Summary
Background
With the use of modern cross-sectional abdominal imaging modalities, an increasing number of cystic pancreatic lesions are identified incidentally. Although there is no pathological diagnosis available in most cases, it is believed that the majority of these lesions display small branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) of the pancreas. Even though a number of large clinical series have been published, many uncertainties remain with regard to this entity of mucinous cystic neoplasms.
Methods
Systematic literature review.
Results
Main-duct (MD) and mixed-type IPMNs harbor a high risk of malignant transformation. It is conceivable that most IPMNs with involvement of the main duct tend to progress to invasive carcinoma over time. Thus, formal oncologic resection is the treatment of choice in surgically fit patients. In contrast, the data regarding BD-IPMN remain equivocal, resulting in conflicting concepts. To date, it is not clear whether and which BD-IPMNs progress to carcinoma and how long this progression takes.
Conclusion
While patients with MD-IPMNs should undergo surgical resection if comorbidities and life expectancy permit this, the management of small BD-IPMNs remains controversial. Population-based studies with long-term follow-up are needed to define which cohort of patients can be observed safely without immediate resection.
Keywords: Intraductal papillary mucinous neoplasm, IPMN, Pancreas, Natural history, Malignant transformation, Cancerogenesis
Introduction
Large cross-sectional studies using modern abdominal imaging modalities such as magnetic resonance imaging (MRI) or computed tomography (CT) have shown that approximately 2% of the general population exhibit cystic lesions in the pancreas [1, 2]. Most of these lesions were found in asymptomatic patients who underwent abdominal imaging for non-pancreas-related abdominal symptoms including urologic, gynecologic, vascular, or other medical conditions [3]. The incidence of cystic pancreatic lesions increases with age and reaches a level of up to 10% in patients of 70 years and older [1]. The majority of authors assume that a large proportion of these lesions represent small intraductal papillary mucinous neoplasms (IPMNs) of the pancreas [2, 4]. However, in the majority of cases there is no histopathological diagnosis available to confirm this assumption. IPMNs are a distinct entity of cystic pancreatic neoplasms characterized by mucin production, cystic dilation of the pancreatic ductal system, and intrapapillary epithelial growth [5, 6]. Moreover, IPMNs are believed to undergo an adenoma-carcinoma sequence eventually culminating in invasive ductal adenocarcinoma over time [3]. Few issues in gastrointestinal medicine are discussed as controversially as the adequate management of patients with these pancreatic lesions [4]. Particularly the role of side-branch IPMNs that exclusively involve the branch duct system of the pancreas is not entirely understood [7, 8, 9]. While a number of clinical series have shown that conservative observation of small branch-duct IPMNs (BD-IPMNs) without specific risk factors is safe over extended time periods [10], other studies have reported that in a significant number of surgically resected BD-IPMNs, high-grade dysplasia or even invasive carcinoma was found on surgical pathology [11, 12, 13, 14]. To date it is not known whether and which IPMNs harbor the risk of malignant transformation and over what time period malignant transformation develops.
The aim of the present article is to summarize current evidence about the natural history of IPMNs. Moreover, the reader should gain insights into the current controversial discussion about the adequate therapeutic management of patients with these lesions.
Epidemiology
IPMNs usually occur between the sixth and seventh decade of life and are mostly located in the head of the pancreas [10, 15, 16]. Considering all IPMN patients, an almost equal gender distribution is found; however, more recent studies suggest that BD-IPMNs may occur slightly more often in females [17, 18]. While older studies have reported that approximately 25% of IPMNs are asymptomatic at the time of discovery [19, 20], recent series found that up to 60% of cystic pancreatic neoplasms are discovered accidentally [18, 21]. When patients with IPMN present with symptoms, they frequently suffer from abdominal pain, weight loss, jaundice, steatorrhea, new onset of diabetes, or pancreatitis [15, 20, 22, 23]. Most IPMNs occur spontaneously and without evidence of a systemic or familiar disease. However, it has been reported that IPMNs may also arise in the background of inherited syndromes such as Peutz-Jeghers syndrome [24] or familial adenomatous polyposis [25]. A small subset of patients with IPMN is associated with a specific phenotype of familial pancreatic cancer [26]. Beside familial pancreatic cancer, patients with IPMN are in general at a higher risk of developing concomitant pancreatic cancer, either synchronously or metachronously. This is an important observation because it indicates that resection of an IPMN only reduces the risk of developing pancreatic cancer but does not abolish it. The risk has been described to be as high as 10% [27], and there is growing evidence that patients with IPMN, as opposed to patients suffering from spontaneous ductal adenocarcinoma, are also at a higher risk of developing primary extrapancreatic malignancy [28]. For example, patients with IPMNs have a significantly higher risk of harboring colon polyps with consecutive colon cancer compared to the general population. Therefore, colonoscopy should be considered in all IPMN patients for screening purposes [29].
Pathological and Histopathological Background
Macroscopically, IPMNs appear as mucin-producing, predominantly papillary cystic neoplasms that probably arise from the epithelium of the main pancreatic duct (MD-IPMN), from its side branches (BD-IPMN), or from both (mixed-type IPMN) [30]. It remains controversial whether mixed-type IPMNs arise from either MD- or BD-IPMNs or if they represent a distinct entity [8]. The majority of IPMNs occur in the head or uncinate process of the pancreas [16, 23]. However, in approximately 15% of cases the lesions are multifocal, eventually involving the entire pancreatic gland [7, 23]. In some cases, the lesion presents as multicentric, forming clusters of cysts [31].
Considering the histopathological examination, surgically resected IPMNs frequently display a wide range of dysplasia ranging from low-grade and moderate epithelial dysplasia (formally called ‘adenoma’ and ‘borderline’ lesions, respectively) way through to high-grade dysplasia (‘carcinoma in situ’) and frank invasive adenocarcinoma. Since a time lag of approximately 5 years was observed between patients presenting with benign IPMN and those presenting with malignant IPMN including high-grade dysplasia and adenocarcinoma [6], current thinking is that a progression from preexisting low-grade dysplastic lesions to frank invasive cancer develops over time [32]. It has been reported that this disease progression is associated with cumulative genetic alterations, including a number of mutations known from sporadic ductal adenocarcinoma such as KRAS and TP53 [33].
Beside the location of the IPMN with regard to involvement of the main pancreatic duct or its side branches, it has recently become clear that four histological subtypes of IPMNs can be distinguished [34]: gastric, intestinal, pancreatobiliary, and oncocytic. These subtypes can be distinguished by morphology only as well as by their characteristic mucin expression pattern, particularly with regard to MUC1, MUC2, and MUC5AC. There is growing evidence that these four subtypes are associated with a different risk of malignant transformation and different clinical outcomes. Concerning invasive carcinoma arising in IPMN, three distinct histological subtypes can be distinguished: tubular, colloid, and oncocytic [30]. Analyzing the outcome of these three subtypes of IPMN, it has been observed that the more favorable outcome of patients with invasive IPMN compared to sporadic ductal adenocarcinoma [6, 20, 23, 35] can only be demonstrated for colloid and oncocytic carcinomas [36]. In contrast, tubular adenocarcinoma was associated with an even poorer outcome compared to that of sporadic pancreatic cancer [36].
Today, it is widely accepted that MD-IPMNs exhibit a significantly higher risk of malignant transformation than BD-IPMNs [10]. The risk of harboring malignancy in MD-IPMNs is as high as 45% for invasive carcinoma, with an additional risk of 20% for harboring high-grade dysplasia (carcinoma in situ) [37]. Therefore, international guidelines recommend surgical resection for MD-and mixed-type IPMNs provided that the patient is an adequate candidate for surgery [10, 38] and that comorbidity and life expectancy do not preclude this option. In contrast, it has been proposed to conservatively observe small BD-IPMNs without immediate resection. In recent years, a number of clinical series have been reported demonstrating the natural course of BD-IPMNs. Due to conflicting results of these studies and the difficulty to correctly diagnose malignancy in BD-IPMNs preoperatively, there remain great differences in the management recommendations – particularly for small and asymptomatic BD-IPMNs [39].
Diagnostic Work-Up for Cystic Pancreatic Lesions
The diagnostic work-up for cystic lesions of the pancreas should definitely include thin-slice cross-sectional studies by CT scan or MRI [40]. Using these modalities, detailed information about the location, cyst size, and potential relationship to blood vessels or other structures can be obtained. A dilation of the main pancreatic duct of ≥1 cm strongly suggests MD-IPMN, whereas a mucinous cystic lesion with communication to the main duct but without main duct dilation indicates BD-IPMN [41]. In addition to cross-sectional imaging, a number of diagnostic means have been described to be useful for correctly diagnosing IPMN. Endoscopic ultrasound (EUS) is widely established and recommended in the preoperative work-up of IPMNs [10] and, together with MRI/MRCP (magnetic resonance cholangiopancreatography), better at detecting a communication between a cystic lesion and a pancreatic duct than CT scans. While some authors report that contrast-enhanced EUS might be the most reliable tool to detect mural nodules within the cystic lesion and to accordingly distinguish benign from high-risk/malignant IPMN [12, 42], others stated that mural nodules were found only in a minority of patients even when malignancy was already established [14, 43]. Furthermore, even though EUS in combination with fine needle aspiration (FNA) is often used to establish malignant transformation, a negative finding does not always rule out malignancy [14]. In any case, it is obvious that the use of EUS and FNA is strongly dependent on the experience of the operator [44]. Likewise, the evaluation of cytology following cyst fluid aspiration correlates with the experience of the involved pathologist. While cyst fluid carcinoembryonic antigen (CEA) was shown to be helpful in differentiating between benign pancreatic pseudocysts and potentially malignant mucinous neoplasms [45], this parameter is of limited value in detecting malignancy in a mucinous lesion [46]. Recently, there has been evidence that the serum tumor markers carbohydrate antigen 19-9 (CA 19-9) and CEA are useful tools in distinguishing between benign and invasive IPMN [18, 47]. However, these markers are not suitable to identify obligate precancerous lesions such as IPMNs with high-grade dysplasia that should be considered for surgical resection before malignant transformation occurs [47]. Once they are significantly elevated, the underlying tumor has often progressed to a stage where it is no longer operable.
In summary, all available preoperative diagnostic tools have their limitations, and to date, there is no method available to discriminate between benign and malignant IPMN with a high degree of certainty [13, 48]. This issue needs to be taken into account for choosing the best therapeutic approach in each individual patient although current guidelines suggest that a diameter of >3 cm and the presence of mural nodules should tip the balance in favor of a surgical approach.
Observational Studies for BD-IPMNs
The revised 2012 international consensus guidelines defined so-called ‘worrisome features’ and ‘high-risk stigmata’ in order to determine treatment recommendations for BD-IPMNs [10]. According to these treatment recommendations, patients identified with cystic lesions with ‘high-risk stigmata’ such as obstructive jaundice, an enhanced solid component within the cyst, or an increasing cyst size over time should undergo resection without further diagnostic evaluation. In contrast, for cysts with ‘worrisome features’ such as a cyst diameter of 3 cm or more, a thickened or enhancing cyst wall, or non-enhancing mural nodules, further diagnostic evaluation using EUS and FNA cytology is recommended. According to the current international guidelines, for asymptomatic cysts without any of these criteria, observation without immediate resection is recommended [10].
There are several large retrospective series available that validated the safety of these recommendations, encompassing more than 250 patients [23, 31, 49]. In none of the patients in these series who met the criteria for conservative observation but still underwent pancreatic resection, cancer was found in the surgical specimen. Moreover, two recent studies confirmed the management guidelines, stating that following the observation recommendations is safe, whereas caution is advised for larger cystic lesions [18]: Jang et al. [50] reported that the new consensus guidelines provided better sensitivity, performance of factors predicting malignancy, and balanced accuracy in the diagnosis of BD-IPMN malignancy. However, in contrast to other studies, Jang et al. [50] found that size alone was of limited accuracy for predicting malignancy. Kobayashi et al. [51] reported that even when small mural nodules of <1 cm in diameter were found, observation was considered safe due to a low risk of malignant transformation. It should be noted here that while the mural nodules are an indicator for the risk of malignant transformation, the cancer does not necessarily arise from these nodules but in other portions of the cystic lesion [51]. Two recent series examined the risk of malignancy in BD-IPMNs with minimal or limited involvement of the main pancreatic duct [52, 53]. The authors of both studies conclude that primary surveillance of mixed-type IPMNs might be reasonable in selected patients. However, in the study of Roch et al. [53], among 70 patients with mixed-type IPMN, 9 (13%) showed a progression to invasive carcinoma at a mean of 3.5 years (range: 1–9 years).
With regard to long-term follow-up studies, it remains unknown when the neoplastic transformation from benign IPMNs to malignant lesions begins [9, 41]. It was suggested that disease progression occurs in 10–40% of the patients over 5 years, and the calculated risk of cancer development is approximately 20% over 10 years [9, 41]. It is well established that patients with IPMN are not only at a higher risk of developing IPMN-associated adenocarcinoma but also of concomitant sporadic pancreatic cancer, either synchronously or metachronously [27]. In these cases, the IPMN indicates only the tip of the iceberg of a pancreatic field defect [54], leading to neoplastic changes over time [7]. It also denotes that resecting the IPMN does not necessarily abolish the risk of pancreatic cancer and that patients have to remain in postoperative surveillance programs. In spite of such a follow-up, the progression to invasive cancer can be missed, as has been reported in patients who developed non-resectable pancreatic cancer during close follow-up after IPMN resection [9]. Not only this issue but also the potential of non-compliance during follow-up always has to be balanced against the risk of surgical resection in each individual patient [55].
Surgical Series of Resected BD-IPMNs
According to the current guidelines, younger patients (<65 years) with a cyst size of >2 cm may also be candidates for resection [10], owing the cumulative risk of malignancy over time [12, 56] and their life expectancy. In recent years, an increasing number of surgical series has been published reporting that also small and unsuspicious BD-IPMNs may harbor a significant risk of malignancy in up to 20% of the cases [11, 12, 13, 57]. Wong et al. [14] found that high-grade dysplasia and adenocarcinoma were found in BD-IPMNs measuring less than 3 cm on EUS. Salvia et al. [48] reported that even in an experienced pancreatic center inaccurate preoperative diagnosis for cystic pancreatic lesions approached 22%. Likewise, Barron et al. [58] found that the preoperative radiographic IPMN type did not correlate with the final pathology in 25% of the patients. The authors suggest considering serum CA 19-9 as a complementary tool within the context of preoperative evaluation of cystic lesions [48]. Indeed, in a recent systematic review by Goh et al. [59], the limitations of the Sendai consensus guidelines were confirmed. The positive predictive value of the guidelines for predicting a malignant BD-IPMN was low, and some malignant lesions might be missed when diagnosis is based on these guidelines [59]. In this study, among 690 surgically resected BD-IPMNs, a malignancy rate of 24% was found. The strength of this study is that for each patient the final histological diagnosis was available. However, the incidence of malignancy only refers to patients who underwent surgery. It is possible that the real incidence of malignancy in BD-IPMNs is much lower because experienced clinicians were more likely to select patients at an increased risk of malignancy for resection. Surgeons often decide on resection based on a number of clinical factors that were not implemented in the international consensus guidelines, such as serum tumor markers, age, and previous extrapancreatic malignancy (e.g. breast cancer), and other unknown criteria [43, 60]. This assumption is also supported by the study of Roch et al. [53] who reported that the criteria mentioned in the international consensus guidelines carried an unequal weight and were not cumulative in the prediction of risk for malignancy or invasiveness in IPMN.
In many cases, instead of a formal oncologic resection, a pancreatic parenchyma-sparing operation such as an enucleation can be considered for BD-IPMNs, even when they present multifocally (fig. 1). Depending on fresh-frozen section histology, the surgeon can decide whether a formal resection is necessary for the adequate treatment (fig. 2). Pancreatic enucleations can be performed with low morbidity and no mortality in high volume centers [61] and therefore represent an alternative to formal oncologic resections. In view of the fact that a significant risk of developing pancreatic cancer remains even after successful oncological IPMN resection, a more limited approach to either confirm or refute the malignant nature of a cystic lesion appears reasonable.
Fig. 1.
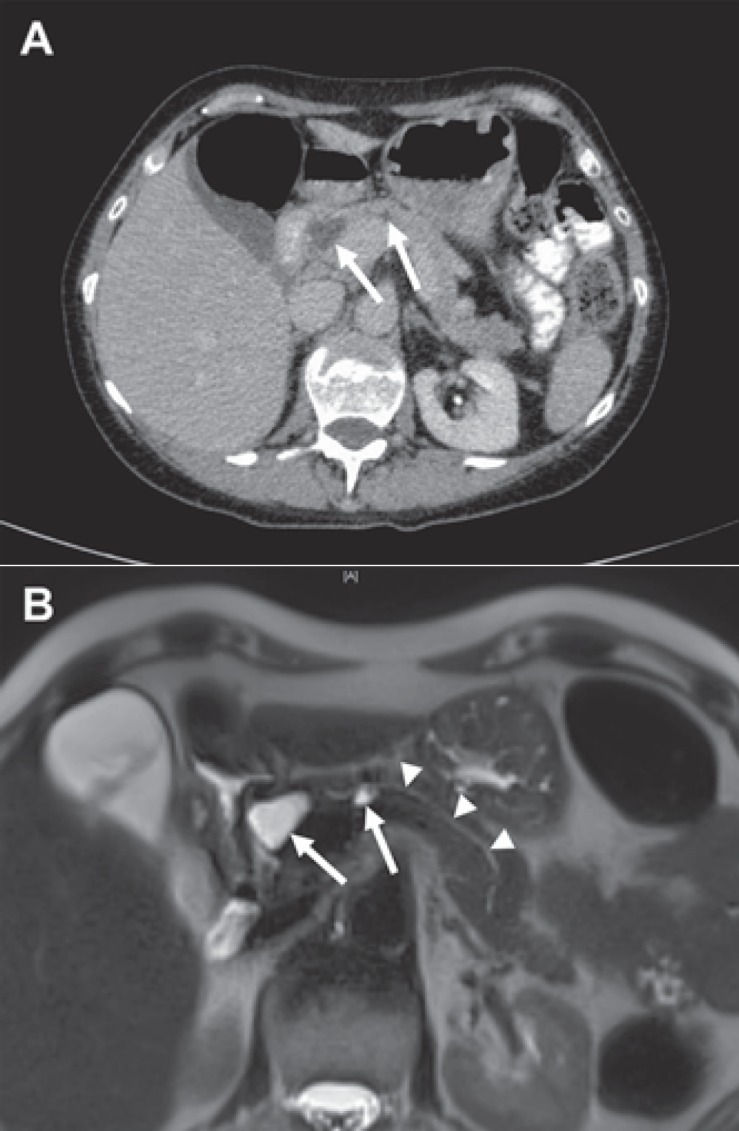
Two small BD-IPMNs in the head and corpus of the pancreas. A CT imaging showing two hypodense cystic lesions in the pancreatic head and corpus (arrows) of a 52-year-old female. In maximal diameter, the lesions measured 2.2 and 1.4 cm, respectively. B T2-weighted MRI exhibiting both lesions (arrows) and an unremarkable and thin pancreatic main duct (arrow heads).
Fig. 2.

Surgical pancreatic enucleation of two small BD-IPMNs. Enucleation of two small BD-IPMNs (arrow heads) in the pancreatic head and corpus of a 52-year-old female (see fig. 1). A Enucleation of the BD-IPMN measuring 2.2 cm in the pancreatic head. B Enucleation of the lesion measuring 1.4 cm in the corpus of the pancreas. For the surgical approach of this lesion, the dorsal part of the pancreatic gland was mobilized by dissection of the splenic vein. Both cystic lesions revealed a visible branch duct with direct connection to the main pancreatic duct (arrows). The final histological examination revealed two BD-IPMNs with low-grade epithelial dysplasia.
Summary of Current Evidence
To date, there remain conflicting results particularly concerning predictors of malignancy in both MD- and BD-IPMNs. Therefore, the natural history of this disease is not entirely understood. It is conceivable that in analogy to the natural history of colon polyps, an adenoma-carcinoma sequence exists in IPMNs. We believe that lesions with high-grade dysplasia have to be considered as high-risk lesions that transform into invasive cancer over time as well as at a significant rate. With regard to current scientific knowledge and the absence of truly population-based (rather than case series-based) studies, it is impossible to determine the real incidence of malignancy in small cystic lesions that have not been resected. It is probable that a number of small BD-IPMNs that are under surveillance already harbor small areas of invasive cancer that would only be detected by histological examination after surgical resection. This would be in analogy to prostate cancer which can be present for many years before becoming clinically apparent [39]. In contrast, it is also conceivable that some BD-IPMNs do never progress or undergo malignant transformation. To date, it remains difficult and sometimes impossible to predict malignancy correctly in IPMNs. Without any doubt, the international consensus guidelines are helpful to identify patients with a high-risk profile. However, the indication for surgical resection always has to be seen individually in each patient. Factors such as age, life expectancy, comorbidity, family history, or the willingness of the patient to undergo surveillance have to be considered for an adequate therapeutic management. The decision making for a colon polyp with its adenoma-carcinoma sequence is easy. A snare polypectomy carries a mortality risk of less than 1: 10,000, and morbidity is also very low. For prostate and breast cancer the decision is more difficult, and the pendulum has swung from concerns about catching all high-risk or malignant lesions early (and resecting them) to a much greater concern about overtreatment because the associated morbidity is significant and may outweigh the benefit of early detection and resection. For potentially malignant lesions of the pancreas the risk assessment is infinitely more complex: Not only is an overlooked pancreatic cancer invariably a death sentence but the risk of overtreatment also carries the risk of a postsurgical mortality of 3–8% and a morbidity of 30%.
It is well known that the prognosis of patients with resected IPMN with low-grade, moderate, or high-grade dysplasia is excellent with a 10-year disease-specific survival of >95% for both MD-and BD-IPMN [37]. Recurrent disease is mainly found in patients with BD-IPMNs or in those with MD-IPMN and a false-negative transection margin because of denuded epithelium. For patients with invasive IPMN, the 5-year overall survival is around 40% [62]. To date, it remains controversial whether this better survival compared to sporadic ductal adenocarcinoma is related to an earlier stage of presentation or due to a different biological behavior of mucinous cystic neoplasms – or both [3].
Conclusion
MD- and mixed-type IPMNs principally have to be considered as precursors to invasive adenocarcinoma of the pancreas. The tumors likely undergo an adenoma-carcinoma sequence eventually culminating in malignant neoplastic transformation. In contrast, the role of BD-IPMNs is not entirely understood. It is not clear whether all BD-IPMNs harbor a risk and malignant potential of malignant transformation and what the timeline of growth and progression might be. Therefore, the indication for surgical resection versus observation has to be individualized depending on a number of clinical factors. Clinical decision making should always be based on the expert opinion of an interdisciplinary team of gastroenterologists, radiologists, and surgeons in a pancreatic high-volume center.
Disclosure Statement
The authors declare no conflict of interests.
References
- 1.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708–713, 713.e701–702. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927–936. doi: 10.1016/s0016-5107(04)02230-8. [DOI] [PubMed] [Google Scholar]
- 5.Longnecker DS. Intraductal papillary-mucinous tumors of the pancreas. Arch Pathol Lab Med. 1995;119:197–198. [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797; discussion 797–789. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanno S, Nakano Y, Koizumi K, et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas. 2010;39:36–40. doi: 10.1097/MPA.0b013e3181b91cd0. [DOI] [PubMed] [Google Scholar]
- 8.Salvia R, Crippa S, Partelli S, et al. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342–346. doi: 10.4240/wjgs.v2.i10.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–657; discussion 657–659. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651; discussion 651–654. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 12.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 13.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256:313–320. doi: 10.1097/SLA.0b013e31825d355f. [DOI] [PubMed] [Google Scholar]
- 14.Wong J, Weber J, Centeno BA, et al. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J Gastrointest Surg. 2013;17:78–84; discussion 84–85. doi: 10.1007/s11605-012-2017-0. [DOI] [PubMed] [Google Scholar]
- 15.Conlon KC. Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol. 2005;23:4518–4523. doi: 10.1200/JCO.2005.22.517. [DOI] [PubMed] [Google Scholar]
- 16.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685; discussion 685–687. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingkakul T, Warshaw AL, Fernandez-Del Castillo C. Epidemiology of intraductal papillary mucinous neoplasms of the pancreas: sex differences between 3 geographic regions. Pancreas. 2011;40:779–780. doi: 10.1097/MPA.0b013e31821f27fb. [DOI] [PubMed] [Google Scholar]
- 18.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–433; discussion 433–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321; discussion 321–322. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79; quiz 309–310. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maire F, Hammel P, Terris B, et al. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446–449. doi: 10.1136/gut.51.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe T, Fukushima N, Brune K, et al. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019–6025. doi: 10.1158/1078-0432.CCR-07-0471. [DOI] [PubMed] [Google Scholar]
- 27.Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70–75. doi: 10.1097/SLA.0b013e3181c5ddc3. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Yokohata K, Noshiro H, Chijiiwa K, Tanaka M. Mucinous cystic neoplasm of the pancreas or intraductal papillary-mucinous tumour of the pancreas. Eur J Surg. 2000;166:141–148. doi: 10.1080/110241500750009492. [DOI] [PubMed] [Google Scholar]
- 29.Reid-Lombardo KM, Mathis KL, Wood CM, Harmsen WS, Sarr MG. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251:64–69. doi: 10.1097/SLA.0b013e3181b5ad1e. [DOI] [PubMed] [Google Scholar]
- 30.Hruben RH, Pitman MB, Klimstra DS. Washington, DC: American Registry of Pathology; 2007. Intraductal neoplasms; in Hruben RH, Pitman MB, Klimstra DS (eds): AFIP Atlas of Tumor Pathology, Series 4: Tumors of the Pancreas; pp. 75–110. [Google Scholar]
- 31.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 32.Kamisawa T, Fujiwara T, Tu Y, et al. Long-term follow-up of intraductal papillary adenoma of the pancreas. J Gastroenterol. 2002;37:868–873. doi: 10.1007/s005350200144. [DOI] [PubMed] [Google Scholar]
- 33.Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40:612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 36.Mino-Kenudson M, Fernandez-Del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–1720. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703–711. doi: 10.1016/j.dld.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Farrell JJ, Fernandez-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 40.Chiu SS, Lim JH, Lee WJ, et al. Intraductal papillary mucinous tumour of the pancreas: differentiation of malignancy and benignancy by CT. Clin Radiol. 2006;61:776–783. doi: 10.1016/j.crad.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 42.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–634. doi: 10.1097/SLA.0b013e3181a189a8. [DOI] [PubMed] [Google Scholar]
- 43.Cone MM, Rea JD, Diggs BS, Douthit MA, Billingsley KG, Sheppard BC. Predicting malignant intraductal papillary mucinous neoplasm: a single-center review. Am J Surg. 2011;201:575–579. doi: 10.1016/j.amjsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Gerke H, Jaffe TA, Mitchell RM, et al. Endoscopic ultrasound and computer tomography are inaccurate methods of classifying cystic pancreatic lesions. Dig Liver Dis. 2006;38:39–44. doi: 10.1016/j.dld.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Walsh RM, Vogt DP, Henderson JM, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677–684; discussion 684–685. doi: 10.1016/j.surg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Pais SA, Attasaranya S, Leblanc JK, Sherman S, Schmidt CM, DeWitt J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489–495. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Fritz S, Hackert T, Hinz U, Hartwig W, Buchler MW, Werner J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg. 2011;98:104–110. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 48.Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: from the surgeon's presumption to the pathologist's reality. Surgery. 2012;152:S135–142. doi: 10.1016/j.surg.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 50.Jang JY, Park T, Lee S, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014;101:686–692. doi: 10.1002/bjs.9491. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi G, Fujita N, Maguchi H, et al. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532–538. doi: 10.1097/MPA.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahora K, Castillo CF, Dong F, et al. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: implications of minimal involvement of the main pancreatic duct. Surgery. 2014;156:611–621. doi: 10.1016/j.surg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roch AM, Ceppa EP, Al-Haddad MA, et al. The natural history of main duct-involved, mixed-type intraductal papillary mucinous neoplasm: parameters predictive of progression. Ann Surg. 2014;260:680–690. doi: 10.1097/SLA.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 54.Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807–1817. doi: 10.1002/1097-0142(20011001)92:7<1807::aid-cncr1697>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Werner J, Fritz S, Buchler MW. Intraductal papillary mucinous neoplasms of the pancreas – a surgical disease. Nat Rev Gastroenterol Hepatol. 2012;9:253–259. doi: 10.1038/nrgastro.2012.31. [DOI] [PubMed] [Google Scholar]
- 56.Weinberg BM, Spiegel BM, Tomlinson JS, Farrell JJ. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology. 2010;138:531–540. doi: 10.1053/j.gastro.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy. 2010;42:1077–1084. doi: 10.1055/s-0030-1255971. [DOI] [PubMed] [Google Scholar]
- 58.Barron MR, Roch AM, Waters JA, et al. Does preoperative cross-sectional imaging accurately predict main duct involvement in intraductal papillary mucinous neoplasm? J Gastrointest Surg. 2014;18:447–455. doi: 10.1007/s11605-013-2444-6. discussion 5455-5456. [DOI] [PubMed] [Google Scholar]
- 59.Goh BK, Tan DM, Ho MM, Lim TK, Chung AY, Ooi LL. Utility of the Sendai consensus guidelines for branch-duct intraductal papillary mucinous neoplasms: a systematic review. J Gastrointest Surg. 2014;18:1350–1357. doi: 10.1007/s11605-014-2510-8. [DOI] [PubMed] [Google Scholar]
- 60.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas ≤3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 61.Hackert T, Hinz U, Fritz S, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396:1197–1203. doi: 10.1007/s00423-011-0801-z. [DOI] [PubMed] [Google Scholar]
- 62.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]


