of only one report of the use of an axially chiral biphenyl-derived phosphepine in asymmetric catalysis (chiral ligand for transition metals): Alberico E, Karandikar S, Gladiali S. ChemCatChem. 2010;2:1395–1398.
- 8.For recent overviews, see: Fan YC, Kwon O. Chem Commun. 2013;49:11588–11619. doi: 10.1039/c3cc47368f.Zhao QY, Lian Z, Wei Y, Shi M. Chem Commun. 2012;48:1724–1732. doi: 10.1039/c1cc15793k.
- 9.For leading references on recent developments in the stereocontrolled synthesis of highly substituted cyclopentanes, including applications in medicinal chemistry, see: Heasley B. Curr Org Chem. 2014;18:641–686.Bazinet RP, editor. Prostaglandins, Leukotrienes and Essential Fatty Acids. (journal)Rassu G, Auzzas L, Pinna L, Battistini L, Curti C. Stud Nat Prod Chem. 2003;29:449–520.
- 10.For the pioneering development of racemic processes, see: Zhang C, Lu X. J Org Chem. 1995;60:2906–2908.Xu Z, Lu X. Tetrahedron Lett. 1999;40:549–552.Lu X, Zhang C, Xu Z. Acc Chem Res. 2001;34:535–544. doi: 10.1021/ar000253x.Du Y, Lu X, Yu Y. J Org Chem. 2002;67:8901–8905. doi: 10.1021/jo026111t.Du Y, Lu X. J Org Chem. 2003;68:6463–6465. doi: 10.1021/jo034281f.Du Y, Lu X, Zhang C. Angew Chem Int Ed. 2003;42:1035–1037. doi: 10.1002/anie.200390266.
- 11.For early examples of enantioselective variants, see: (a) Reference 2b. (b) Reference 5b.
- 12.Christoffers J, Baro A, editors. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Wiley–VCH; New York: 2005. [Google Scholar]
- 13.For early examples, see: (a) Reference 5b. Wallace DJ, Sidda RL, Reamer RA. J Org Chem. 2007;72:1051–1054. doi: 10.1021/jo062170l.Cowen BJ, Miller SJ. J Am Chem Soc. 2007;129:10988–10989. doi: 10.1021/ja0734243.Voituriez A, Panossian A, Fleury-Brégeot N, Retailleau P, Marinetti A. J Am Chem Soc. 2008;130:14030–14031. doi: 10.1021/ja806060a.
- 14.For exceptions (≥70% ee), see: Xiao H, Chai Z, Zheng CW, Yang YQ, Liu W, Zhang JK, Zhao G. Angew Chem Int Ed. 2010;49:4467–4470. doi: 10.1002/anie.201000446.Han X, Wang Y, Zhong F, Lu Y. J Am Chem Soc. 2011;133:1726–1729. doi: 10.1021/ja1106282.Fujiwara Y, Fu GC. J Am Chem Soc. 2011;133:12293–12297. doi: 10.1021/ja2049012.Neel M, Gouin J, Voituriez A, Marinetti A. Synthesis. 2011:2003–2009.Dakas PY, Parga JA, Höing S, Schöler HR, Sterneckert J, Kumar K, Waldmann H. Angew Chem Int Ed. 2013;52:9576–9518. doi: 10.1002/anie.201302045. (f) Reference 13c.
- 15.Zhang Q, Yang L, Tong X. J Am Chem Soc. 2010;132:2550–2551. doi: 10.1021/ja100432m. [DOI] [PubMed] [Google Scholar]
- 16.For an example of an enantioselective phosphine-catalyzed [4+1] annulation that involves different coupling partners, see: Zhang X-n, Deng H-P, Huang L, Wei Y, Shi M. Chem Commun. 2012;48:8664–8666. doi: 10.1039/c2cc34619b.
- 17.For a few other early examples of non-enantioselective phosphine-catalyzed [4+1] annulations, see: Chen Z, Zhang J. Chem Asian J. 2010;5:1542–1545. doi: 10.1002/asia.201000193.Xie P, Huang Y, Chen R. Org Lett. 2010;12:3768–3771. doi: 10.1021/ol101611v.Tan J, Zhou R, Sun H, Song H, He Z. J Org Chem. 2011;76:2374–2378. doi: 10.1021/jo200164v.
- 18.For example, see: Ruble JC, Latham HA, Fu GC. J Am Chem Soc. 1997;119:1492–1493.Fu GC. Acc Chem Res. 2004;37:542–547. doi: 10.1021/ar030051b.Lee SY, Murphy JM, Ukai A, Fu GC. J Am Chem Soc. 2012;134:15149–15153. doi: 10.1021/ja307425g. (d) Reference 5.
- 19.Ziegler DT, Fu GC. Chiral Phosphine-Catalyzed Asymmetric [4+1] Annulations of 2-(Acetoxymethyl)buta-2,3-dienoates with 1,1-Bisnucleophiles Abstracts of Papers. 244th ACS National Meeting; Philadelphia, PA. Aug 19–23, 2012; ORGN 35. The investigation was completed in 2013. [Google Scholar]
- 20.Han X, Yao W, Wang T, Tan YR, Yan Z, Kwiatkowski J, Lu Y. Angew Chem Int Ed. 2014;53:5643–5647. doi: 10.1002/anie.201311214. [DOI] [PubMed] [Google Scholar]
- 21.For reviews of bioactive compounds/natural products that include a cyano group, see: Fleming FF. Nat Prod Rep. 1999;16:597–606.Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. J Med Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r.
- 22.The cyano group serves as a useful precursor to a wide array of other functional groups, including amines, aldehydes, ketones, amides, carboxylic acids, and heterocycles. For leading references, see: Murahashi S-I, editor. Science of Synthesis. Georg Thieme Verlag; Stuttgart, Germany: 2004. p. 19.
- 23.Although we have not yet pursued systematic studies of 3,3′-substituted biphenyl-derived phosphepines, our investigations of binaphthyl-based phosphepines suggest that the substituted aromatic groups in the 3 and 3′ positions may play a significant role in enhancing enantioselectivity.
- 24.Exposure of phosphepines 3 and 4 (as solids) to air for 21 days led to essentially no phosphine oxide (<5%) according to 31P NMR spectroscopy. Exposure of toluene solutions to air for 8 days led to ~10% oxidation of phosphepine 3 and ~55% oxidation of phosphepine 4.
- 25.Notes: (a) On a gram-scale, the reaction illustrated in Table 2, entry 1 proceeded in 93% ee and 76% yield (1.12 g), with 88% recovery of the catalyst as the phosphine oxide (after oxidation with t-BuOOH). (b) The absolute configurations of nine of the [4+1] annulation products described herein were determined by X-ray crystallography (see the Supporting Information).
- 26.For applications of related compounds to the synthesis of bioactive molecules, see: Ung AT, Pyne SG, Bischoff F, Lesage ASJ, Skelton BW, White AH. Tetrahedron. 2013;69:2577–2587.
- 27.Under our standard conditions, n-hexylsulfonylacetonitrile undergoes this [4+1] annulation in 78% ee and 94% yield (determined by 1H NMR spectroscopy with the aid of an internal standard).
- 28.Notes: (a) The minor diastereomer is formed in 28% ee. (b) We have not yet examined the impact of the choice of ester on the ee, dr, and yield.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.