Abstract
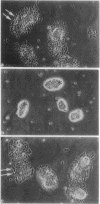
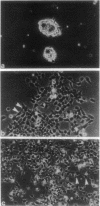
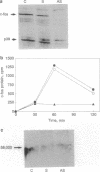
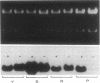
When Sertoli cells of the seminiferous epithelium in the testis were cultured on Matrigel (a reconstituted basement membrane), laminin, or one of the biologically active laminin-derived peptides (YIGSR, SIKVAV, or RGD), they exhibited dramatic changes in morphology accompanied by changes in protein secretion and gene expression, including a rapidly induced stimulation of c-fos mRNA. To examine the role of c-fos in Sertoli cell attachment, spreading, and differentiation on extracellular matrix, we constructed sense and antisense c-fos phosphorothioate oligodeoxynucleotides (ODNs). Sertoli cells, in small clumps of 10-20 cells, cultured on Matrigel or laminin in the presence of ODNs antisense to c-fos (30 micrograms/ml) did not spread for the first 10-20 hr. After that time, normal spreading occurred, probably as a result of ODN degradation. Cells cultured in medium supplemented with ODNs sense to c-fos (30 micrograms/ml), or without ODNs, spread within 30 min to 1 hr, and after 12 hr a monolayer was established. Furthermore, incubation of Sertoli cells with ODNs antisense to c-fos resulted in a significant reduction of c-fos protein levels, whereas treatment with ODNs sense to c-fos barely affected c-fos protein expression. These data indicate that c-fos may mediate the events involved in Sertoli cell attachment and spreading upon contact of the cells with extracellular matrix.
Full text
PDF
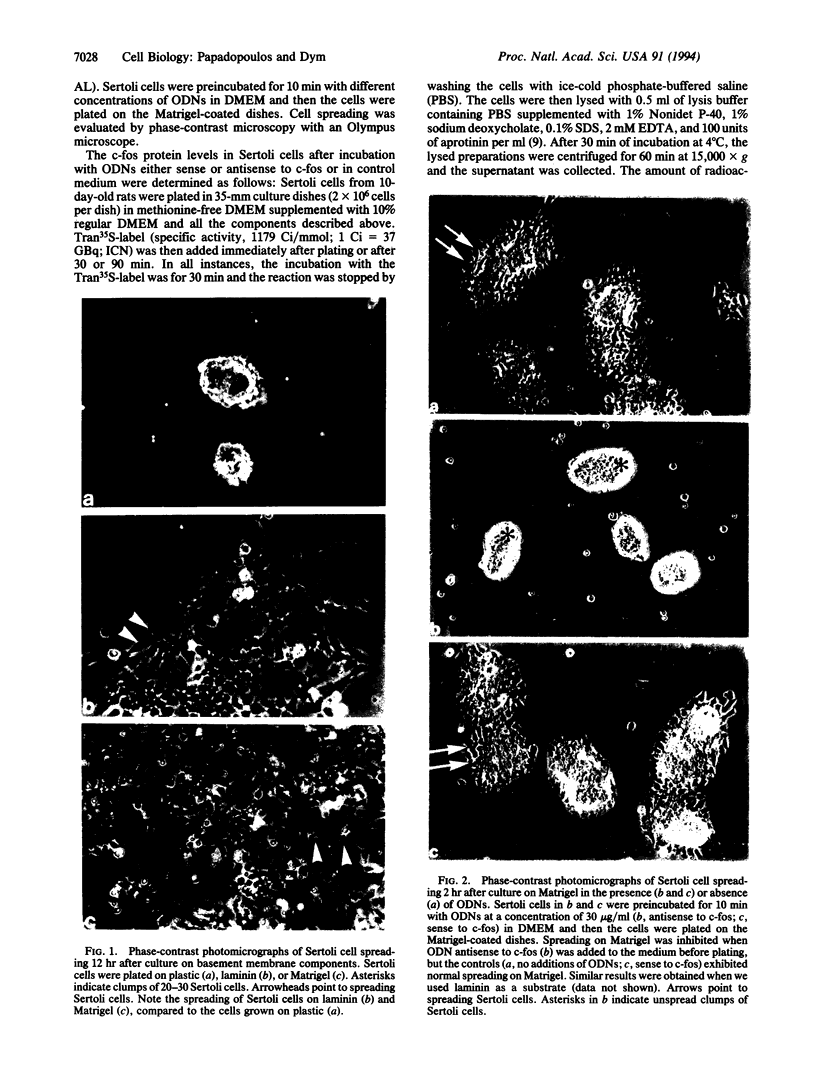
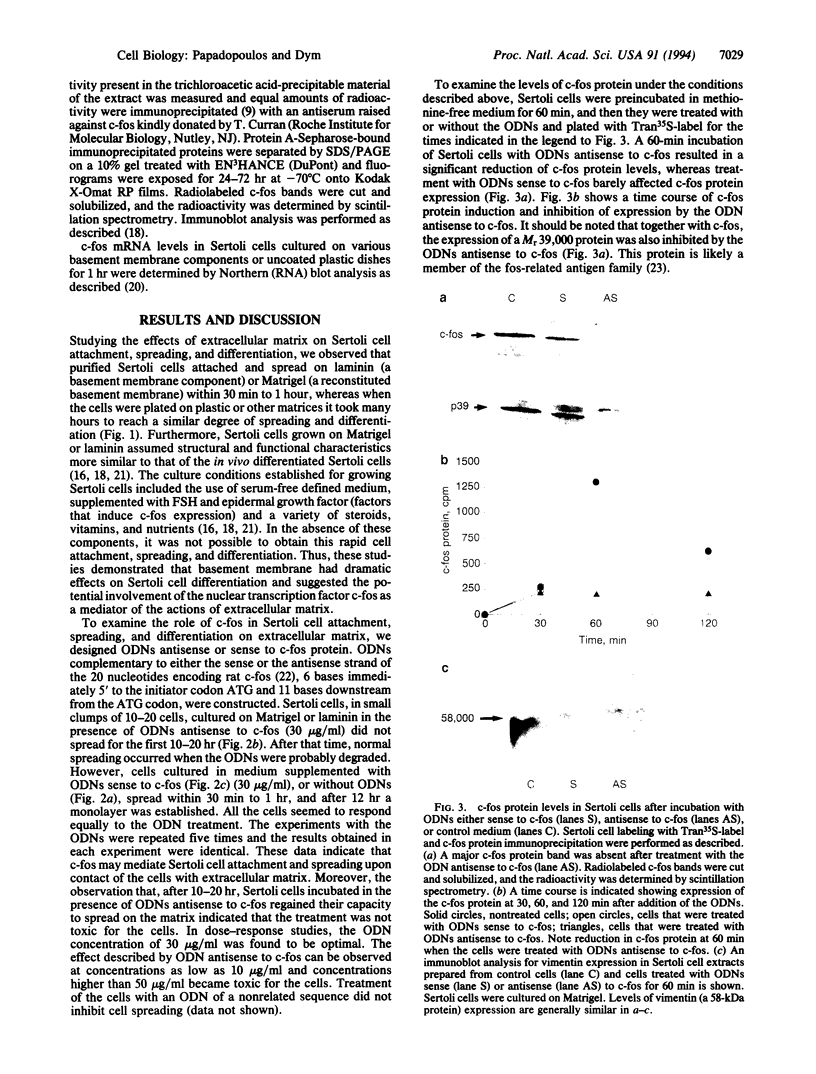


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M., Lamsam-Casalotti S., Jia M. C., Kleinman H. K., Papadopoulos V. Basement membrane increases G-protein levels and follicle-stimulating hormone responsiveness of Sertoli cell adenylyl cyclase activity. Endocrinology. 1991 Feb;128(2):1167–1176. doi: 10.1210/endo-128-2-1167. [DOI] [PubMed] [Google Scholar]
- Hadley M. A., Byers S. W., Suárez-Quian C. A., Djakiew D., Dym M. In vitro models of differentiated Sertoli cell structure and function. In Vitro Cell Dev Biol. 1988 Jun;24(6):550–557. doi: 10.1007/BF02629090. [DOI] [PubMed] [Google Scholar]
- Hadley M. A., Byers S. W., Suárez-Quian C. A., Kleinman H. K., Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985 Oct;101(4):1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. H., Joseph D. R., French F. S., Conti M. Follicle-stimulating hormone induces transient expression of the protooncogene c-fos in primary Sertoli cell cultures. Mol Endocrinol. 1988 Jan;2(1):55–61. doi: 10.1210/mend-2-1-55. [DOI] [PubMed] [Google Scholar]
- Janecki A., Steinberger A. Vectorial secretion of transferrin and androgen binding protein in Sertoli cell cultures: effect of extracellular matrix, peritubular myoid cells and medium composition. Mol Cell Endocrinol. 1987 Jul;52(1-2):125–135. doi: 10.1016/0303-7207(87)90105-5. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kubota S., Tashiro K., Yamada Y. Signaling site of laminin with mitogenic activity. J Biol Chem. 1992 Mar 5;267(7):4285–4288. [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Igarashi M. Demonstration of high molecular weight forms of inhibin in bovine follicular fluid (bFF) by using monoclonal antibodies to bFF 32K inhibin. Biochem Biophys Res Commun. 1986 May 14;136(3):1103–1109. doi: 10.1016/0006-291x(86)90447-x. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Norton J. N., Skinner M. K. Regulation of Sertoli cell differentiation by the testicular paracrine factor PModS: potential role of immediate-early genes. Mol Endocrinol. 1992 Dec;6(12):2018–2026. doi: 10.1210/mend.6.12.1491688. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Jia M. C., Culty M., Hall P. F., Dym M. Rat Sertoli cell aromatase cytochrome P450: regulation by cell culture conditions and relationship to the state of cell differentiation. In Vitro Cell Dev Biol Anim. 1993 Dec;29A(12):943–949. doi: 10.1007/BF02634233. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993 Feb;120(4):1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H. K., Luyten F. P., Roberts A. B., Roche N. S., Reddi A. H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992 Sep;202(1):1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]