Abstract
Recruiting sufficient numbers of participants for physical activity trials for individuals with diabetes can be difficult because there are often many behavioral demands for participants, and inclusion and exclusion criteria can be extensive. This study examined the recruitment strategies used for a randomized, controlled trial designed to investigate the efficacy of an automated telephone intervention to promote physical activity in adults with type 2 diabetes in an urban Veterans Administration health care system. Traditional recruitment approaches of posting flyers and obtaining referrals from clinicians did not yield sufficient numbers of interested patients. Using the electronic medical record system to identify patients with uncontrolled diabetes allowed staff to send targeted mailings to participants, and 77% of participants were recruited using this method. The targeted mailing approach elicited a positive response rate of 12% (328 of 2,764 potential participants identified) and appeared to produce a more representative and appropriate sample than other recruitment methods used. Lessons learned in this study may be helpful to researchers in future trials who attempt to recruit participants with diabetes for physical activity protocols.
Efficient recruitment of adequate numbers of participants is crucial to the success of randomized, controlled trials (RCTs). Investigators unable to meet recruitment goals in a timely manner may have difficulty completing their trial, detecting significant effects, or even retaining their funding (1–3). Recruitment can be particularly challenging when targeting populations with chronic illnesses such as diabetes for physical activity trials because there are additional medical and safety concerns to consider. Although difficulties with recruitment are common, few studies elaborate on the thorny issue of recruitment in a way that clarifies the obstacles and offers potential solutions (4,5).
Although there are many benefits associated with physical activity for individuals with diabetes (6), it has been shown that people with diabetes are less likely than people without diabetes to meet either national or American Diabetes Association recommendations for physical activity (7). Furthermore, the prevalence rates of diabetes in the U.S. population are rising (8), and nearly one in four patients in the Veterans Administration (VA) health care system is diagnosed with diabetes (9). Consequently, there is a great need for research trials to inform the development of effective strategies to increase physical activity for individuals with diabetes in the United States, and for veterans in particular. To achieve this goal, it is imperative that diabetes researchers recruit enough eligible participants to complete their trials successfully.
Physical activity interventions are often long and demanding and require significant changes in participants’ day-to-day routines, so identifying patients who are willing to participate can be challenging. When targeting patients with diabetes and other medical complexities, the inclusion, exclusion, and safety requirements become more limiting, making recruitment even more difficult (10–13). Given the tremendous time and effort it can take to screen and identify an appropriate participant pool for a randomized trial for patients with diabetes, it behooves researchers to use recruitment methods that most effectively and efficiently identify eligible and interested participants.
Posting flyers, providing brochures, sending out mass mailings, and advertising in the media are commonly used recruitment methods, each with pros and cons and varying levels of success in different settings (14–20). All of these methods have the advantage of requiring minimal initial effort and staff time. However, these methods have two major disadvantages. First, they require potential participants to take the initiative to contact the research staff and may favor patients who are highly motivated to exercise and more proactive. This can result in a sample that is unrepresentative of the larger population and thus threaten the external validity of the research findings (21). Second, these methods require large amounts of staff time to filter through initial responses for appropriate and eligible individuals.
With the increasing availability of electronic medical records (EMRs), a more focused approach to recruitment can be taken. Identifying patients who meet key inclusion criteria can be relatively simple and allows research staff to take the initiative and contact potential participants directly, promoting greater external validity with minimal burden to either patients or research staff (22,23).
This article illustrates the advantages of a targeted, staff-initiated recruitment method by describing recruitment in an RCT to assess the efficacy of an automated, interactive voice-response telephone intervention to promote physical activity behaviors in adults with type 2 diabetes. We will review recruitment methods used in this trial and discuss the challenges and lessons learned in recruiting participants with type 2 diabetes for a physical activity trial.
Methods
Sedentary overweight or obese patients with type 2 diabetes from a large, urban VA health care system were recruited to participate in a 12-month RCT aimed at promoting physical activity. As with most physical activity studies with compromised medical populations, the inclusion and exclusion criteria were extensive to ensure that individuals would be able to exercise in a safe manner. Inclusion criteria were 1) veteran status, 2) BMI ≥25 kg/m2 (e.g., overweight or obese), 3) a current prescription for insulin or oral medications to control blood glucose, 4) A1C ≥7% but no more than 10% to enhance homogeneity of the sample, 5) physical activity in the sedentary range (i.e., not engaging in aerobic exercise at least three times per week for 30 minutes/day for the past 6 months), 6) interest in increasing physical activity level, and 7) approval from a health care provider (HCP) to participate in this physical activity trial. Exclusion criteria were 1) orthopedic problems that prevented participation in a walking program; 2) significant peripheral neuropathy; 3) active psychosis, substance abuse, or psychiatric hospitalizations in the past 6 months; 4) severe cognitive impairment; 5) inability to understand conversational English; and 6) lack of direct access to a telephone. Women who were pregnant, breastfeeding, or likely to become pregnant within the next 6 months were also excluded. In addition, the study cardiologist and exercise physiologist carefully reviewed each potential participant’s chart for any additional medical factors that would limit the participant’s ability to take part in a physical activity program (e.g., significant cardiac disease, sores on the feet, history of falls, hip fracture, moderate to severe chronic obstructive pulmonary disease, unstable blood glucose, or general medical instability).
During the first few months of recruitment, staff used traditional recruitment strategies; flyers and brochures that provided a basic description of the study were posted and distributed in high-traffic areas within the health care system. All recruitment materials included information regarding participant remuneration ($240 for full participation over the course of the year-long study). In addition, HCPs and other research staff received information and brochures about the study and were encouraged to refer overweight or obese patients with type 2 diabetes. For both of these methods, individuals who were interested in learning more about the study were asked to contact study staff directly using the contact information provided.
Although >25% of interested individuals screened were identified in this manner, many of the individuals who contacted study staff did not meet the basic inclusion criteria. Although not recorded systematically, research staff observed that a large proportion of potential participants who established initial contact either were not veterans, did not have a diabetes diagnosis, or did not qualify as sedentary. Given that these approaches appeared to be attracting a large number of ineligible participants, study staff reexamined the recruitment strategies being used and decided to take a more targeted approach by identifying potential participants who met some key inclusion criteria using the EMR system.
For this more targeted approach, potential participants with type 2 diabetes were identified and sent a letter from study staff. This approach, approved by the health care system’s institutional review board, involved identifying patients with a recent A1C test result in the targeted range (7–10%) using automated queries of the EMR and mailing letters to the identified patients providing a basic description of the study and the remuneration offered to participants. To maintain Health Insurance Portability and Accountability Act compliance, the letter described the study intervention but did not specify that the study was for individuals with type 2 diabetes. A preaddressed, stamped postcard was enclosed that could be sent back to the investigators, allowing recipients to indicate whether they were interested in being contacted to learn more about the study. The letter also provided telephone numbers so recipients could initiate contact with study staff. Importantly, the letter also stated that if the research team did not hear from a recipient within 2 weeks, someone from the study staff might call to inquire about interest in the study. Phone calls to individuals who expressed interest (either by telephone or postcard) were prioritized. When recruitment flow waned and study staff had time to devote to additional outreach to patients, telephone calls were made to some of the letter recipients who had not responded by telephone or postcard.
All participants who expressed an interest in participating received a telephone screening during which basic inclusion and exclusion criteria were reviewed. For those who met initial screening criteria over the telephone, a more thorough medical chart review was then conducted to identify medical contraindications and other exclusions not identified during the phone screening. In addition, all participants had to obtain approval to participate in the study from one of their HCPs. Those who were deemed eligible and interested were then scheduled for a baseline assessment during which they were officially enrolled and signed an informed consent form.
Results
Recruitment Process
Of the 2,764 veterans to whom recruitment letters were sent, 29% (n = 794) responded either by returning the enclosed postcard or by calling study staff. Approximately 35% of these respondents (10% of the individuals to whom letters were sent, n = 282) indicated interest in learning more about the study, responding via postcard (23%, n = 183) or via telephone (12%, n = 99).
Study staff initiated calls to 26% (n = 521) of the individuals who did not respond to the letter and reached more than half (55%, n = 284). Of those reached by telephone, 16% expressed interest in participating (n = 46).
Eligibility Screening
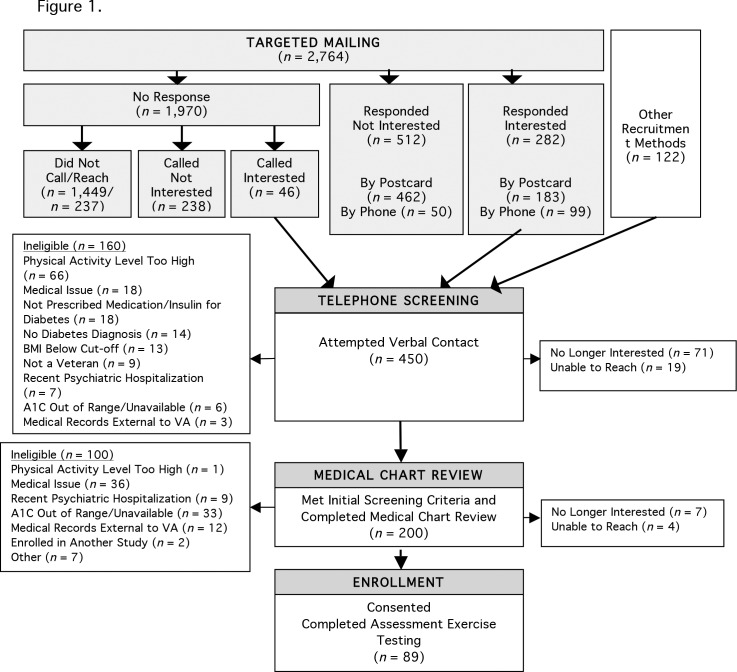
More than one-third (160 of 450, 36%) of interested individuals were deemed ineligible during initial telephone screening for a range of reasons. In addition, after initially expressing interest in the study, 71 of the 450 individuals reported that they were not interested when called for the initial telephone screening, and 19 could not be reached via telephone. Figure 1 provides detailed information about reasons for ineligibility at each phase of recruitment.
FIGURE 1.
Consort flow diagram leading to enrollment.
The order of the initial screening questions was adjusted to enhance efficiency over the course of recruitment based on experiences of the study staff. Criteria that could be gathered easily (i.e., height, weight, medication use, and veteran status) or resulted in a high rate of exclusions (e.g., physical activity level) were queried earlier in the telephone conversation to minimize the likelihood that potential participants would learn that they were not eligible after a lengthy call with research staff. The screening process stopped without further questions if a necessary criterion was not met. As a result, excluded individuals may also have met additional criteria for ineligibility, likely resulting in an underestimation in each category of reasons for ineligibility.
Before scheduling the first baseline assessment visit, the exercise physiologist and study cardiologist reviewed medical records of the 200 potential participants who completed the telephone screening to determine the medical appropriateness of their participation in regular physical activity. At this phase of the recruitment process, one-half (100 of 200) of potential participants were found to be ineligible (Figure 1). Another 11 (6%) were no longer interested or were unreachable.
Table 1 provides baseline demographics for 88 of the 89 participants who attended the first baseline appointment and completed the consent process. (One participant was excluded after consent and before completing demographic information.)
TABLE 1.
Baseline Characteristics by Recruitment Type
| Characteristic | All Participants (n) | Targeted Recruitment (n) | Non-Targeted Recruitment (n) | P |
| Age (years; mean ± SD) | 62.8 ± 9.7 (88) | 63.9 ± 9.7 (70) | 58.4 ± 8.5 (18) | 0.03 |
| Female (%) | 3 (88) | 4.3 (70) | 0 (18) | |
| Race/ethnicity (%) | ||||
| Hispanic white | 2 (87) | 2.9 (69) | 0 (18) | |
| Black/African American | 15 (87) | 15.9 (69) | 11.1 (18) | |
| White | 81 (87) | 78.3 (69) | 88.9 (18) | |
| Other | 2 (87) | 2.9 (69) | 0 (18) | |
| Education beyond high school (%) | 71.5 (88) | 69.9 (70) | 77.9 (18) | |
| Employed (%) | 22.7 (88) | 24.3 (70) | 16.7 (18) | |
| Current smoker (%) | 17.0 (88) | 17.1 (70) | 16.7 (18) | |
| Heart disease (%) | 48.8 (86) | 48.5 (68) | 50 (18) | |
| BMI (kg/m2; mean ± SD) | 35.0 ± 5.8 (86) | 34.4 ± 5.4 (67) | 37.1 ± 6.7 (19) | 0.08 |
| Waist circumference (cm; mean ± SD) | 119.6 ± 15.2 (79) | 117.9 ± 13.7 (62) | 125.8 ± 18.7 (17) | 0.06 |
| A1C (%; mean ± SD) | 7.8 ± 0.9 (84) | 7.9 ± 0.9 (65) | 7.5 ± 0.9 (19) | 0.10 |
| Ever engaged in regular physical activity (%) | 78 (88) | 80.0 (70) | 72.2 (18) | |
| Time since regular physical activity (months; mean ± SD) | 63.6 ± 94.8 (75) | 53.5 ± 95.2 (59) | 100.9 ± 86.2 (16) | 0.08 |
Analysis of Enrolled Participants
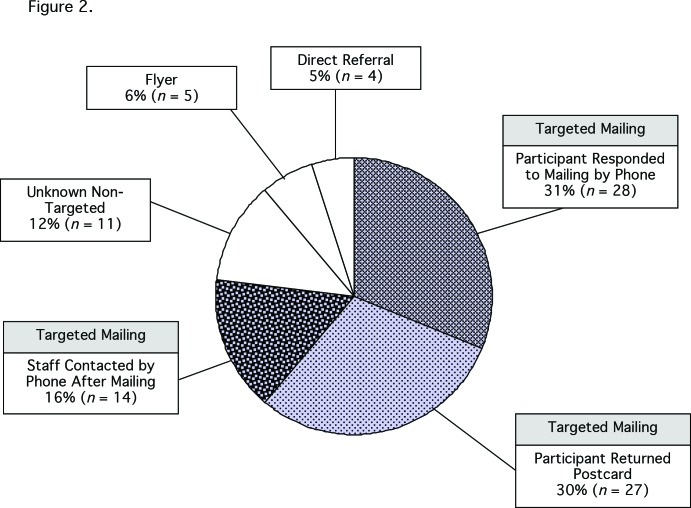
To evaluate the success of the various recruitment strategies for this study, recruitment characteristics for the participants who enrolled in the study were examined. Of the 89 participants who consented to participate and enrolled, 77% (n = 69) were recruited via targeted mailings, 31% (n = 28) responded to the mailing by telephone, 30% (n = 27) responded by postcard, and 16% (n = 14) responded after receiving a call initiated by study staff (Figure 2).
FIGURE 2.
Recruitment methods for enrolled participants (n = 89).
Table 1 compares baseline demographics for the targeted and non-targeted recruitment groups. A significant difference between participants recruited by the two methods was detected for age (t [86] = –2.20, P = 0.03), indicating that participants who were recruited through the targeted mailing were significantly older (mean age 63.9 ± 9.7 years) than those recruited via non-targeted means (mean age 58.4 ± 8.5 years). BMI and waist circumference differences trended toward significance (t [84] = 1.80, P = 0.08; (t [77] = 1.94, P = 0.06, respectively), with higher BMI and waist circumference in the non-targeted group. χ2 tests could not be performed for the categorical variables sex, race, employment, or education level because >80% of cells had expected frequencies <5. However, it is interesting to note that most of the minority participants (88%) enrolled in the study were recruited through targeted efforts. Additionally, although only 3% of the sample was female, all female participants were recruited via targeted methods.
Discussion
Given the extensive exclusion criteria related to physical activity safety concerns for patients with diabetes, investigators conducting physical activity trials in this population should be prepared to encounter high numbers of patients who may not meet study criteria. Investigators can maximize recruitment efficiency by anticipating challenges and taking a proactive and targeted approach to recruitment, while maintaining flexibility to revise the plan if it proves to be inadequate.
Investigators in this study were ultimately satisfied with a targeted mailing approach after shifting from earlier methods that did not yield the needed level of response. Although the inclusion criteria were clearly stated in early recruitment materials, these initial methods drew a high number of individuals interested in being considered for an exercise program who failed to meet the basic inclusion criteria (e.g., no diabetes diagnosis, not a veteran, or not sedentary). Non-targeted recruitment methods for physical activity trials appear to attract volunteers who are motivated to exercise but may not be representative of the patient population of interest (21). Conversely, the targeted approach was successful in identifying a more representative sample, as evidenced by the recruitment of more women, minorities, and older patients than through more traditional methods.
Using a targeted mailing approach required significant staff resources on the front end but proved to be an effective method of identifying participants; 77% of those who ultimately enrolled were recruited via mailings. Although staff time was required to identify appropriate patients in the EMR, this was achieved with minimal effort by the investigators and research staff. This targeted method narrowed the pool of potential participants to those who met some of the key criteria for the study, resulting in fewer screenings of patients who were ineligible. These results are consistent with findings from other researchers within the VA health care system who used mailings to a targeted group with follow-up telephone contact to enhance efficiency and effectiveness of recruitment in medical populations. For example, Resio et al. (24) found use of targeted mailings and telephone contact to be highly effective in reaching prospective veteran participants compared to other recruitment methods (i.e., provider referrals and media advertisements).
Finding an efficient recruitment method for physical activity trials targeting patients with type 2 diabetes is particularly important given the numerous exclusion criteria related to safety requirements. Investigators could refine their search of the EMR further than was done in this study by including more restrictive search parameters that more closely reflect the inclusion and exclusion criteria. Had that approach been taken for this study, it may have reduced the number of potential participants who were excluded during the phone screening or medical chart review.
In this study, queries in the EMR to identify veterans with elevated A1C levels generated large numbers of patients because of the high prevalence of type 2 diabetes in the veteran population. Staff attempted to call only 26% of the letter recipients who did not respond (by calling or returning the enclosed postcard) because this strategy was only used when staff were not engaged in other recruitment activities (e.g., responding to potential participants who had expressed an interest). Although the telephone calls were not used consistently, they did prove to be a fruitful method for recruitment; 16% of those reached by telephone were interested in the study and proceeded to the screening call, and these individuals comprised 16% of those eventually enrolled.
In this study, potential participants may have been attracted by the prospect of receiving a personalized exercise plan and exercise training sessions with an exercise physiologist and may have responded more favorably to the letters than they would have for other types of physical activity trials. For trials in which the number of available potential participants is much lower or in which the intervention is less appealing, it may be necessary for investigators to take more initiative in contacting potential participants, such as calling all those who do not respond to letters (25) or going to medical clinics for face-to-face contacts with providers and patients (26–28).
Several limitations of this study should be noted. First, this trial was conducted in a VA health care system, which limits the extent to which these results can be generalized outside of a veteran population (i.e., a largely male population in an urban setting and with greater medical complexity) (29,30). Second, we did not obtain a complete list of reasons patients may have been excluded from participation because assessments ceased as soon as they met their first exclusion criterion. Third, our recruitment approach was not completely systematic; we started targeted mailings after initial recruitment began and called only a subset of individuals who did not respond to the targeted mailing. And finally, we were not able to conduct a cost analysis of staff time spent on recruitment. Several members of the research team contributed to recruitment efforts while also juggling a multitude of other study responsibilities. Consequently, we were unable to obtain an accurate breakdown of time spent solely on each recruitment strategy, impeding our ability to conduct meaningful cost analyses. It would be an important next step to investigate the cost-effectiveness of this targeted recruitment approach.
Conclusion
Our recruitment experience has promising implications for the use of targeted mailings generated from EMRs to recruit individuals with type 2 diabetes to participate in physical activity trials. Given the widespread use of EMRs, opportunities for such recruitment strategies should be broadly accessible. The issues discussed in this article highlight the importance of considering challenges to recruitment during initial study design. Our initial low recruitment response necessitated an adjustment in recruitment efforts that resulted in a more successful approach. Other researchers may wish to consider using a targeted approach as they design studies, which could enhance recruitment flow from the outset. Further investigation is warranted to determine the types of studies and research settings for which the use of EMRs and a targeted recruitment approach may be most effective.
Acknowledgments
This research was supported by a grant titled “Telehealth Intervention to Promote Exercise for Diabetes” from the Rehabilitation Research and Development Service of the U.S. Department of Veterans Affairs (No. F4202) to Drs. Mori and Niles. The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Fisher L, Hessler D, Naranjo D, Polonsky W. AASAP: a program to increase recruitment and retention in clinical trials. Patient Educ Couns 2012;86:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters-Lawrence MH, Bell MC, Hsu LL, et al. ; Sickle Cell Disease Clinical Research Network (SCDCRN). Clinical trial implementation and recruitment: lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials 2012;33:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan J. Subject recruitment and retention: barriers to success. Appl Clin Trials 2004;13:50–54 [Google Scholar]
- 4.McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomized controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken L, Gallagher R, Madronio C. Principles of recruitment and retention in clinical trials. Int J Nurs Pract 2003;9:338–346 [DOI] [PubMed] [Google Scholar]
- 6.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 2010;33:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao G, Ford ES, Li C, Mokdad AH. Compliance with physical activity recommendations in US adults with diabetes. Diabet Med 2008;25:221–227 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and pre-diabetes in the United States, 2011. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 21 November 2013
- 9.Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI): Diabetes QUERI Center 2011 strategic plan Available from http://www.queri.research.va.gov/about/strategic_plans/dm.pdf. Accessed 21 November 2013.
- 10.Plotnikoff RC, Courneya KS, Sigal RJ, et al. Alberta Diabetes and Physical Activity Trial (ADAPT): a randomized theory-based efficacy trial for adults with type 2 diabetes: rationale, design, recruitment, evaluation and dissemination. Trials 2010;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eakin EG, Reeves MM, Lawler SP, et al. The Logan Healthy Living Program: a cluster randomized trial of a telephone-delivered physical activity and dietary behavior intervention for primary care patients with type 2 diabetes or hypertension from a socially disadvantaged community: rationale, design and recruitment. Contemp Clin Trials 2008;29:439–454 [DOI] [PubMed] [Google Scholar]
- 12.Heiney SP, Adams SA, Drake BF, Bryant LH, Bridges L, Hebert JR. Successful subject recruitment for a prostate cancer behavioral intervention trial. Clin Trials 2010;7:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow D, Gottlieb DJ, DeMolles D, Fielding RA. Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci 2011;66A:1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korde LA, Micheli A, Smith AW, et al. Recruitment to a physical activity intervention study in women at increased risk of breast cancer. BMC Med Res Methodol 2009;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin RR, Fujimoto WY, Marrero DG, et al. ; DPP Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials 2002;23:157–171 [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing and staff experience in a total recruitment program for a clinical trial: the SHEP experience. Control Clin Trials 999;20:133–148 [DOI] [PubMed] [Google Scholar]
- 17.Wisdom K, Neighbors K, Williams VH, Havstad SL, Tilley BC. Recruitment of African Americans with type 2 diabetes to a randomized controlled trial using three sources. Ethn Health 2002;7:267–278 [DOI] [PubMed] [Google Scholar]
- 18.Amthauer H, Gagilo B, Glasgow RE, Dortch W, King DK. Lessons learned: patient strategies for a type 2 diabetes intervention in a primary care setting. Diabetes Educ 2003;29:673–681 [DOI] [PubMed] [Google Scholar]
- 19.Kapp JM, Peters C, Oliver DP. Research recruitment using Facebook advertising: big potential, big challenges. J Cancer Educ 2013;28:134–137 [DOI] [PubMed] [Google Scholar]
- 20.Gordon JS, Akers L, Severson HH, Danaher BG, Boles SM. Successful participant recruitment strategies for an online smokeless tobacco cessation program. Nicotine Tob Res 2006;8(Suppl. 1):S35–S41 [DOI] [PubMed] [Google Scholar]
- 21.Glasgow RE, Strycker LA, Kurz D, et al. Recruitment for an Internet-based diabetes self-management program: scientific and ethical implications. Ann Behav Med 2010;40:40–48 [DOI] [PubMed] [Google Scholar]
- 22.Thadani SR, Weng C, Bigger JT, Ennever JF, Wajngurt D. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc 2009;16:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmickl CN, Li M, Li G, et al. The accuracy and efficiency of electronic screening for recruitment into a clinical trial on COPD. Respir Med 2011;105:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resio MA, Baltch AL, Smith RP. Mass mailing and telephone contact were effective in recruiting veterans into an antibiotic treatment randomized clinical trial. J Clin Epidemiol 2004;57:1063–1070 [DOI] [PubMed] [Google Scholar]
- 25.Pinto BM, Friedman R, Marcus BH, Kelley H, Tennstedt S, Gillman MW. Effects of a computer-based, telephone-counseling system on physical activity. Am J Prev Med 2002;23:113–120 [DOI] [PubMed] [Google Scholar]
- 26.Eakin EG, Lawler SP, Winkler EAH, Hayes SC. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: Exercise for Health. Ann Behav Med 2012;43:229–238 [DOI] [PubMed] [Google Scholar]
- 27.Piette JD, McPhee SJ, Weinberger M, Mah CA, Kraemer FB. Use of automated telephone disease management calls in an ethnically diverse sample of low-income patients with diabetes. Diabetes Care 1999;22:1302–1309 [DOI] [PubMed] [Google Scholar]
- 28.Pinto BM, Trunzo JJ, Rabin C, et al. Recruitment strategies for a home-based physical activity intervention for breast cancer patients. J Clin Psychol Med Settings 2004;11:171–178 [Google Scholar]
- 29.Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. military, veteran and civilian men. Am J Prev Med 2012;43:483–489 [DOI] [PubMed] [Google Scholar]
- 30.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? Arch Intern Med 2000;160:3252–3257 [DOI] [PubMed] [Google Scholar]