Summary
Background
Increased usage of computed tomography and magnetic resonance imaging has led to a large increase in identified pancreatic cysts of up to 25% in population-based studies. The clinical and economic relevance of identifying so many cystic lesions has not been established. Compared to other organs such as liver or kidney, dysontogenetic pancreatic cysts are rare. Pancreatic cysts comprise a variety of benign, premalignant or malignant lesions; however, precise diagnosis before resection has an accuracy of only 80%. The focus of recent research was the malignant potential of intraductal papillary mucinous neoplasms (IPMN) with the aim of establishing clinical pathways addressing risk of malignancy, age and comorbidity, treatment-related morbidity and mortality as well as cost-effectiveness of treatment and surveillance. The focus of this review is to analyze the clinical and socio-economic relevance as well as the cost-benefit relation for IPMNs.
Methods
For analysis, the following MESH terms were used to identify original articles, reviews, and guidelines in PubMed: (‘intraductal papillary mucinous neoplasm’ OR ‘pancreatic cysts’) and (incidence OR relevance OR socio-economic OR economic OR cost-effectiveness OR cost-benefit). The retrieved publications were reviewed with a focus on clinical and socio-economic relevance in relation to the increasing incidence of IPMN.
Results
Addressing the increasing prevalence of pancreatic cystic lesions, recent consensus guidelines suggested criteria for risk stratification according to ‘worrisome features’ and ‘high-risk stigmata’. Recent prospective cohort studies evaluated whether these can be applied in clinical practice. Evaluation of three different clinical scenarios with regard to costs and quality-adjusted life years suggested a better effectiveness of surveillance after initial risk stratification by endoscopic ultrasound-guided fine-needle aspiration with cyst fluid analysis compared with immediate resection or follow-up without further intervention. Of interest, the ‘immediate surgery’ strategy was lowest for cost-effectiveness.
Conclusions
The increasing incidence of identified pancreatic cysts requires an improved strategy for non-invasive risk stratification based on advanced imaging strategies. In light of a malignancy risk of 2% for branch-duct IPMN, the socio-economic necessity of a balance between surveillance and resection has to be agreed on.
Keywords: Intraductal papillary mucinous neoplasm, IPMN, Pancreatic cystic lesion, Cost-effectiveness, Incremental cost-effectiveness ratio, ICER, Quality-adjusted life years, QUALI
Introduction
The increasing sensitivity of imaging modalities gave rise to an increase of pancreatic cystic lesions (PCL) since the mid-nineties. In parallel, an increased number of resected intraductal papillary mucinous neoplasm (IPMN) was reported [1]. Autopsy series detected small pancreatic cysts in a range of 24.3–27.5% and hereby determined the prevalence [2, 3]. Only in a minority of cases the pathologist regarded these lesions as harboring a potential risk of malignancy (3%). In the context of epidemiological studies and in cross-sectional imaging for other indications, the number of PCL increased to 2.6% on computed tomography scans (CT) and to 27.7% in secretin-enhanced magnetic resonance imaging (MRI) studies [4, 5, 6], suggesting that MRI is now as sensitive as autopsy studies. In the Rochester Epidemiology Project, a 14-fold increase in the incidence rate of IPMNs between 1985 and 2005 was observed (0.31–4.35 cases per 100,000 persons) [7] which was most likely caused by two effects: the improvement in detection rate as well as the aging population. The aging population contributes to the higher prevalence of PCL as well as to mucin-producing adenocarcinoma in patients with pancreatic cysts at a rate of 33.2 per 100,000 (95% confidence interval (CI): 21.6–44.0) [8]. There is a linear increase in the rate of malignant transformation with increasing age up to 38.6 per 100,000 in 80- to 84-year-old men. In the absence of a PCL, the prevalence of mucin-producing adenocarcinomas in US adults between 40 and 84 years of age was 0.83 per 100,000. Of note, the mortality rate for pancreatic adenocarcinoma and IPMN-associated pancreatic adenocarcinoma did not increase over the same time period, suggesting a stable number of PCL now detected by improved imaging modalities rather than a net increase in incidence [9, 10]. IPMN-associated carcinoma increased from 0.008 to 0.032 per 100,000 persons in 2001 to 0.06 per 100,000 persons in 2007. Because overall pancreatic cancer mortality remained stable between 1975 and 2007, the effect is again caused by a better detection of the IPMN association of pancreatic cancer. Thus, the increased detection of IPMN had no impact on pancreatic cancer-related mortality. These observations suggest that unfortunately most PCL including IPMNs are of no overall clinical relevance since mortality is considered the most relevant outcome or study endpoint. Nevertheless, the increased detection of PCL in the recent past triggered extensive diagnostic investigations to clarify the potential risk of malignancy as well as the consequences of surgical resection which does not benefit all patients.
Methods
A literature search was undertaken in PubMed for original publications, reviews, and guidelines with the following keywords (‘intraductal papillary mucinous neoplasm’ OR ‘pancreatic cysts’) and (incidence OR relevance OR socio-economic OR economic OR cost-effectiveness OR cost-benefit). The retrieved publications were reviewed with a focus on clinical and socio-economic outcomes as well as on the increasing incidence and detection of IPMN.
IPMN Subtypes
IPMNs are characterized by an intraductal proliferation of epithelia combined with the production and secretion of mucus. Depending on the localization of the IPMN, they are divided into main-duct IPMN (MD-IPMN), branch-duct IPMN (BD-IPMN), and mixed-type IPMN [11, 12]. The majority of IPMNs are BD-IPMNs and occur at a single site. In 20–30%, they present as multifocal lesions, and in 5–10% they can be found spread over the entire pancreas in a field defect [4, 13]. Considering the localization of the lesion, the potential of malignancy varies between 57–92% in MD-IPMN and 6–42% in BD-IPMN [14]. There is almost general agreement that the diagnosis of MD-IPMN requires resection and that this strategy is cost-effective. In the case of a mixed-type IPMN, the main duct lesion determines the prognosis.
In the largest prospective cohort study, BD-IPMNs were diagnosed as an incidental finding in 57%, with a predominance in the 7th decade of life. The risk of progression was below 5%, and the risk of invasive growth during the surveillance period was below 2% [15]. In contrast, the operative mortality ranged between 1.3 and 8%, highlighting the necessity of accurate preoperative diagnosis. Besides imaging criteria such as size or mural nodules, BD-IPMNs can be classified into different histological subtypes. These include an intestinal, a pancreatobiliary, an oncocytic, and a gastric cellular subtype. The first three mainly present as MD-IPMNs and are localized in the pancreatic head and body. The pancreatobiliary subtype is burdened with the worst outcome due to its high probability of the development of pancreatic adenocarcinoma [16, 17]. The gastric subtype is often identified in BD-IPMNs and harbors a lower risk of malignancy with a longer time interval of progression to pancreatic adenocarcinoma. However, those histological subclassifications are not considered for risk stratification, because they cannot be determined prior to resection.
The aim of the IPMN guidelines from 2006 [14] and 2012 [18] was to establish criteria predicting invasive IPMN or IPMN with high-grade dysplasia. With the help of extended criteria named ‘high-risk stigmata’ (obstructive jaundice in a patient with cystic lesion of the head of the pancreas, enhancing solid components within a cyst, main pancreatic duct >10 mm in size) and ‘worrisome features’ (clinical pancreatitis, imaging: cyst >3 cm, thickened/enhancing cyst walls, main duct size 5–9 mm, non-enhancing mural nodule, abrupt change in caliber of pancreatic duct with distal pancreatic atrophy), a better discrimination was achieved. In a retrospective analysis of 100 resected patients with IPMN, the number of high-risk stigmata correlated well with the grade of malignancy of BD-IPMNs and therefore independently validated the consensus criteria [19].
Clinical Value of the Consensus Guidelines
Fernandez-Castillo and co-workers have used the revised international consensus guideline [18] in a prospective database including 762 patients with BD-IPMN [20]. In this cohort and in adherence to the guideline, 20% of the patients underwent surgical resection at the time of diagnosis. An additional 21% of the patients were resected during the 5-year surveillance period. In summary, high-grade dysplasia or invasive cancer was associated with an age above 65 years, a cyst size of more than 3 cm (56.3 vs. 38.5%; p = 0.028), main duct dilation or stricture, as well as multifocal disease (52.4 vs. 31.1%; p = 0.049). Jaundice, mural nodules or masses, and an increase of serum carbohydrate antigen (CA) 19-9 over 37 kU/l were associated with invasive cancer (26 vs. 13%; p = 0.031). CA 19-9 was elevated in 89% of all resected patients, in only 35% of resected patients with invasive BD-IPMN, but only in 14% of patients with benign lesions. Ultimately, CA 19-9 was unable to discriminate between invasive or non-invasive IPMN [20, 21, 22]. Among all resected patients, invasive cancer was present in only 10% and high-grade dysplasia in a further 14%, suggesting that 76% of the patients underwent surgery in vain. When a cyst size smaller than 3 cm was taken into account, high-grade dysplasia and invasive cancer were found in 10 and 7%. No cancer was diagnosed in cysts smaller than 2 cm. No dysplasia was found in lesions smaller than 1 cm. In 27.6% of all IPMNs, worrisome features were detected and those underwent surgery in nearly all cases (97%). In only 29% of them, however, high-grade dysplasia or invasive cancer was detected on resection specimen. Therefore, in retrospect, more than 70% of resected patients underwent surgery without potential benefit. In contrast, in the absence of worrisome features and thus Sendai/Fukuoka criteria-negative BD-IPMN (guidelines from 2012 [18]), the rate of invasive cancer was 0.26% (1 in 500) and high-grade dysplasia was found in 9%. Worrisome features were significantly more common in patients with histologically diagnosed high-grade dysplasia or invasive carcinoma (85 vs. 59%, p = 0.001) in comparison to those with low-grade or moderate dysplasia. Of note, no patient developed pancreatic cancer during surveillance. Median survival in resected invasive carcinoma arising from BD-IPMN was 110 months (95% CI: 5–214 months; 2-year survival: 84%) compared to 13 months for patients with BD-IPMN and concurrent pancreatic ductal adenocarcinoma (95% CI: 5–21 months; 2-year survival: 23%). The data outlined here in detail reflect findings from all prospective studies so far reported and include 1,502 patients [20, 21, 22, 23, 24, 25, 26, 27, 28].
Cost-Effectiveness Analysis
The data above demonstrate the applicability and clinical relevance of the revised consensus criteria for the follow-up of IPMNs [18]. The remaining question is whether this strategy is cost-effective. The above data also clarify approaches that the sensitivity for the detection of malignancy according to the consensus guidelines is 100%, but the specificity is only 23–31%, resulting in a high number of false-positive findings and unnecessary surgery [29, 30]. Given the direct and indirect cost incurred by surgical resection, the strategy might not be cost-effective. A recent study focused on the cost-effectiveness of the 2006 consensus guidelines in the management of BD-IPMN, comparing surveillance to no surveillance and immediate surgery [31]. The hypothetical cohort recruited 60-year-old patients with BD-IPMN in the head of the pancreas.
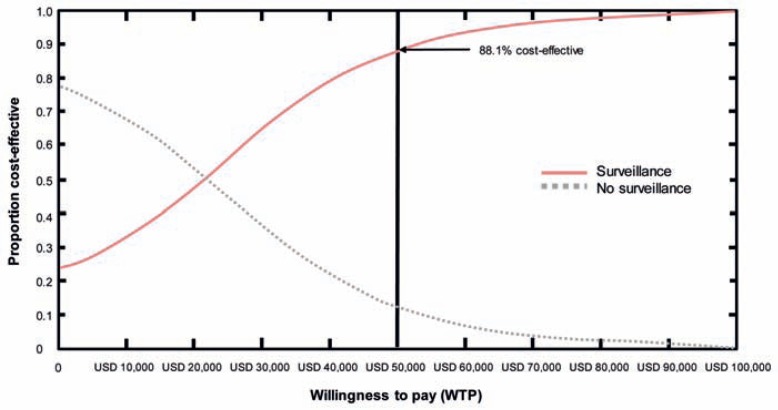
Three strategies were generated: The first included surveillance following the consensus guidelines from 2006 and establishing a surveillance strategy with possible surgical resection whenever indicated. The second strategy was based on intervention only in the case of symptoms without surveillance (no surveillance strategy). The third model calculated the effect of immediate surgery in all cases (surgery strategy). Employing the model of Markov, a lifetime horizon was evaluated. The results indicated that the no surveillance strategy was the least effective with regard to quality-adjusted life years (QALY) gained but the cheapest. The surveillance strategy was more effective in terms of survival but more costly. In this study, the most costly but most effective strategy with regard to QALY gained was immediate surgery, in which only 5.4% died from invasive cancer whereas 4.7% died as a consequence of surgery. Of note, 1 in 20 patients died from surgical complications. In the surveillance strategy, 9.7% died from cancer and 4.5% following surgery in comparison to the no surveillance strategy where 11.2 and 4.4% died from cancer or surgery, respectively. Since the number of patients undergoing surgery was, of course, very different between the groups, the surveillance strategy had the lowest risk of death. Although the surgery strategy had the highest QALY gained, it was associated with the highest cost of up to USD 132,436 per QALY in comparison to the surveillance strategy. Depending on the health care setting, a QALY of USD 25,000–50,000 is accepted by society. Immediate surgery in the setting of IPMN would exceed this amount by far. The USD 50,000/QALY was used as a benchmark for hemodialysis. In summary, employing the surveillance strategy suggested by the consensus guidelines would result in 88.1% of patients being covered by the set benchmark of USD 50,000/QALY (fig. 1). The reason why immediate surgery is not cost-effective is due to the low specificity of 23–30% achieved by preoperative diagnostic measures, which lead to false-positive diagnosis with 70–77% of patients undergoing unnecessary surgery. Based on a careful consideration of the cost per life year saved, employing the surveillance strategy suggested by the consensus guidelines indicates that it is in the cost range of colorectal cancer screening programs [32, 33]. Moreover, as direct mortality and morbidity of pancreatic surgery should not be disregarded, the surveillance strategy should be preferred because it allows for a higher sensitivity to diagnose malignancy at lower costs. A caveat should be mentioned in this context: At the age of 78 years, the cost for one QALY rises above USD 50,000 and the no surveillance strategy is more cost-effective. In this situation, comorbidity drives outcome.
Fig. 1.
Acceptability curve of no surveillance versus surveillance strategies. Based on willingness-to-pay (WTP) threshold (USD per QALY) on the horizontal axis. The axis represents the proportion of simulated trials in the Monte Carlo probabilistic sensitivity analysis that would fall within a specifled budget. For example, a USD 50,000 WTP threshold results in 88.1% cost-effectiveness proportion in the simulated trials if a surveillance strategy would be pursued. In addition, the net health benefit is positive at a WTP threshold of USD 20,291 for the surveillance strategy (modified from [30]).
A second study used a similar approach to analyze cost-effectiveness following the release of the consensus guidelines in 2006 [34]. In this analysis, which also employed a Markov model, the clinical course of the patients were adjusted for age, sex, and mortality with regard to individual health and disease states including cancer-related mortality [35]. Again the authors created three different scenarios: The first (strategy I) recruited patients with probable serous cystic neoplasms on cross-sectional imaging. These were followed as a benign disease using a ‘watch and wait’ strategy. In the second group (strategy II), all patients were resected if they were fit for surgery. The patients did not undergo further surveillance after surgery. The third group (strategy III) underwent preoperative endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) and cyst fluid analysis. Depending on the individual surgical risk and the result of the FNA (mucinous vs. serous cystic lesion), patients underwent surgical resection. EUS with FNA and fluid analysis distinguish between mucinous and non-mucinous cysts [36]. EUS was also used to identify high-risk stigmata and worrisome features in order to increase the diagnostic accuracy. The standard patient was 65 years of age with a 3-cm incidental cystic lesion in the tail of the pancreas and an ASA (American Society of Anesthesiologists) score of III. The analysis revealed that strategy II is the cheapest (USD 13,200 per patient) but gains the lowest QALY (9.66). Strategy I resulted in a QALY of 10.34 at slightly higher costs (USD 18,883 per patient). Strategy III was burdened with the highest costs (USD 23,337 per patient) but also the highest QALY (10.73). Finally, the ICER (incremental cost-effectiveness ratio) of group III was only USD 11,394 and that of group II amounted to USD 9,474, emphasizing that the additional costs were well invested and remained in an acceptable range.
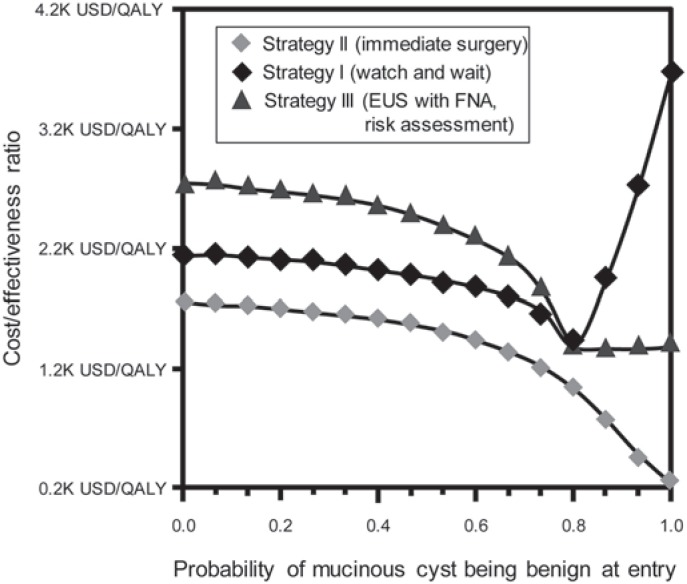
For the allocation of an optimal management strategy, the most important factor remains the risk of surgery. In a group with a low risk for developing malignancy or a higher risk for operative complications, resection was never the best strategy regardless of the model used. The discrimination between a serous and a mucinous lesion is important to determine a cost-effective strategy. If the preoperative diagnosis falls short in the discrimination between benign and premalignant lesion and the probability drops to less than 27%, EUS-FNA stratification (strategy III) is no longer cost-effective. If the preoperative accuracy for a benign cyst is higher than 77%, then a ‘watch and wait’ strategy becomes the most cost-effective (fig. 2). It is therefore important that each institution involved in the surveillance and treatment of IPMNs is aware of the diagnostic accuracy of its own institutional tools. As suggested by Huang et al. [30], in the case of older patients burdened with comorbidity (ASA II–III) as well as localization of the cysts in the pancreatic head, a ‘watch and wait’ strategy should be preferred from a cost-effectiveness standpoint. Nevertheless, in a Monte Carlo analysis, there is a higher risk for this group to develop unresectable pancreatic cancer if compared to the FNA strategy. Whether that is of clinical relevance needs to be decided by the multidisciplinary team involved in the treatment and is based on risk assessment and risk stratification. Finally, initial EUS with FNA and cyst fluid analysis was found to have the most beneficial effect with the highest QALY gained as well as ICER, but only if the mortality risk of surgery was at or below 4.7%.
Fig. 2.
Result of a one-way sensitivity analysis with the X-axis showing the probability of a mucinous cystic lesion being benign at entry into the model and the Y-axis showing the corresponding ICER. Strategy III is consistently more cost-effective – except when the probability of a mucinous cystic lesion being benign exceeds 77%. In this case, strategy I (watch and wait) becomes more cost-effective (K = 1,000) (modified from [33]).
In summary, in these analyses, surgical resection for all patients with an incidental solitary PCL was not the preferred option on the basis of cost-effectiveness. A preoperative diagnostic work-up by EUS with FNA and cyst fluid analysis should be employed to lower the rate of unnecessary surgery and to decrease surgical morbidity and mortality. Risk stratification is therefore mandatory and it should be kept in mind that the risk of a SB(side branch)-IPMN progressing to invasive cancer is 2% per year and corresponds to the risk of stroke associated with atrial fibrillation. We assess the risk of bleeding before we prescribe an anticoagulant in this situation and should likewise assess the risk in SB-IPMN before advising surgery. An individual strategy for each cyst and each patient is necessary in order to achieve optimal but cost-effective therapy.
Conclusion
On the one hand, the widespread use of cross-sectional imaging during the last two decades revealed a rapidly increasing number of PCL harboring the potentially life-threatening risk of pancreatic cancer for the patient. On the other hand, the incidence rate of pancreatic malignancy has been stable over the same two decades. We have reached the point where advanced imaging modalities reach the sensitivity of autopsy studies and therefore the prevalence peak of PCL. However, preoperative diagnostic specificity and accuracy for classifying these cystic lesions remains poor. Therefore, the management of potentially premalignant lesions such as BD-IPMN remains challenging. A critical appraisal of the published literature suggests that the treatment outcome of what is presumed to be a BD-IPMN remains unsatisfactory, with a large rate of surgically overtreated patients. Until we have collated a more comprehensive understanding of the natural history of BD-IPMNs, the management of patients with pancreatic cysts should be individually tailored and preferably carried out in centers with a high caseload. At present, the authors confirm that the Sendai consensus guidelines revised in Fukuoka are not only clinically relevant and applicable but also cost-effective and of sufficient concern regarding socio-economic factors.
A cancer prevention strategy for PCL greatly differs from the management of other premalignant lesions in the gastrointestinal tract in two important ways. A snare polypectomy reduces the risk of developing colorectal cancer by 70% and has been shown to increase overall survival; compared to respective pancreatic surgery, the procedural risk is minimal. Moreover, IPMNs are field defects of the pancreas and, as in the colon, they have a tendency, or they are multifocal in up to 30%, resulting in extended surgery with an even higher operative risk [37]. Lastly, BD-IPMNs are in 4% associated with synchronous or metachronous pancreatic ductal adenocarcinoma arising from the non-IPMN-affected pancreatic tissue. Surgery of an IPMN therefore only reduces but does not abolish the risk of developing pancreatic cancer in the remaining organ [38]. After surgery, lifelong surveillance is thus recommended, and a second operation, if it is not a total pancreatectomy, is challenging for both the patient and the surgeon. Careful consideration of probable complications is required as they involve significant morbidity and mortality.
One aspect of enhancing diagnostic specificity is the identification of the BD-IPMN subtypes by EUS-FNA [17, 39]. Ultimately it is our goal to increase the diagnostic accuracy for PCL to improve risk stratification and management, and to reduce health care expenditure but still not to miss the 1 in 500 of the BD-IPMN patients who will have invasive cancer and who are burdened with the poorest prognosis of all gastrointestinal malignancies.
Disclosure Statement
The authors declare that they have no relevant or material financial interest that relate to the research described in this paper.
References
- 1.Sohn TA, Yeo CJ, Camern JL, et al. Intraductal papillary mucinous neoplasms of the pancreas. An updated experience. Ann Surg. 2004;239:788–799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 3.Kimura W. How many millimeters do atypical epithelia of the pancreas spread intraductally before beginning to infiltrate? Hepatogastroenterology. 2003;50:2218–2224. [PubMed] [Google Scholar]
- 4.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 6.Bülow R, Simon P, Thiel R, et al. Anatomic variants of the pancreatic duct and their clinical relevance: an MR-guided study in the general population. Eur Radiol. 2014;24:3142–3149. doi: 10.1007/s00330-014-3359-7. [DOI] [PubMed] [Google Scholar]
- 7.Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555–558. doi: 10.1016/j.cgh.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner TB, Glass LM, Smith KD, Ripple GH, Barth RJ, Klibansky DA, Colacchio TA, Tsapakos MJ, Suriawinata AA, Tsongalis GJ, Pipas JM, Gordon SR. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in United States adults. Am J Gastroenterol. 2013;108:1546–1550. doi: 10.1038/ajg.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klibansky DA, Reid-Lombardo KM, Gordon SR, et al. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555–558. doi: 10.1016/j.cgh.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosmahl M, Pauser U, Anlauf M, et al. Cystic pancreas tumors and their classification: features old and new (article in German) Pathologe. 2005;26:22–30. doi: 10.1007/s00292-004-0734-1. [DOI] [PubMed] [Google Scholar]
- 12.Kloppel G, Kosmahl M, Luttges J. Intraductal neoplasms of the pancreas: cystic and common (article in German) Pathologe. 2005;26:31–36. doi: 10.1007/s00292-004-0728-z. [DOI] [PubMed] [Google Scholar]
- 13.Nagai K, Doi R, Kida A, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg. 2008;32:271–278; discussion 279–280. doi: 10.1007/s00268-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International Association of Pancreatology: International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 15.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–2349. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 16.Distler M, Kersting S, Niedergethmann M, et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324–330. doi: 10.1097/SLA.0b013e318287ab73. [DOI] [PubMed] [Google Scholar]
- 17.Kloppel G, Basturk O, Schlitter AM, Konukiewitz B, Esposito I. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–466. doi: 10.1053/j.semdp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Aso T, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Ideno N, Osoegawa T, Takahata S, Shindo K, Ushijima Y, Aishima S, Oda Y, Ito T, Mizumoto K, Tanaka M. ‘High-risk stigmata’ of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014;43:1239–1243. doi: 10.1097/MPA.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 20.Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, Pitman MB, Warshaw AL, Lillemoe KD, Fernandez-del Castillo CF. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 21.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952–959. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 22.Fritz S, Hackert T, Hinz U, et al. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg. 2011;98:104–110. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 23.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long term follow-up results. Gut. 2008;57:339–343. doi: 10.1136/gut.2007.129684. [DOI] [PubMed] [Google Scholar]
- 25.Woo SM, Ryu JK, Lee SH, et al. Branch duct intraductal papillary mucinous neoplasms in a prospective series of 190 patients. Br J Surg. 2009;96:405–411. doi: 10.1002/bjs.6557. [DOI] [PubMed] [Google Scholar]
- 26.Uehara H, Ishikawa O, Katayama K, et al. Size of mural nodule as an indicator of surgery for branch-duct intraductal papillary mucinous neoplasm of the pancreas during follow-up. J Gastroenterol. 2011;46:657–663. doi: 10.1007/s00535-010-0343-0. [DOI] [PubMed] [Google Scholar]
- 27.Maguchi H, Tanno S, Mizuno N. Natural history of branch duct intraductal papillary mucinous neoplasm: a multicenter study in Japan. Pancreas. 2011;40:364–370. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 28.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 29.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang ES, Gazelle GS, Hur C. Consensus guidelines in the management of branch duct intraductal papillary mucinous neoplasm: a cost-effectiveness analysis. Dig Dis Sci. 2010;55:852–860. doi: 10.1007/s10620-009-1014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provenzale D, Lipscomb J. Cost-effectiveness: definitions and use in the gastroenterology literature. Am J Gastroenterol. 1996;91:1488–1493. [PubMed] [Google Scholar]
- 32.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lerch MM, Braun J, Harder M, et al. Posteroperative adaptation of the small intestine after total colectomy and J-pouch-anal anastomosis. Dis Colon Rectum. 1989;32:600–608. doi: 10.1007/BF02554181. [DOI] [PubMed] [Google Scholar]
- 34.Das A, Ngamruengphong S, Nagendra S, Chak A. Asymptomatic pancreatic cystic neoplasm: a cost-effectiveness analysis of different strategies of management. Gastrointest Endosc. 2009;70:690–699. doi: 10.1016/j.gie.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- 36.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–2349. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, Razo O, McGrath D, Pederzoli P, Fernández-Del Castillo C. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Fujii T, Shimoyama Y, Kanda M, Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Nakao A, Kodera Y. Clinical implication of morphological subtypes in management of intraductal papillary mucinous neoplasm. Ann Surg Oncol. 2014;21:2444–2452. doi: 10.1245/s10434-014-3565-1. [DOI] [PubMed] [Google Scholar]