Abstract
Background
Recent studies of children and adolescents exposed to radioactive iodine-131 (I-131) after the 1986 Chernobyl nuclear accident in Ukraine showed significant dose-related increase in the risk of thyroid cancer, but the association of radiation doses with tumor histological and morphological features is not clear.
Methods
A cohort of 11,664 individuals in Belarus ≤18 years at the time of the accident underwent three cycles of thyroid screening in 1997-2008. I-131 thyroid doses were estimated from individual thyroid activity measurements taken within two months after the accident and dosimetric data from questionnaires. Demographic, clinical and pathological characteristics of thyroid cancer cases were analyzed using one-way analysis of variance, chi-square or Fisher's exact tests, and logistic regression.
Results
As a result of screening, 158 thyroid cancers were identified. The majority of cases had T1a and T1b tumors (93.7%), with many regional N1 (60.6%) but few distant M1 (<1%) metastases. Higher I-131 doses were associated with higher frequency of solid or diffuse sclerosing variants of thyroid cancer (P<0.01) and histological features of cancer aggressiveness, such as lymphatic vessel invasion, intrathyroidal infiltration, and multifocality (all P<0.03). Latency was not correlated with radiation dose. Fifty-two cases of self-reported thyroid cancers diagnosed prior to 1997 were younger at the time of the accident and had a higher percentage of solid variant cancers compared to screening-detected cases (all P<0.0001).
Conclusions
I-131 thyroid radiation doses were associated with significantly higher frequency of solid or diffuse sclerosing variants of thyroid cancer and various features of tumor aggressiveness.
Keywords: thyroid cancer, pathology, morphology, thyroid neoplasms, Chernobyl nuclear accident, papillary carcinoma, radiation, latency
Introduction
The Chernobyl nuclear power plant accident in Ukraine on April 26, 1986 released large amounts of radiation, in particular radioactive iodines, into the atmosphere.1 Children and adolescents living in the contaminated areas in Ukraine and Belarus were exposed to substantial doses of radiation to the thyroid gland from ingestion of iodine-131 (I-131).1 At the time, convincing evidence existed of an association between exposure to external x-ray and gamma radiation in childhood and adolescence and an increased risk of thyroid cancer.2 However, risks from internally deposited radioactive iodines were not well understood.
Previous studies of mostly adult patients exposed to I-131 from diagnostic and therapeutic procedures had largely negative findings3,4 Four-five years after the accident,5 ecological6,7 and then analytical epidemiological studies8,9 reported increased risks of thyroid cancer among those living in Chernobyl-contaminated areas. It was not clear, however, whether the observed increases were due to radiation doses or to intensive and wide-spread screening for thyroid abnormalities.10 These post-Chernobyl thyroid cancers were characterized by predominance of papillary carcinoma (93-98% of all post-Chernobyl cases11,12 compared to 60-70% in other studies of childhood thyroid cancer13,14). The prevalence of solid morphology,15 high rates of extrathyroidal spread and lymph node involvement16 and distant metastases, and changes in histology with time and increasing latency10 were noted in many early publications. However, it was unknown whether the observed unusual features were due to exposures to Chernobyl-related radiation or to endemic iodine deficiency.17
We conducted two cohort studies of children and adolescents who lived on the territories contaminated by the Chernobyl fallout in Ukraine and Belarus and were systematically screened irrespective of radiation dose.18 Radiation risks of thyroid cancer were significantly increased in both cohorts. Our group has analyzed clinical and pathomorphological features of 45 screening-detected thyroid cancer cases from Ukraine.19 The cases had a high proportion of cancers with solid structure and intrathyroidal infiltration. However, no analyses were carried out on the relationship between tumor characteristics and radiation dose to the thyroid. Here we report findings on the relationship of I-131 thyroid dose and pathomorphological properties of 158 thyroid cancer cases detected during three sequential screenings of the parallel cohort from Belarus.18 To compare clinical and pathological features of screening-detected cancers with cancers identified during routine medical care, we also analyzed 52 cases diagnosed in this cohort before the initiation of standardized screening in 1997.
Materials and Methods
Study Population
A detailed description of the Belarus-American (BelAm) study population and methods has been published previously.18,20 In brief, the cohort includes 11,664 individuals aged 18 years or younger at the time of the Chernobyl accident on April 26, 1986. All study subjects had thyroid radioactivity measurements taken in Belarus within two months after the accident and were screened for the first time in 1997-2000.20 The cohort was screened two more times in 2002-2004 and 2004-2006, with final follow up extended until the end of September 2008 to account for patients referred for additional biopsies and surgeries.
The study was approved by institutional review boards in Belarus and the United States. Informed consent was provided by the study participants or by accompanying guardians for minors.
Screening Procedures
The details of the screening are presented in Figure 1. The majority of study subjects resided in Minsk and Gomel oblasts (an oblast is an administrative subdivision similar in size to a state or province) and were screened at study centers in Minsk and Gomel cities or at local medical clinics by visiting mobile screening teams. Thyroid screening consisted of ultrasound examination and palpation by a sonographer and a clinical examination with independent palpation by an endocrinologist.18 Any discrepancies were resolved by a third examination conducted jointly by both doctors. At the time of screening, participants were administered questionnaires to ascertain demographic, residential, dietary and medical history. Individual estimates of I-131 dose to the thyroid were assigned to each subject based on their thyroid radioactivity measurement taken in 1986, the interview data on factors contributing to dose (residential locations, diet), and radioecological models of the environmental transfer of I-131.21
Figure 1.
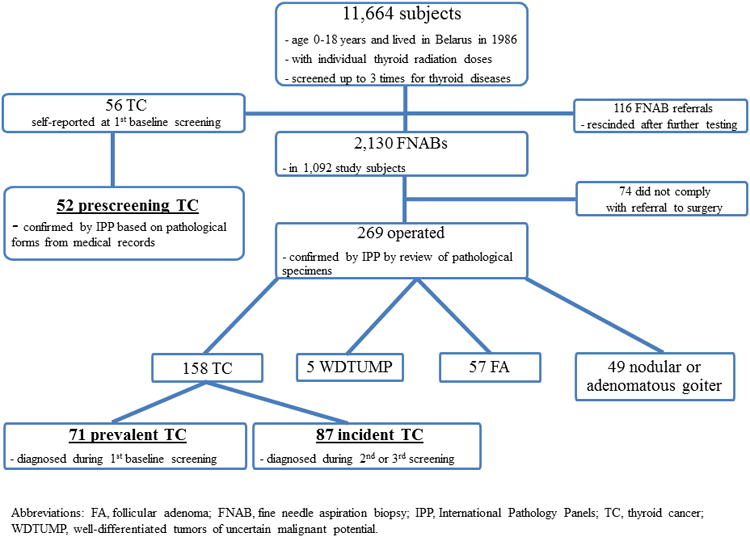
The CONSORT (Consolidated Standards of Reporting Trials) diagram showing the flow of BelAm study subjects through each stage of systematic screening.
Patients with a) thyroid nodules detected either on palpation or sonogram that measured ≥10 mm in greatest dimension and b) all nodules that measured 5-10 mm and were sonographically suspicious for malignancy (hypoechoic, indistinct borders, calcified inclusions, extension through the thyroid capsule, or suspicious lymph nodes) or with diffusely abnormal thyroid tissue accompanied by unexplained cervical lymphadenopathy were referred to study centers for further evaluation and ultrasound-guided fine needle aspiration biopsy (FNAB). Patients were further referred for surgery if cytology was diagnostic or suspicious for malignancy in either a nodule or a lymph node or for a follicular neoplasm in a nodule.
When the intraoperative frozen-section diagnosis was carcinoma, the patient underwent a total or near total thyroidectomy, followed by the usual standard of care. When the frozen-section diagnosis was follicular neoplasm, the patient underwent a hemithyroidectomy, which was followed by a completion thyroidectomy when indicated. All specimens were fixed in 10% neutral buffered formalin and embedded in serial 3–4 mm paraffin blocks; 4-mm-thick sections were stained with hematoxylin and eosin for histologic examination. Pathological specimens of all cases were reviewed and thyroid cancer diagnoses confirmed by the International Pathology Panels (IPP), initially established in the framework of the Chernobyl Tissue Bank 22 and later on convened specifically for this study. Panel members were unaware of patients' thyroid radiation dose.
Cancers were staged according to TNM classification system (5th edition) which was later converted into TNM 6th edition.23 Papillary thyroid cancers (PTC) were subdivided into several different variants, depending on the dominant structural component: papillary, follicular, or solid when >80% of the surface of the slides had the сorresponding structure.24 As in several recent publications by members of the IPP,19,25 we classified PTCs as mixed when they were composed of a combination of 2 patterns (papillary-follicular, papillary-solid, solid-follicular) at a ratio of 50%:50%, 50%:40%, or 60%:30%, allowing for <10% of the tumor to have a third pattern.
Screening-detected thyroid cancers were further subdivided into prevalent (cancers surgically removed within three years of initial screening and located within the same area of the thyroid gland where thyroid pathology was identified during initial screening) and incident (all other) cases (Figure 1).
Prescreening cases
Prescreening thyroid cancers were defined as cases diagnosed after the accident and reported by cohort subjects at initial screening in 1997. The route to diagnosis for the prescreening cases was primarily through ultrasound- or palpation-detected thyroid nodule during routine medical care or through one of the screening programs instituted in Belarus by national or international organizations. Pathological forms for self-reported cases were retrieved from medical records and reviewed by the IPP (see above).
Statistical Analysis
We examined means and distributions of demographic, clinical and pathological characteristics across three groups of thyroid cancer cases diagnosed in the BelAm cohort (screening-detected prevalent and incident cases and prescreening cases) and tested for statistically significant associations using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests or Fisher's exact tests for categorical variables. Thyroid radiation dose was log-normally distributed. Therefore, all further univariate tests were done using a Wilcoxon-Mann-Whitney test. Correlations of thyroid radiation dose with other continuously distributed variables were evaluated by the Spearman rank correlation test.
Adjusted unconditional logistic regression models were used to compute odds ratios (ORs) and 95% confidence intervals (CI) for the associations of radiation doses with various tumor properties. For screening-detected cases, latency was defined as time from exposure to surgery.
All statistical tests were 2-sided and considered statistically significant for P<0.05. Statistical analyses were conducted using SAS software.26
Results
Screening-detected and prescreening thyroid cancers
Over the three cycles of screening of 11,664 subjects, 2,130 FNABs were completed in 1,092 subjects (out of 2,246 referrals, 94.8%)(Figure 1). Following the FNAB, 343 patients were referred for surgery, and 269 (78.4%) complied. In total, the expert IPP reviewed surgical specimens and agreed upon diagnosis of thyroid cancer for 158 patients (157 papillary and 1 follicular types). The panel was not able to classify 5 cases as definitely benign or malignant and labeled them as “well-differentiated tumors of uncertain malignant potential.”25 These cases are not included in subsequent analyses.
The IPP used pathological forms extracted from medical records to confirm thyroid cancer diagnoses for 52 (all papillary type) out of 56 thyroid cancers reported during interview at initial screening. The histology form was missing for one patient and diagnoses were not confirmed for 3 self-reported cases.
Demographic and clinical characteristics of thyroid cancer cases
The demographic characteristics of the total screened study population (n=11,664) have been published previously.20 In brief, men and women were equally represented in the cohort, ∼70% were screened after age 18 years, and ∼2% each had history of diffuse or nodular goiter before screening. At the time of first screening in 1997-2000, the majority of study subjects (65%) had urinary iodine concentrations <100 mcg/L, defined by the WHO as iodine deficiency.27
Compared to prevalent and incident screening cases (n=158), the prescreening cases (n=52) were significantly younger at the time of the accident (P<0.01), significantly younger at the time of surgery (P<0.0001) and significantly more likely to have lived in rural areas at the time of the accident (P=0.02, Table 1). Prescreening cases were significantly more likely to have a more advanced tumor stage at histology (71% with T3 and above), whereas the majority (94%) of screening cases had tumors in less advanced stages T1a and T1b (P<0.0001, Table 1). There were no differences between prescreening and screening cases, or between prevalent and incident cases, in the stage of metastases to the regional lymphatic nodes or distant metastases.
Table 1. Demographic and clinical characteristics of thyroid cancer cases.
| Characteristics | Prescreening cases | Screening cases | P-valuea | ||
|---|---|---|---|---|---|
|
| |||||
| Prevalent | Incident | Prescreening vs. screening | Prevalent vs. incident | ||
| N (%) | 52 (100) | 87 (100) | 71 (100) | ||
| Age at time of the accident, years | |||||
| Mean±SD | 5.4±3.7 | 8.7±5.4 | 6.4±5.11 | ||
| <7 | 38 (73.1) | 35 (40.2) | 41 (57.8) | <0.01 | 0.07 |
| 7-12 | 12 (23.1) | 29 (33.3) | 19 (26.8) | ||
| 13-18 | 2 (3.8) | 23 (26.5) | 11 (15.4) | ||
| Age at surgery, years | |||||
| Mean±SD | 15.0±4.4 | 23.0±5.9 | 24.4±6.1 | ||
| <15 | 27 (51.9) | 9 (10.3) | 3 (4.2) | <0.0001 | 0.18 |
| 15-19 | 17 (32.7) | 21 (24.1) | 14 (19.7) | ||
| 20-24 | 8 (15.4) | 19 (21.8) | 25 (35.2) | ||
| 25-38 | 0 (0) | 38 (43.7) | 29 (40.9) | ||
| Sex | 0.42 | 0.17 | |||
| Male | 28 (53.8) | 37 (42.5) | 38 (53.5) | ||
| Female | 24 (46.2) | 50 (57.5) | 33 (46.5) | ||
| Place of residence at time of the accident | 0.02 | 0.21 | |||
| Urban | 16 (30.8) | 39 (44.8) | 39 (54.9) | ||
| Rural | 36 (69.8) | 48 (55.2) | 32 (45.1) | ||
| Oblast of residence at time of the accident | 0.28 | 0.15 | |||
| Gomel oblast | 46 (88.5) | 78 (89.7) | 62 (87.3) | ||
| Minsk city/oblast | 6 (11.5) | 4 (4.6) | 8 (11.3) | ||
| Other | 0 | 5 (5.7) | 1 (1.4) | ||
| Family history of thyroid cancer | |||||
| Yes | 2 (4.0) | 1 (1.2) | 1 (1.4) | 0.26 | 0.99 |
| No | 50 (96.0) | 86 (98.8) | 70 (98.6) | ||
| Tumor staging | |||||
| T1a (≤10 mm) | 11 (21.2) | 53 (60.9) | 52 (73.2) | <0.0001 | 0.28 |
| T1b (11-20 mm) | 4 (7.7) | 28 (32.2) | 15 (21.1) | ||
| T2 | 0 (0) | 5 (5.8) | 4 (5.6) | ||
| T3 | 35 (67.3) | 1 (1.1) | 0 | ||
| T4a | 2 (3.8) | 0 | 0 | ||
| T4b | 0 | 0 | 0 | ||
| Metastases to regional lymph nodes | 0.06 | 0.25 | |||
| N0 | 13 (25) | 30 (34.5) | 31 (43.7) | ||
| N1 | 39 (75) | 55 (63.2) | 39 (54.9) | ||
| Unknown | 0 (0) | 2 (2.3) | 1 (1.4) | ||
| Distant metastases | 0.43 | 0.45 | |||
| M0 | 50 (92.3) | 87 (100) | 70 (98.6) | ||
| M1 | 1 (5.8) | 0 | 1 (1.4) | ||
| Unknown | 1 (1.9) | 0 | 0 | ||
Abbreviations: SD, standard deviation.
P-values from one-way ANOVA for continuous variables and chi-square tests or Fisher's exact tests of heterogeneity for categorical variables.
Histopathological features of thyroid cancer cases
While, overall, screening and prescreening cases had a similar proportion of tumors >10 mm at pathology (P=0.92, Table 2), prevalent tumors were significantly more likely to be >10 mm compared to incident cases (P=0.04). Prescreening cases were more likely to be multifocal compared to cancers detected during serial screenings of the cohort (P=0.06). At the same time, screening-detected cancers more frequently than prescreening cases had non-cancer thyroid nodular pathology such as hyperplastic nodules, follicular adenomas, and adenomatous goiter, diagnosed along with cancer (P<0.01), had a significantly higher proportion of a solid histopathological variant of PTC and were less likely to have a papillary variant (P<0.0001). There were no cases of classic papillary variant among the prescreening cancers, but histopathological features were unknown for about 31% of cases. Prescreening cases also were significantly more likely to have thyroid capsule invasion and extrathyroidal spread than screening cases (both P<0.0001). However, there was no significant difference between prescreening and screening cases in intrathyroidal infiltration (P=0.13).
Table 2. Histopathological features of TC cases.
| Features | Prescreening cases | Screening cases | P-valuef | ||
|---|---|---|---|---|---|
|
| |||||
| Prevalent | Incident | Prescreening vs. screening | Prevalent vs. incident | ||
| N (%) | 52 (100) | 87 (100) | 71 (100) | ||
| Tumor size at pathology, mm | 0.92 | 0.04 | |||
| ≤10 | 31 (59.6) | 45 (51.7) | 48 (67.6) | ||
| 11-47 | 21 (40.4) | 42 (48.3) | 23 (32.4) | ||
| Multifocalitya | 0.06 | 0.54 | |||
| No | 35 (67.3) | 72 (82.8) | 56 (78.9) | ||
| Yes | 16 (30.8) | 15 (17.2) | 15 (21.1) | ||
| Unknown | 1 (1.9) | 0 | |||
| TC with other nodular pathologyb | <0.01 | 0.75 | |||
| No | 49 (94.2) | 63 (72.4) | 53 (74.6) | ||
| Yes | 3 (5.8) | 24 (27.6) | 18 (25.4) | ||
| Histopathological variant of PTCc | <0.0001 | 0.72 | |||
| Follicular | 10 (19.2) | 21 (24.4) | 20 (28.2) | ||
| Papillary | 0 (0) | 23 (26.7) | 23 (32.4) | ||
| F-P Mixedd | 4 (7.7) | 17 (19.8) | 11 (15.5) | ||
| Solide | 18 (34.6) | 24 (27.9) | 17 (23.9) | ||
| Diffuse Sclerosing | 4 (7.7) | 1 (1.2) | 0 | ||
| Unknown | 16 (30.8) | 0 | 0 | ||
| Intrathyroidal infiltration | 0.13 | 0.48 | |||
| No | 22 (42.3) | 25 (28.7) | 24 (33.8) | ||
| Yes | 29 (55.8) | 61 (70.1) | 46 (64.8) | ||
| Unknown | 1 (1.9) | 1 (1.2) | 1 (1.4) | ||
| Thyroid capsule invasion | <0.0001 | 0.84 | |||
| No | 16 (30.8) | 76 (87.4) | 62 (87.3) | ||
| Yes | 36 (69.2) | 10 (11.5) | 9 (12.7) | ||
| Unknown | 0 (0) | 1 (1.1) | 0 (0) | ||
| Extrathyroidal spread | <0.0001 | 0.81 | |||
| No | 40 (76.9) | 83 (95.4) | 68 (95.8) | ||
| Yes | 12 (23.1) | 3 (3.5) | 3 (4.2) | ||
| Unknown | 0 (0) | 1 (1.1) | 0 | ||
Abbreviations: F-P, follicular-papillary; mm, millimeters; PTC, papillary thyroid cancer; TC, thyroid cancer.
TC cases with more than one carcinoma at pathology.
TC cases with one or more thyroid follicular adenoma, nodular goiter or adenomatous goiter.
One prevalent TC was identified as follicular subtype; all other prescreening and screening cases were papillary subtype.
Includes follicular-papillary and papillary-follicular histopathological variants.
Includes solid variants and any mixed variants with solid component.
P-values from chi-square tests or Fisher's exact tests of heterogeneity for categorical variables.
Association of histopathological features of screening-detected thyroid cancers with thyroid radiation dose
The distribution of thyroid cancer cases by individual thyroid doses is presented in Figure 2. Overall, in analyses adjusted for age at exposure, thyroid I-131 doses in prescreening cases were similar to screening-detected cases (P=0.61, not shown). The median thyroid dose for screening-detected cancers was 529 mGy (mean=1,296; range: 1-17,472, not shown).
Figure 2.
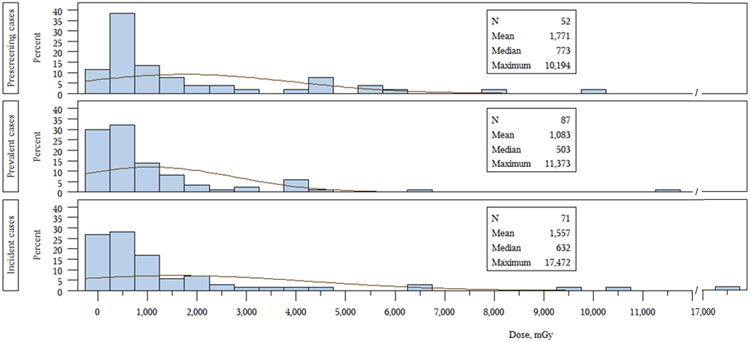
Comparison of thyroid doses for prescreening, prevalent and incident thyroid cancer cases in BelAm cohort.
Table 3 shows that frequencies of lymphatic invasion (P=0.03) and intrathyroidal infiltration (P=0.02) increased significantly with increasing radiation doses among screening-detected cancers, but other invasive properties of thyroid cancer, such as presence of tumor capsule and vascular invasion, thyroid capsule invasion, and extrathyroidal spread, were not associated with radiation dose. Cases in the highest dose category of 950+ mGy were almost five times more likely to have more than one distinct cancer at pathology compared to those with doses <200 mGy (RR=4.86, 95%CI: 1.30-18.1, P=0.02,Table 3). In addition, subjects with higher doses were significantly more likely to be diagnosed with a solid or diffuse sclerosing variant of PTC than with any other variants combined (P<0.01). Thyroid cancer cases associated with high radiation doses were more likely to have other nodular pathology, although not significantly so (P=0.44).
Table 3. Hiostopathological features of screening-detected thyroid cancers (N=158) by categories of thyroid radiation dosea.
| Radiation dose, mGy | Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| <200 | 200-949 | 950-17,472 | P-valueb | |
| Tumor size at pathology, mm: ≤10 vs. 11-47 | 1 | 0.90 (0.38-2.15) | 1.06 (0.41-2.72) | 0.77 |
| Presence of tumor capsule: No vs. Yes | 1 | 0.50 (0.19-1.28) | 0.70 (0.24-2.10) | 0.96 |
| Lymphatic invasion: Yes vs. No | 1 | 1.04 (0.44-2.43) | 2.65 (0.96-7.36) | 0.03 |
| Blood vessel invasion: Yes vs. No | 1 | 1.76 (0.43-7.20) | 1.53 (0.32-7.24) | 0.88 |
| Intrathyroidal infiltration: Yes vs. No | 1 | 1.23 (0.53-2.86) | 2.96 (1.09-8.03) | 0.02 |
| Thyroid capsule invasion: Yes vs. No | 1 | 1.63 (0.39-6.79) | 2.10 (0.46-9.50) | 0.43 |
| Extrathyroidal spread: Yes vs. No | 1 | 0.44 (0.06-3.56) | 0.35 (0.04-3.17) | 0.52 |
| Multifocalityc: Yes vs. No | 1 | 2.24 (0.64-7.79) | 4.86 (1.30-18.1) | 0.02 |
| TC with other nodular pathologyd: Yes vs. No | 1 | 1.28 (0.48-3.39) | 1.57 (0.56-4.41) | 0.44 |
| Histopathological variant of PTC: Solid and Diffuse Sclerosing vs. Other | 1 | 0.64 (0.23-1.75) | 2.75 (0.99-7.65) | <0.01 |
Abbreviations: mm, millimeters; mGy, milligray; PTC, papillary thyroid cancer; TC, thyroid cancer.
Logistic regression models adjusted for age at the time of surgery and sex.
P values from the test of linear trend.
TC cases with more than one carcinoma at pathology.
TC cases with one or more thyroid follicular adenoma, nodular goiter or adenomatous goiter.
Nine cases of screening-detected thyroid cancers could be classified as occult (5 were referred for surgery because of suspicion for follicular adenoma and 4 were detected in one of many suspicious nodules). Results of analyses with and without these cases were generally similar.
Associations with other factors
Urinary iodine concentrations during baseline screening were not associated with tumor size and aggressiveness of incident thyroid cancers (all P>0.10, not shown). Mean (SD) for latency period for screening-detected prevalent and incident cases was 14.2 (1.4) and 18.1 (2.4) years, respectively (not shown). Latency was not correlated with thyroid dose (Spearman's r= - 0.12, P=0.15, not shown).
Discussion
In a large prospective cohort study of children and adolescents from Belarus exposed to I-131 after the Chernobyl accident, we found that among 158 screening-detected thyroid cancer cases, higher radiation doses were associated with a significantly higher frequency of solid variant PTC and with several histopathological features of tumor aggressiveness, such as lymphatic invasion and intrathyroidal tissue invasion. Subjects exposed at the highest dose level were five times more likely to have cancer multifocality. Compared to screening-detected cases, 52 prescreening cases were significantly younger at the time of the accident and had more advanced cancer at the time of diagnosis. Prescreening cases were significantly more likely than screened cases to have thyroid capsule invasion and extrathyroidal spread, although they were less likely to have concurrent non-cancer thyroid pathology such as hyperplastic thyroid nodules, follicular adenomas, or adenomatous goiter.
To our knowledge, this is the first study to systematically evaluate the association between thyroid cancer pathology detected using similar screening methods and individual I-131 thyroid doses. Although larger series of post-Chernobyl thyroid cancer cases have been published recently,12,25 the cases lacked individual thyroid doses and diagnoses were made during routine medical care, not by means of standardized detection procedures.
The Belarusian cohort analyzed here is one of two screening cohorts established in the mid-1990s to follow up children and adolescents who lived on territories contaminated by Chernobyl fallout in Ukraine and Belarus and had thyroid radioactivity measurements taken within two months after the accident.18 The Belarusian cohort is comparable to the Ukrainian cohort in size (11,664 and 13,243, respectively), age at exposure and sex distribution.20,28 Thyroid I-131 radiation doses in the BelAm cohort were significantly lower than in the UkrAm cohort, both for all screened subjects (560 mGy (SD=1,180)20 and 780 mGy (1,850),28 respectively, P<0.0001), and for prevalent cases only (mean=1,083 mGy (SD=1,660)20 and 2,000 mGy (2,520),28 respectively, P<0.01). The number of self-reported cases of thyroid cancer diagnosed before initial screening was much higher in Belarus (56 vs. 14 in Ukraine) and included more tumors ≤10 mm in size (59.6% vs. none in Ukraine). The number of thyroid cancers identified during the first round of screening was also much higher in Belarus (87 vs. 45 in Ukraine). Screening-detected prevalent cases in Ukraine19 tended to have larger tumors at pathology (76.7% with tumors >10 mm vs. 48.3% in Belarus) and to be diagnosed at a later tumor stage (44.2% T4 vs. 0 in this study). While thyroid cancer cases detected during initial screening in Ukraine and Belarus had similar proportions of cancers with solid structure (34.9% vs. 27.9%, respectively) and intrathyroidal infiltration (72.1% vs. 70.1%), extrathyroidal spread was significantly higher among cases diagnosed in the Ukrainian cohort (46.5% vs. 3.5%). The larger number of prescreening cases in Belarus and the smaller size and earlier stage of the prevalent cases might reflect the widespread screenings that took place prior to initiation of our screening study, but the earlier screenings would not seem to explain the larger number of prevalent cases in Belarus. This suggests that some factors other than screening may have played a role in the observed differences in thyroid cancer pathology between the two cohorts.
In terms of tumor stage at presentation and number of regional and distant metastases, our results are similar to those in a large consecutive series of 738 cases of thyroid cancer among children <15 years of age from Belarus29 (72.6% T1, 30.4% N0, 97.6% M0). Although the majority of cases in that series had a history of residing in areas contaminated by the Chernobyl accident, individual doses to the thyroid gland were not available, and some were diagnosed shortly before the accident or were born after the accident.
Similar to our findings, a large series of 2,658 thyroid cancers from Ukraine12 reported a high percentage of PTCs (>90%) and increasing proportions (∼20%) of small cancers over time. In contrast to our findings, Bogdanova et al.12 did not find any association between radiation exposure status based on the age and place of residence at the time of the accident, and tumor characteristics of PTCs in age-matched groups of patients born before and after Chernobyl. In our analyses adjusted for age and sex, we observed significant associations between several characteristics of tumor aggressiveness and individual I-131 radiation doses.
Our findings related to radiation dose also stand in contrast to other recent analyses of morphological characteristics and aggressiveness of post-Chernobyl childhood thyroid cancers.17,30 Williams et al. reported differences in differentiation and invasiveness of thyroid cancers diagnosed in children from Belarus, Ukraine, and the Russian Federation compared to childhood thyroid cancers diagnosed in England, Wales, and Japan, suggesting that they could be due not to radiation exposure, but to differing levels of iodine deficiency, which can increase incidence, reduce latency, and influence tumor morphology and aggressiveness.17 However, the study did not measure either individual thyroid doses or iodine concentrations. Although we did not have urinary iodine measurements at the time of the accident, urinary iodine levels during baseline screening showed no association with morphologic characteristics of incident thyroid cancers.
In other large series of thyroid cancer among subjects not known to be exposed to radiation, from Greece,31 Mallinckrodt Institute of Radiology in St. Louis, U.S.,32 and pooled U.S.,33 and Japan34 data, a higher proportion of follicular thyroid cancer (9%,31 5%32 and 10%33 vs. 1%), multifocality (48%31 and 57%32 vs. 19%) and family history of thyroid cancer (10%33 and 9%34 vs. 2%) have been reported compared to our study.
The histological pattern of thyroid cancers in our cohort appeared to change over time, with a gradual reduction in the solid variant of PTC, similar to other studies of Chernobyl-associated thyroid cancers,25,30 and reflective of shorter latency for this variant compared to typical follicular or papillary variants. As expected, the reduction in the proportion of solid variant from prescreening to screening cases in our study was accompanied by a reduction in aggressive biological behavior. In particular, there were significantly lower rates of thyroid capsule invasion and extrathyroidal spread in screening cases compared to prescreening cases. It is possible that the apparent change in histopathological variant observed over time may be due to differences in nodule conspicuity, whereby a relatively more aggressive solid variant of papillary type carcinoma is more conspicuous than other variants and is more likely to be diagnosed earlier.35
Our study has some notable strengths. These include systematic screening according to a strict protocol; referral to FNAB and surgery according to well-defined standardized criteria; a high rate of compliance for FNAB (94.8%) and surgery (78.4%); short interval between FNAB and surgery (average 4 months); and independent review and confirmation of all histopathological specimens by the international panels of experts.
The study also has some limitations. About 20% of those referred to surgery after the FNAB did not comply. However, study subjects were not aware of their I-131 thyroid doses and follow-up and referrals were done without regards to thyroid dose. Among prescreening cases, dose-dependent participation cannot be excluded. However, prescreening cases were diagnosed at more advanced tumor stages and had somewhat higher proportion of regional but not distant metastases, arguing against dose-related self-selection. Furthermore, all statistical analyses were adjusted for other potential risk factors, such as age at the time of surgery and sex, to eliminate possible effects of differential selection.
In summary, systematic screening of a cohort of 11,664 subjects exposed to I-131 after the Chernobyl accident in Belarus identified a large number of thyroid cancers. We found that higher radiation doses to the thyroid gland were associated with solid and diffuse sclerosing variants of PTCs, more biologically aggressive cancers and a higher probability of multifocal cancers and multiple nodular pathology. The biologic explanation for these radiation dose-dependent findings should be pursued in genetic/molecular studies. Study findings provide important evidence about clinical follow-up of patients exposed to environmental radiation doses of I-131.
Acknowledgments
Funding for this study was provided by the National Cancer Institute (grant CA132918 to LBZ and Intramural Research Program from the Division of Cancer Epidemiology and Genetics to AB, MPL, EO, AB, VD, KM, MH.)
We gratefully acknowledge the late Dr. Jacob Robbins for his important contributions to the study over the years. We would like to thank Drs. Ellen Greenebaum, Eugeny Cherstvoy, Vladimir Gapanovich, Nataliya Kamysh, Vladimir Masyakin, Victor Minenko, Sergei Petrenko, George Romanov, Yuri Sidorov, Ms. Liliya Starostenko and the staff of the Republican Research Centre for Radiation Medicine and Human Ecology, Gomel, Belarus, for their dedication and commitment to the success of the study.
We express our deep appreciation of the work of the members of the International Pathology Panel of the Chernobyl Tissue Bank and the ad hoc Panel: Drs. Alexander Abrosimov, Tatiana Bogdanova, Ronald Ghossein, Masahiro Ito, Virginia LiVolsi, Alexander Nerovnya, Juan Rosai, Geraldine Thomas, and Dillwyn Williams (Chair).
Over the years we have greatly benefited from important advice of the members of the Thyroid Advisory Group: Drs. Oksana Baltarowich, Evelyn Bromet, Maria Demkowicz, Christine Durbak, Shirley Fry, Richard Hornung, Bruce Napier (Chair), Genevieve Roessler, and Arthur Schneider.
Footnotes
The competing financial interests' declaration: The authors declare they have no actual or potential competing financial interests.
References
- 1.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Annex D: Health Effects Due to Radiation from the Chernobyl Accident. II. New York: United Nations; 2011. 2008 Report to the General Assembly with Scientific Annexes. [Google Scholar]
- 2.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 3.Holm LE, Hall P, Wiklund K, et al. Cancer risk after iodine-131 therapy for hyperthyroidism. J Natl Cancer Inst. 1991;83:1072–1077. doi: 10.1093/jnci/83.15.1072. [DOI] [PubMed] [Google Scholar]
- 4.Holm LE, Wiklund KE, Lundell GE, et al. Cancer risk in population examined with diagnostic doses of 131I. J Natl Cancer Inst. 1989;81:302–306. doi: 10.1093/jnci/81.4.302. [DOI] [PubMed] [Google Scholar]
- 5.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 6.Abelin T, Egger M, Ruchti C. Fallout from Chernobyl. Belarus increase was probably caused by Chernobyl. BMJ. 1994;309:1298. author reply 1300. [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Yamashita S, Ashizawa K, et al. Pediatric thyroid diseases around Chernobyl: Morphological aspects of the Chernobyl Sasakawa Health and Medical Cooperation Project. In: Yamashita S, Shibata Y, editors. Chernobyl: A Decade. Netherlands: Elsevier Science B.V.; 1997. [Google Scholar]
- 8.Astakhova LN, Anspaugh LR, Beebe GW, et al. Chernobyl-related thyroid cancer in children of Belarus: a case-control study. Radiat Res. 1998;150:349–356. [PubMed] [Google Scholar]
- 9.Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 10.Williams D. Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat Rev Cancer. 2002;2:543–549. doi: 10.1038/nrc845. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991-1992) from the Republic of Belarus. Cancer. 1994;74:748–766. doi: 10.1002/1097-0142(19940715)74:2<748::aid-cncr2820740231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanova T, Zurnadzhy LY, LiVolsi V, et al. Thyroid cancer pathology in Ukraine after Chernobyl. In: Tronko M, Bogdanova T, Saenko VA, Thomas GA, Likhtarev I, Yamashita S, editors. Thyroid Cancer In Ukraine after Chernobyl: dosimetry, epidemiology, pathology, molecular biology. Nagasaki, Japan: Nagasaki Association for Hibakushas' Medical Care (NASHIM); 2014. [Google Scholar]
- 13.Harach HR, Williams ED. Childhood thyroid cancer in England and Wales. Br J Cancer. 1995;72:777–783. doi: 10.1038/bjc.1995.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–172. doi: 10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 15.Williams D. Twenty years' experience with post-Chernobyl thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:1061–1073. doi: 10.1016/j.beem.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Rybakov SJ, Komissarenko IV, Tronko ND, et al. Thyroid cancer in children of Ukraine after the Chernobyl accident. World J Surg. 2000;24:1446–1449. doi: 10.1007/s002680010239. [DOI] [PubMed] [Google Scholar]
- 17.Williams ED, Abrosimov A, Bogdanova T, et al. Morphologic characteristics of Chernobyl-related childhood papillary thyroid carcinomas are independent of radiation exposure but vary with iodine intake. Thyroid. 2008;18:847–852. doi: 10.1089/thy.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stezhko VA, Buglova EE, Danilova LI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 19.Bogdanova TI, Zurnadzhy LY, Greenebaum E, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: pathology analysis of thyroid cancer cases in Ukraine detected during the first screening (1998-2000) Cancer. 2006;107:2559–2566. doi: 10.1002/cncr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zablotska LB, Ron E, Rozhko AV, et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;104:181–187. doi: 10.1038/sj.bjc.6605967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drozdovitch V, Minenko V, Khrouch V, et al. Thyroid dose estimates for a cohort of Belarusian children exposed to radiation from the Chernobyl accident. Radiat Res. 2013;179:597–609. doi: 10.1667/RR3153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas GA. The Chernobyl Tissue Bank: integrating research on radiation-induced thyroid cancer. J Radiol Prot. 2012;32:N77–80. doi: 10.1088/0952-4746/32/1/N77. [DOI] [PubMed] [Google Scholar]
- 23.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 6th. New York: John Wiley & Sons; 2002. [Google Scholar]
- 24.Hedinger C, Williams ED. L S World Health Organization Histological Typing of Thyroid Tumours. Berlin: Springer Inc.; 1988. [Google Scholar]
- 25.LiVolsi VA, Abrosimov AA, Bogdanova T, et al. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol) 2011;23:261–267. doi: 10.1016/j.clon.2011.01.160. [DOI] [PubMed] [Google Scholar]
- 26.SAS Institute Inc. SAS Version 9.3 for Windows. Cary, NC 2012.
- 27.World Health Organization (WHO) A guide for programme managers. Lyon, France: International Agency for Research on Cancer; 2007. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination. [Google Scholar]
- 28.Tronko MD, Howe GR, Bogdanova TI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 29.Demidchik YE, Demidchik EP, Reiners C, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243:525–532. doi: 10.1097/01.sla.0000205977.74806.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams ED, Abrosimov A, Bogdanova T, et al. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. Br J Cancer. 2004;90:2219–2224. doi: 10.1038/sj.bjc.6601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pazaitou-Panayiotou K, Kaprara A, Boudina M, et al. Thyroid carcinoma in children and adolescents: presentation, clinical course, and outcome of therapy in 23 children and adolescents in Northern Greece. Hormones (Athens) 2005;4:213–220. doi: 10.14310/horm.2002.11160. [DOI] [PubMed] [Google Scholar]
- 32.Grigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002;95:724–729. doi: 10.1002/cncr.10725. [DOI] [PubMed] [Google Scholar]
- 33.Newman KD, Black T, Heller G, et al. Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children's Cancer Group. Ann Surg. 1998;227:533–541. doi: 10.1097/00000658-199804000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enomoto Y, Enomoto K, Uchino S, Shibuya H, Watanabe S, Noguchi S. Clinical features, treatment, and long-term outcome of papillary thyroid cancer in children and adolescents without radiation exposure. World J Surg. 2012;36:1241–1246. doi: 10.1007/s00268-012-1558-4. [DOI] [PubMed] [Google Scholar]
- 35.O'Kane P, Shelkovoy E, McConnell RJ, et al. Differences in sonographic conspicuity according to papillary thyroid cancer subtype: results of the Ukrainian-American cohort study after the Chornobyl accident. Am J Roentgenol. 2008;191:W293–298. doi: 10.2214/AJR.07.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]


