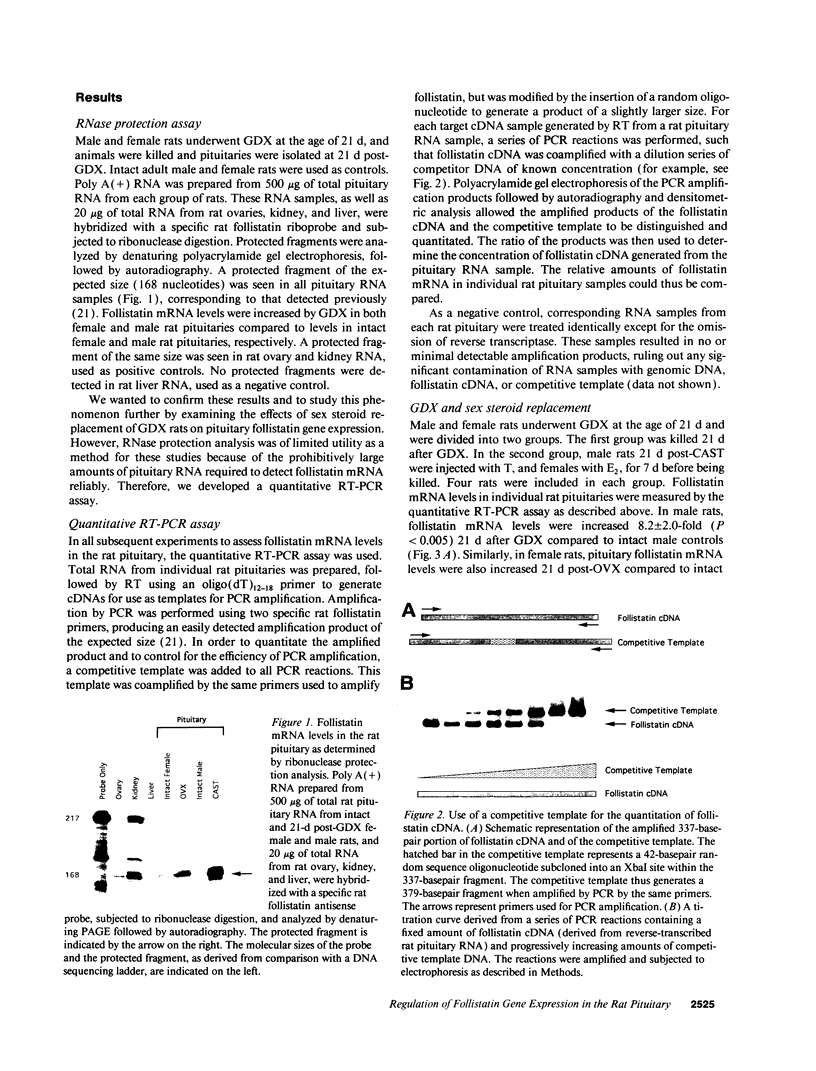
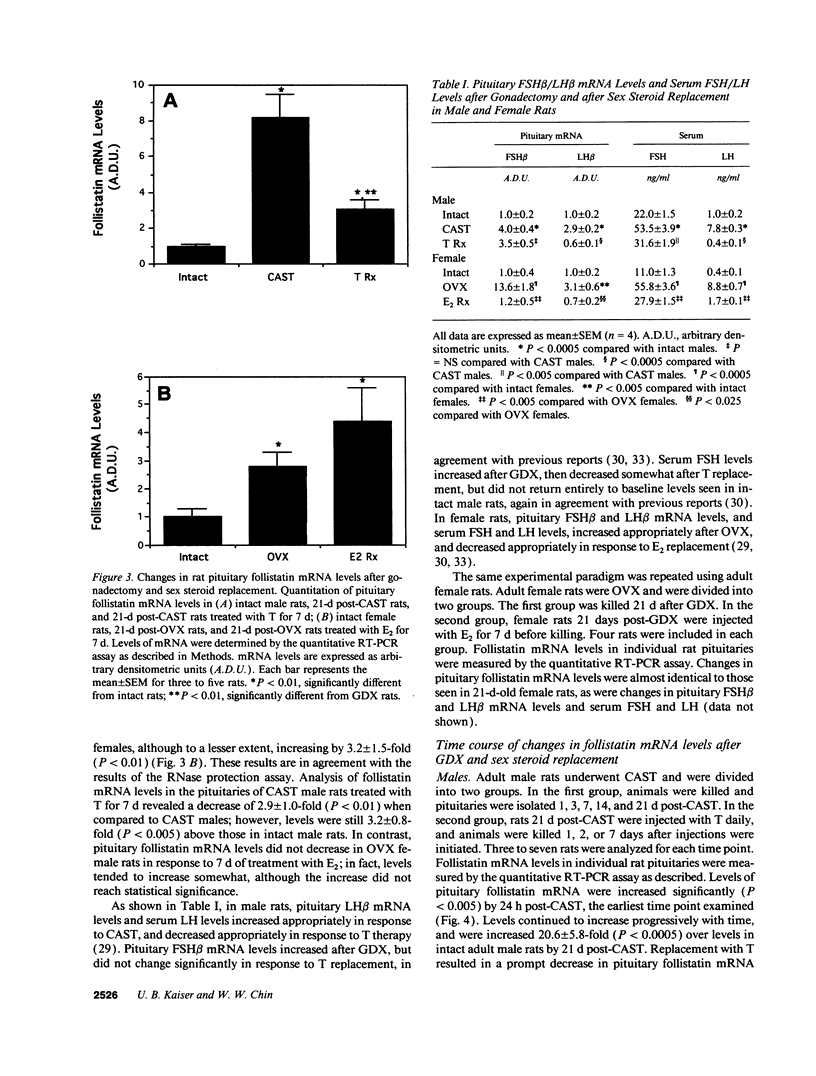
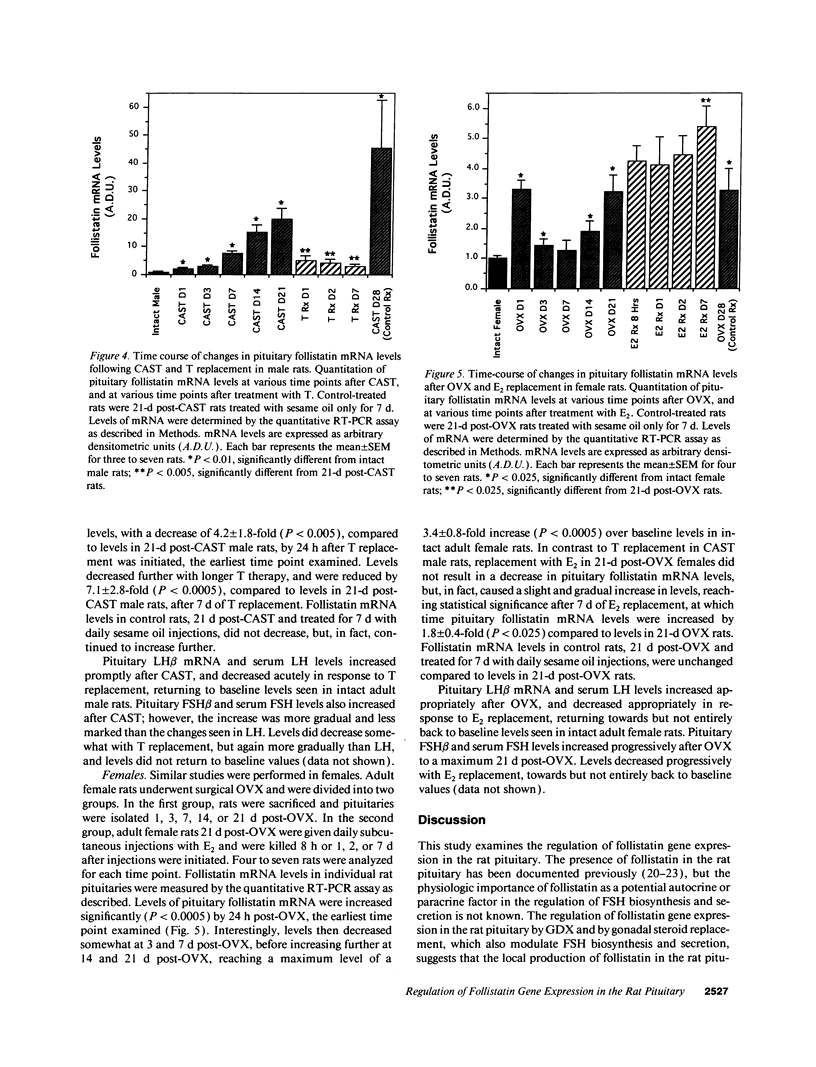
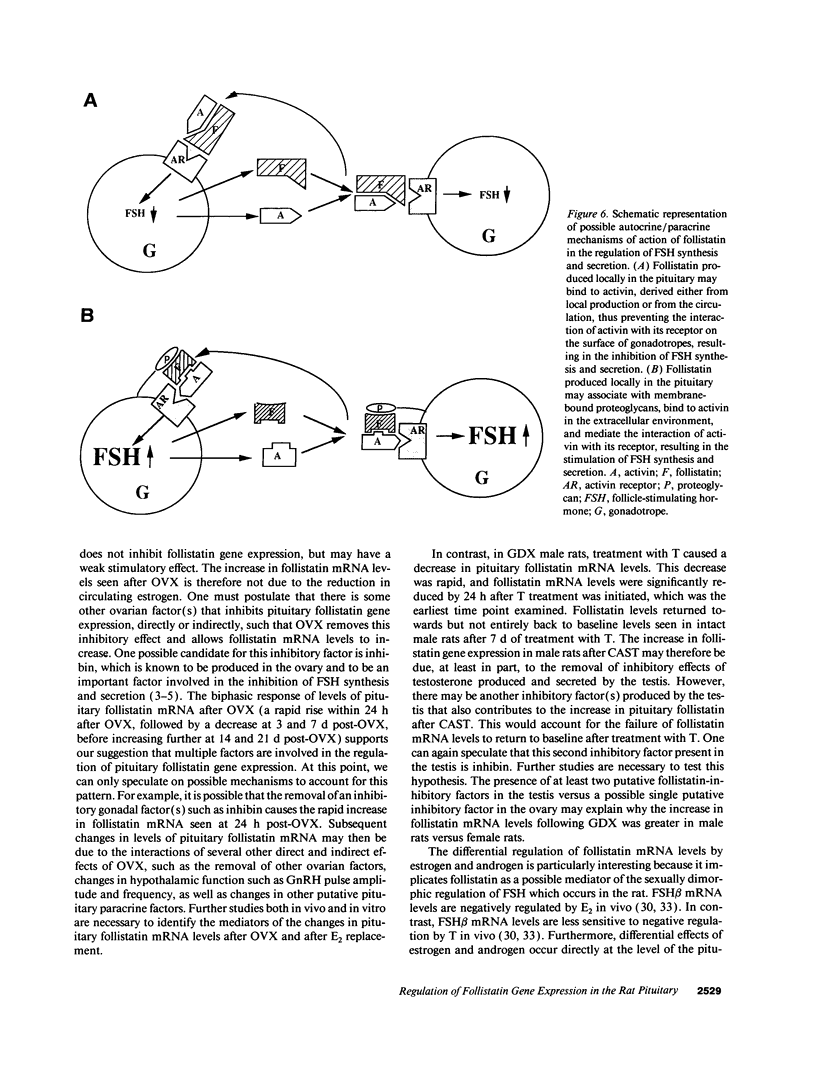
Abstract
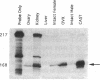
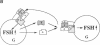
Follistatin is a glycoprotein, originally isolated from the gonads, that specifically inhibits follicle-stimulating hormone (FSH) biosynthesis and secretion. We have previously detected follistatin mRNA in rat pituitary gonadotropes. To assess the potential physiologic role of follistatin in the rat pituitary, we have investigated the effects of gonadectomy (GDX) and of sex steroid replacement on pituitary follistatin gene expression. Follistatin mRNA levels in individual rat pituitaries were measured by a quantitative reverse transcription-polymerase chain reaction assay. Female and male rats 21 d old underwent surgical GDX and were then killed 21 d after GDX. Follistatin mRNA levels in rat pituitary increased 3.2 +/- 1.5-fold (P < 0.01) in GDX female rats and 8.2 +/- 2.0-fold (P < 0.005) in GDX male rats, compared with intact female and male controls, respectively. Replacement therapy with 17 beta-estradiol-3-benzoate (10 micrograms/100 g body weight) subcutaneously daily for 7 d in GDX female rats resulted in a slight further increase in follistatin mRNA levels compared to GDX females. In contrast, therapy with testosterone propionate (500 micrograms/100 g body weight) subcutaneously daily for 7 d in GDX male rats resulted in a decrease in follistatin mRNA levels, towards but not completely back to baseline levels in intact males. Time-course studies in adult male and female rats showed that the increase in follistatin mRNA levels after GDX is rapid, with significant increases occurring within 24 h after GDX, and parallels or precedes increases in FSH beta mRNA levels and FSH secretion. The regulation of follistatin mRNA levels in the rat pituitary by GDX and by sex steroids suggests that follistatin may be important as an autocrine or paracrine factor in the regulation of FSH.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi B., Keeping H. S., Winters S. J., Kotsuji F., Maurer R. A., Troen P. Rapid and profound suppression of messenger ribonucleic acid encoding follicle-stimulating hormone beta by inhibin from primate Sertoli cells. Mol Endocrinol. 1989 Feb;3(2):280–287. doi: 10.1210/mend-3-2-280. [DOI] [PubMed] [Google Scholar]
- Attardi B., Vaughan J., Vale W. Regulation of FSH beta messenger ribonucleic acid levels in the rat by endogenous inhibin. Endocrinology. 1992 Jan;130(1):557–559. doi: 10.1210/endo.130.1.1727723. [DOI] [PubMed] [Google Scholar]
- Badger T. M., Wilcox C. E., Meyer E. R., Bell R. D., Cicero T. J. Simultaneous changes in tissue and serum levels of luteinizing hormone, follicle-stimulating hormone, and luteinizing hormone/follicle-stimulating hormone releasing factor after castration in the male rat. Endocrinology. 1978 Jan;102(1):136–141. doi: 10.1210/endo-102-1-136. [DOI] [PubMed] [Google Scholar]
- Carroll R. S., Corrigan A. Z., Gharib S. D., Vale W., Chin W. W. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol. 1989 Dec;3(12):1969–1976. doi: 10.1210/mend-3-12-1969. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Ling N., Guillemin R., Massagué J. A surface component on GH3 pituitary cells that recognizes transforming growth factor-beta, activin, and inhibin. J Biol Chem. 1988 Nov 25;263(33):17225–17228. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Correa-Rotter R., Mariash C. N., Rosenberg M. E. Loading and transfer control for Northern hybridization. Biotechniques. 1992 Feb;12(2):154–158. [PubMed] [Google Scholar]
- Corrigan A. Z., Bilezikjian L. M., Carroll R. S., Bald L. N., Schmelzer C. H., Fendly B. M., Mason A. J., Chin W. W., Schwall R. H., Vale W. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology. 1991 Mar;128(3):1682–1684. doi: 10.1210/endo-128-3-1682. [DOI] [PubMed] [Google Scholar]
- DePaolo L. V., Shimonaka M., Schwall R. H., Ling N. In vivo comparison of the follicle-stimulating hormone-suppressing activity of follistatin and inhibin in ovariectomized rats. Endocrinology. 1991 Feb;128(2):668–674. doi: 10.1210/endo-128-2-668. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Shimasaki S., Mercado M., Cooksey K., Ling N., Ying S., Ueno N., Guillemin R. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987 Nov;1(11):849–855. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- Fink G. Feedback actions of target hormones on hypothalamus and pituitary with special reference to gonadal steroids. Annu Rev Physiol. 1979;41:571–585. doi: 10.1146/annurev.ph.41.030179.003035. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Bowers S. M., Need L. R., Chin W. W. Regulation of rat luteinizing hormone subunit messenger ribonucleic acids by gonadal steroid hormones. J Clin Invest. 1986 Feb;77(2):582–589. doi: 10.1172/JCI112340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib S. D., Leung P. C., Carroll R. S., Chin W. W. Androgens positively regulate follicle-stimulating hormone beta-subunit mRNA levels in rat pituitary cells. Mol Endocrinol. 1990 Nov;4(11):1620–1626. doi: 10.1210/mend-4-11-1620. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Wierman M. E., Badger T. M., Chin W. W. Sex steroid hormone regulation of follicle-stimulating hormone subunit messenger ribonucleic acid (mRNA) levels in the rat. J Clin Invest. 1987 Aug;80(2):294–299. doi: 10.1172/JCI113072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Lau K. Pituitary follicular cells secrete both vascular endothelial growth factor and follistatin. Biochem Biophys Res Commun. 1989 Nov 30;165(1):292–298. doi: 10.1016/0006-291x(89)91068-1. [DOI] [PubMed] [Google Scholar]
- Kaiser M., Gibori G., Mayo K. E. The rat follistatin gene is highly expressed in decidual tissue. Endocrinology. 1990 May;126(5):2768–2770. doi: 10.1210/endo-126-5-2768. [DOI] [PubMed] [Google Scholar]
- Kaiser U. B., Lee B. L., Carroll R. S., Unabia G., Chin W. W., Childs G. V. Follistatin gene expression in the pituitary: localization in gonadotropes and folliculostellate cells in diestrous rats. Endocrinology. 1992 May;130(5):3048–3056. doi: 10.1210/endo.130.5.1572312. [DOI] [PubMed] [Google Scholar]
- Kogawa K., Nakamura T., Sugino K., Takio K., Titani K., Sugino H. Activin-binding protein is present in pituitary. Endocrinology. 1991 Mar;128(3):1434–1440. doi: 10.1210/endo-128-3-1434. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Boyd F. T., Andres J. L. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59–72. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Meunier H., Rivier C., Evans R. M., Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel U., Albiston A., Findlay J. K. Rat follistatin: gonadal and extragonadal expression and evidence for alternative splicing. Biochem Biophys Res Commun. 1990 Nov 30;173(1):401–407. doi: 10.1016/s0006-291x(05)81072-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Sugino K., Titani K., Sugino H. Follistatin, an activin-binding protein, associates with heparan sulfate chains of proteoglycans on follicular granulosa cells. J Biol Chem. 1991 Oct 15;266(29):19432–19437. [PubMed] [Google Scholar]
- Nakamura T., Takio K., Eto Y., Shibai H., Titani K., Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990 Feb 16;247(4944):836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Farnworth P. G., Clarke L., Jacobsen J., Cahir N. F., Burger H. G., de Kretser D. M. Effects of bovine 35 kDa FSH-suppressing protein on FSH and LH in rat pituitary cells in vitro: comparison with bovine 31 kDa inhibin. J Endocrinol. 1990 Mar;124(3):417–423. doi: 10.1677/joe.0.1240417. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Klein R., de Vos F. L., McLachlan R. I., Wettenhall R. E., Hearn M. T., Burger H. G., de Kretser D. M. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem Biophys Res Commun. 1987 Dec 16;149(2):744–749. doi: 10.1016/0006-291x(87)90430-x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shimasaki S., Koga M., Buscaglia M. L., Simmons D. M., Bicsak T. A., Ling N. Follistatin gene expression in the ovary and extragonadal tissues. Mol Endocrinol. 1989 Apr;3(4):651–659. doi: 10.1210/mend-3-4-651. [DOI] [PubMed] [Google Scholar]
- Shimonaka M., Inouye S., Shimasaki S., Ling N. Follistatin binds to both activin and inhibin through the common subunit. Endocrinology. 1991 Jun;128(6):3313–3315. doi: 10.1210/endo-128-6-3313. [DOI] [PubMed] [Google Scholar]
- Shiozaki M., Sakai R., Tabuchi M., Nakamura T., Sugino K., Sugino H., Eto Y. Evidence for the participation of endogenous activin A/erythroid differentiation factor in the regulation of erythropoiesis. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1553–1556. doi: 10.1073/pnas.89.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukovski L., Findlay J. K., Robertson D. M. The effect of follicle-stimulating hormone-suppressing protein or follistatin on luteinizing bovine granulosa cells in vitro and its antagonistic effect on the action of activin. Endocrinology. 1991 Dec;129(6):3395–3402. doi: 10.1210/endo-129-6-3395. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Ling N., Ying S. Y., Esch F., Shimasaki S., Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. F., Farnworth P. G., Findlay J. K., Burger H. G. Chronic inhibitory effect of follicle-stimulating hormone (FSH)-suppressing protein (FSP) or follistatin on activin- and gonadotropin-releasing hormone-stimulated FSH synthesis and secretion in cultured rat anterior pituitary cells. Endocrinology. 1990 Sep;127(3):1385–1393. doi: 10.1210/endo-127-3-1385. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wierman M. E., Gharib S. D., LaRovere J. M., Badger T. M., Chin W. W. Selective failure of androgens to regulate follicle stimulating hormone beta messenger ribonucleic acid levels in the male rat. Mol Endocrinol. 1988 Jun;2(6):492–498. doi: 10.1210/mend-2-6-492. [DOI] [PubMed] [Google Scholar]
- Wierman M. E., Gharib S. D., Wang C., LaRovere J. M., Badger T. M., Chin W. W. Divergent regulation of gonadotropin subunit mRNA levels by androgens in the female rat. Biol Reprod. 1990 Aug;43(2):191–195. doi: 10.1095/biolreprod43.2.191. [DOI] [PubMed] [Google Scholar]
- Xiao S., Findlay J. K. Interactions between activin and follicle-stimulating hormone-suppressing protein and their mechanisms of action on cultured rat granulosa cells. Mol Cell Endocrinol. 1991 Aug;79(1-3):99–107. doi: 10.1016/0303-7207(91)90100-7. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Swanson G., Tan P., Ling N., Esch F., Ueno N., Shimasaki S., Guillemin R. Follistatin specifically inhibits pituitary follicle stimulating hormone release in vitro. Biochem Biophys Res Commun. 1987 Nov 30;149(1):133–139. doi: 10.1016/0006-291x(87)91614-7. [DOI] [PubMed] [Google Scholar]
- Ying S. Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988 May;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]