Summary
Background
Intraductal papillary mucinous neoplasms (IPMNs) display diverse macroscopic, histological, and immunohistochemical characteristics with typical morphological appearance in magnetic resonance imaging. Depending on those, IPMNs may show progression into invasive carcinomas with variable frequency. Overall, IPMN-associated invasive carcinomas are found in about 30% of all IPMNs, revealing phenotpyes comparable with conventional ductal adenocarcinomas or mucinous (colloid) carcinomas of the pancreas. In Sendai-negative side-branch IPMNs, however, the annual risk of the development of invasive cancer is 2%; thus, risk stratification with regard to imaging and preoperative biomarkers and cytology is mandatory.
Methods and Results
The present study addresses the radiological and interventional preoperative measures including histological features to determine the risk of malignancy and the prognosis of IPMNs.
Conclusion
While preoperative imaging largely relies on the detection of macroscopic features of IPMNs, which are associated with a divergent risk of malignant behavior, in resected specimens the determination of the grade of dysplasia and the detection of an invasive component are the most important features to estimate the prognosis of IPMNs.
Keywords: Intraductal papillary mucinous neoplasm, IPMN, Cystic lesion, Pancreas
Pancreatic Cystic Neoplasms: Introduction and Background
There is a plethora of cystic lesions in the pancreas, ranging from the most common finding of pancreatic pseudocysts to rare abnormities such as congenital cysts, as well as various cystic neoplasms [1, 2, 3]. Whilst some cystic neoplasms, such as serous cystic neoplasms (SCN), can generally be considered of benign origin, others, like mucinous cystic neoplasms (MCN) or intraductal papillary mucinous neoplasms (IPMNs), harbor a significant malignant potential [1]. An overview of cystic lesions and their frequency can be found in table 1. The radiological distinction between malignant, premalignant, and benign lesions as well as the classification of non-neoplastic cystic pancreatic masses such as congenital cysts and pseudocysts can be complex [1, 2, 3]. However, the exclusion of malignancy in any pancreatic lesion is essential. This may involve imaging, endosonography-guided fine needle aspiration (FNA) to obtain cytology and biochemical markers of mucinous, serous, or inflammatory cystic lesions and surgical resection, followed by histopathological evaluation [4].
Table 1.
Frequency of cystic lesions of the pancreas of resected cases [54]
| Cystic lesion | Frequency |
|---|---|
| Overall frequency (autopsy cases) | 24.3% (73/300) |
| Serous cystadenoma (SCN) | 10% |
| Mucinous cystadenoma (MCN) | 8% |
| Solid pseudopapillary neoplasm | 10% |
| IPMN | 24% |
| Ductal adenocarcinoma with cystic features | 21% |
| Pancreatic pseudocyst | 34% |
Regarding potentially malignant cystic pancreatic neoplasms that are lined by a mucinous epithelium, several discriminating criteria evolved during the past years, not least due to improved radiological techniques and thorough pathological workup [4]. Thus, MCN, which histologically contain an ovarian-like stroma in general, typically do not involve pancreatic ducts, while IPMNs were shown to arise from the pancreatic ductal system [1, 5, 6]. As subsequently described in detail, IPMNs are regarded as premalignant lesions going through a cascade of malignant transformation, starting as IPMN with low-grade dysplasia (also termed adenomas) and possibly progressing into IPMNs with associated invasive carcinoma.
IPMNs are cystic lesions of the pancreas that are derived from the pancreatic ducts [3, 4, 7]. They can affect the main pancreatic duct (MPD), the branch ducts (BD), or both. IPMNs arising from the MPD are called MD-IPMNs, lesions arising from the BDs are called BD-IPMNs, and lesions involving both the MPD as well as the BDs are called mixed-type IPMNs [8]. The majority of IPMNs are found in the head but they can also be found in the body, tail, or throughout the pancreas, with the majority of the lesions being of the BD-IPMN subtype [6].
Epidemiology and Etiology
The incidence of IPMNs in the population is difficult to assess due to the increasing awareness of them as well as the increasing quality of imaging modalities. However, studies currently predict an incidence of 2 cases per 100,000. Imaging of the pancreas shows cystic lesions in 2.5% of asymptomatic patients and in 10% if a population older than 80 years is screened [9, 10]. The median size of cystic lesions is 8 mm, and in up to 30% multifocal cystic lesions are found [11]. Autopsy studies reveal side-branch IPMN (BD-IPMN) in 20% of the patients without significant dysplasia [12]. IPMNs mostly occur at an older age (mean age 64–67 years), with a slightly higher risk in men according to the literature [2, 13]. While 95.8% of all MD-IPMNs present either as high-grade dysplasia or invasive cancer, the rate of malignancy in BD-IPMNs is much lower, which poses the necessity for risk stratification. The risk of malignancy development in BD-IPMN is estimated with 2% per year [11].
The etiology of IPMNs is largely unknown. Nevertheless, some genetic factors could be associated with the genesis of IPMNs, and although occurring sporadically in most cases, some IPMNs were found to arise within hereditary syndromes [13, 14, 15, 16, 17, 18]. The latter included familial adenomatous polyposis (FAP), an inherited disease that mainly affects the colon and rectum – classically due to mutations in the APC gene, which codes for the adenomatous polyposis coli (APC) protein [13, 14]. It is the loss of function of this gene that results in pathology. Various case studies have shown that in those patients with FAP and IPMN there is almost an identical immunohistochemical staining, with those lesions found in FAP and the IPMNs showing loss of the APC protein [19]. Another inherited gastrointestinal tumor syndrome is Lynch syndrome, also known as hereditary nonpolyposis colorectal carcinoma (HNPCC) [17]. HNPCC is associated with microsatellite instability (MSI) and lack of MSH2 and MSH6 expression (depending on HNPCC type). Studies have revealed that IPMNs are more often associated with other nonpancreatic cancer manifestations and are sometimes associated with MSI, MSH2, or MSH6 [17, 18] as well as BRCA2 mutations, which were found in 25% of IPMN patients with a family history of pancreatic cancer in a study by Lubezky et al. [17]. Associations with Peutz-Jeghers syndrome have also been described in the literature [5]. Furthermore, studies examining the rate of extrapancreatic neoplasms have shown that patients with an IPMN have an increased rate of extrapancreatic neoplasms in 3.5–9.3% depending on the follow-up time, the most frequent being colonic, gastric, prostatic, and breast carcinomas [2, 4, 5, 16, 17, 20]. In a number of patients with McCune-Albright syndrome, characterized by fibrous dysplasia, precocious puberty, and café au lait spots, IPMNs have been described as a McCune-Albright syndrome-associated tumor, present in about 15% of the patients. In these patients, germline GNAS-activating mutations are reported which lead to IPMNs, underlining the concept of somatic GNAS mutations being diagnostic in IPMN [20, 21].
Pathological Features
Generally, IPMNs may display a dilatation of ducts or have a multicystic appearance, especially if they arise from BDs (WHO 2010). Within the cysts, papillary structures may be found [2, 7, 21]. Furthermore, IPMNs are typically filled with mucin of viscous consistency [2, 4, 22]. In some cases, mucous secretion into the duodenum can be seen [23, 24, 25]. The size of the cystic ducts ranges from 10 to 80 mm in diameter (WHO 2010). Due to intraductal obstruction caused by the tumors, the remaining pancreatic parenchyma may display a marked atrophy [22].
IPMNs can arise in the entire pancreas but most frequently affect the pancreatic head [6]. In a significant subset of IPMNs, multicentricity has been described [26]. Based on their ductal involvement, as also mirrored in radiological investigations, IPMNs may macroscopically be subclassified into MD-type, BD-type, or mixed-type IPMNs [6, 8]. While this classification is important for preoperative risk assessment, as obtained by radiographic imaging, it has been suggested to be of minor importance for the pathological workup, since microscopically most tumors show involvement of both MD and BD [8].
These different types may display slightly different morphologic features. Thus, MD-IPMNs are more likely to contain intramural nodules or present as a mass lesion in a dilated duct [2, 3]. They are more frequent in the pancreatic head but may affect the entire pancreatic main duct, eventually progressing into the branches [22]. BD-IPMNs can have a multicystic appearance and usually do not show intracystic papillary features macroscopically [2, 27].
Histological Features
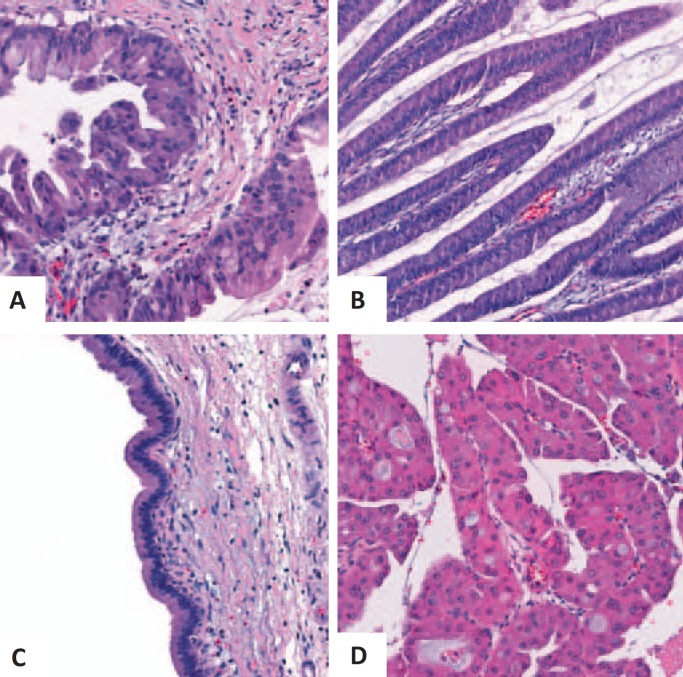
Overall, IPMNs consist of intraductal proliferations of mucin-producing, columnar epithelial cells [6]. As summarized in table 2 and figure 1, based on histological and immunohistochemical features, IPMNs may be subclassified into pancreatobiliary-type, intestinal-type, gastric-type, and oncocytic-type IPMNs [6].
Table 2.
| MUC1 | MUC2 | MUC5AC | MUC6 | CDX2 | |
|---|---|---|---|---|---|
| Pancreatobiliary | + | – | + | rarely + | – |
| Intestinal | – | + | + | (+) | + |
| Gastric | – | – | + | + | – |
| Oncocytic | + | goblet cells | goblet cells | + | – |
Fig. 1.
Various histological types of IPMNs. A Pancreatobiliary type, B intestinal type, C gastric type, and D oncocytic type.
The intestinal subtype represents the most common subtype of the MD-IPMN. It is characterized by a villous growth pattern with tall columnar epithelial cells with elongated nuclei and goblet cells, similar as in colonic adenomas [2, 28]. Immunohistochemically, the tumor cells typically express MUC(mucin)2, MUC5, and caudal-type homeobox 2 (CDX2) [28, 29].
The pancreatobiliary type also typically involves the MD in the pancreatic head but produces comparably little mucin. It is characterized by complex arborizing papillae lined by cuboidal cells that resemble the pancreatic and biliary duct cells [6]. Immunohistochemically, the tumor cells are positive for MUC1 and MUC5 [30].
The gastric type is typically found in BD-IPMNs [28, 29]. These cells resemble gastric foveolar cells, form pyloric gland-like structures at the base of the papillae, and express MUC5 and MUC6 [4, 28, 30].
Oncocytic-type IPMNs typically display complex, arborizing papillae with delicate stroma, lined by two or more layers of oncocytic cells [6]. Immunohistochemically, the tumor cells reveal positivity for MUC1 and MUC6 [6].
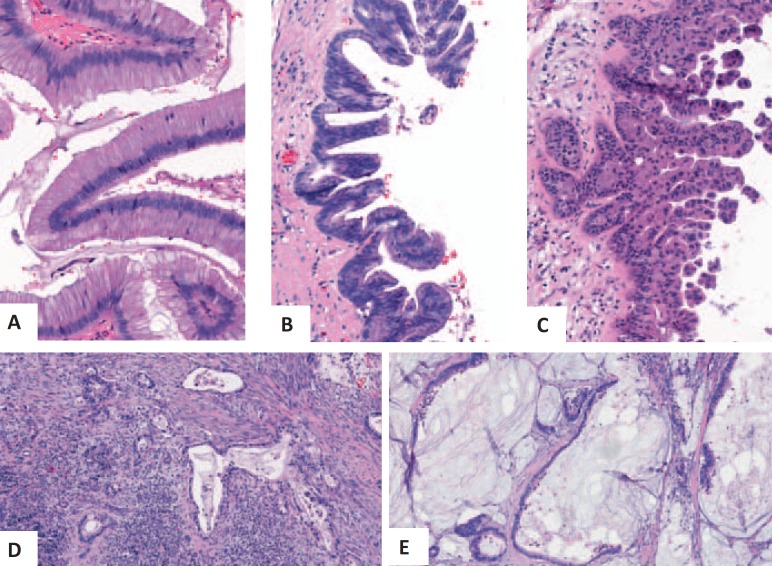
Irrespective of the macroscopic or histological subtypes, IPMNs are classified according to their degree of dysplasia (fig. 2) [6]. According to the current WHO classification of tumors [6], the grade of dysplasia is determined as low-grade (formerly referred to as adenoma), intermediate-grade (borderline type), or high-grade (carcinoma in situ) in noninvasive IPMN. About 30% of resected IPMNs reveal an association with uni- or multifocal invasive carcinomas [6]. In 59–75% of these cases, the invasive tumor component resembles conventional ductal adenocarcinomas, while 24–41% display the phenotype of a mucinous (colloid) carcinoma [31, 32, 33, 34]. The phenotype of the invasive tumor component depends on the IPMN subtype: thus, IPMNs of the pancreatobiliary, gastric, or oncycytic type result in an invasive phenotype resembling pancreatic ductal adenocarcinomas, while intestinal IPMNs may progress into invasive cancer either showing a colloid phenotype or resembling ductal adenocarcinoma [6].
Fig. 2.
Histological findings in noninvasive (A–C) and invasive (D, E) IPMNs, revealing low-grade dysplasia (A), intermediate-grade dysplasia (B), and high-grade dysplasia (C), as well as invasive growth with the phenotypes of a ductal adenocarcinoma (D) and of a mucinous (colloid) carcinoma (E).
Of note, IPMNs are frequently heterogeneous regarding the degree of dysplasia. Therefore, a histological workup of the entire lesion may be necessary to exclude malignancy.
The prognosis of IPMN is mainly determined by the presence and extent of an invasive carcinoma. In matched-pair analyses, IPMNs showed a significantly better survival than conventional pancreatic ductal adenocarcinomas, with 5-year survival rates of 38–47 versus 16% and median survival times of 32–47 versus 17–19 months, respectively [31, 34, 35]. IPMN-associated carcinomas with colloid or oncocytic phenotpyes showed a significantly better prognosis than IPMN-associated or conventional ductal adenocarcinomas. However, while this survival benefit was prominent in early tumor stages, it was lost in more progressed tumors with nodal metastases. This finding was explained by the observation that colloid carcinomas were more frequently resected at lower T stages and showed less nymph node metastases, highly differentiated tumor grades, as well as less neural and vessel invasion [31, 34, 35].
Clinical Symptoms and Risk Factors
Symptoms rarely appear in low-grade IPMNs and often arise in advanced stages of IPMN only when malignant transformation has already occurred [2]. The symptoms that these patients present with may include abdominal pain, jaundice, weight loss, or episodic pancreatitis-like symptoms [2]. Furthermore, the development of diabetes early in the course of disease is a large clinical risk factor for malignancy and has been demonstrated in multiple studies. In 2012, the revised Sendai criteria proposed a classification including clinical and radiological findings to predict malignancies. These guidelines suggested two categories called ‘high-risk stigmata’ (obstructive jaundice, enhanced solid components, dilatation of main pancreatic duct greater than 10 mm) and ‘worrisome features’ (history of pancreatitis, maximal cyst diameter greater than 30 mm, thickened and enhanced cyst wall, MPD diameter 5–9 mm, non-enhanced mural nodules, abrupt change of caliber of the MPD with distal pancreatic atrophy and lymphadenopathy). Unlike the presence of high-risk stigmata, the presence of worrisome features does not necessarily lead to the recommendation of surgical intervention [2]. The Fukuoka guidelines (revised Sendai guidelines) do not indicate whether the number of factors in either category correlates with the likelihood of malignancy. A recent study stratified patients with BD-IPMN into three groups with regard to high-risk stigmata or worrisome features. The presence of one high-risk stigmata justified pancreatic resection, while in contrast there was no significant correlation between the number of worrisome features and the grade of malignancy [36].
Imaging Features of IPMNs
It is commonly the case that patients with IPMN have no symptoms and that the neoplasm is detected incidentally when imaging studies are performed for unrelated indications [2, 5]. As such, there is often a delay in diagnosis due to the insidious nature of this entity [37, 38].
As IPMNs are usually incidental radiological findings it is important to determine the risk of malignancy and subsequent management; however, this is a difficult task. There has been much research into the predictors of malignancy of IPMNs, including two revisions of the international consensus guidelines [2, 8].
The imaging predictors of malignancy in IPMNs can be complicated; however, they can be subdivided into the following headings: ‘type’, ‘size’, ‘focal cyst findings’, ‘MPD dilation’, and ‘associated pancreatic findings’. All of these can be obtained with conventional imaging techniques. It is important to note that all of these findings as well as the patients’ history have to be taken into consideration when assessing the risk of malignancy.
Type
As previously described, IPMNs are cystic lesions that arise from the MPD (MD-IPMNs), its BDs (BD-IPMNs), or both (mixed-type IPMN). MD-IPMNs can be differentiated from BD-IPMNs by their location. Dilation of the MD ≥10 mm (or 5–6 mm as a suspicious/worrisome feature) without an evident reason leads to the differential diagnosis of MD-IPMN [4, 7, 39]. IPMNs arising from the MPD have a higher risk of malignancy, and this finding indicates surgical resection. BD-IPMNs are recognized as cystic dilations of pancreatic BDs, usually showing a grape-like appearance in magnetic resonance cholangiopancreatography (MRCP) with the stalk of the grape representing the small, non-dilated connection duct between the BD and the MPD (fig. 3). These BD dilations can be more tubular, too. Mixed-type IPMNs are diagnosed when both features of BD- and MD-IPMNs are present. In general, the connection to the pancreatic duct can be best visualized by MRCP with a sensitivity of 91.4 to 100% and a specificity of 89.7% [40]. If a connection of the duct to the cystic lesion can be visualized, the only remaining differential diagnosis is a pancreatic pseudocyst or a BD-IPMN. Endosonographic ultrasound (EUS) might be able to suggest a duct connection to the cystic lesion; however, sensitivity and specificity are lower when compared to magnetic resonance pancreatography or endoscopic retrograde pancreatography.
Fig. 3.
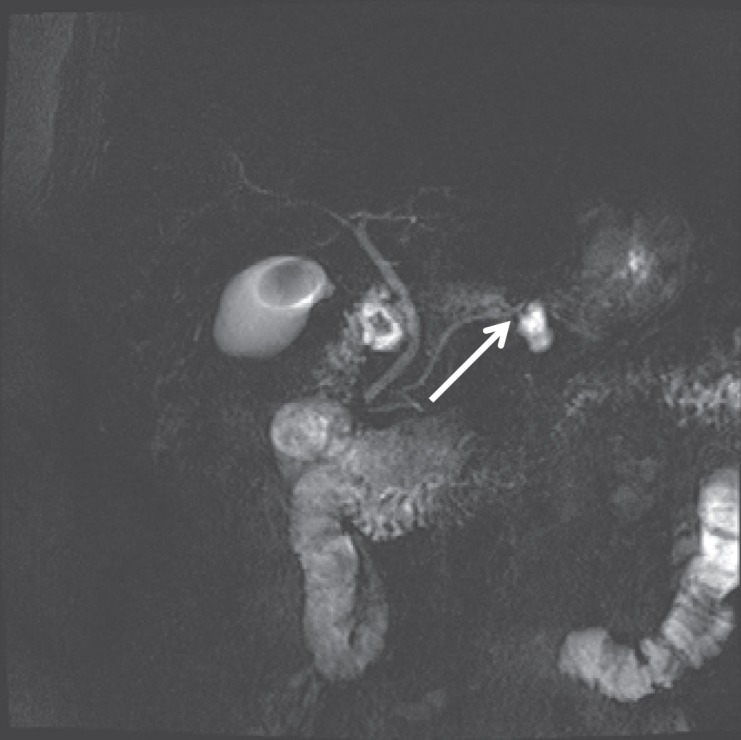
BD-IPMN – note the connection of the lesion via a branch duct to the main duct; the arrowhead points at the BD from which the IPMN arises.
The study by Manfredi et al. [3] showed that the 5-year survival rate is not statistically significant between MD-IPMNs and mixed-type IPMNs; thus, they should be more diligently approached with a lower threshold for resection.
Size
One of the most controversial aspects of BD-IPMNs is the topic of cyst size. It was initially thought that all lesions ≥30 mm in diameter should be resected due to their perceived high malignancy rate. However, size alone is not a significant predictor of malignancy in MD-, BD-, or mixed-type IPMNs [41]. A study by Fritz et al. [27] has shown that IPMNs under 10 mm in size may still confer malignancy. Although size is an unreliable predictor of malignancy, it should be emphasized that especially those patients with larger lesions should be monitored, and the Fukuoka criteria have kept a size of 30 mm as a worrisome feature.
Focal Cyst Findings
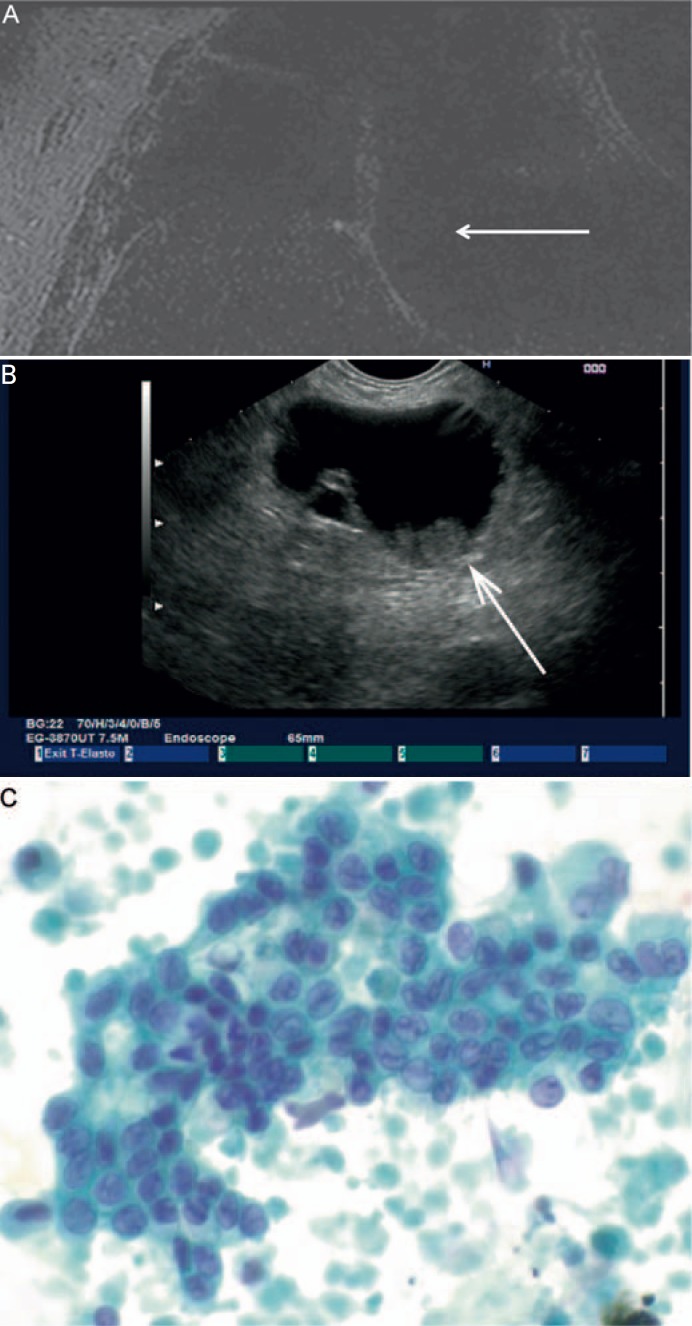
Some of the strongest predictors of malignancy in all types of IPMNs involve focal cyst findings. In studies using magnetic resonance imaging to look at the characteristics of the duct in relation to all IPMNs, it was found that mural nodules along the walls of the pancreatic ducts and duct wall enhancement with increased cyst wall thickness are predictors of malignancy (fig. 4A). In a summary statistic comprising 539 patients with BD-IPMN, 165 patients displayed a cyst greater than 3 cm, and 54 of them harbored nodules. 83.3% of those patients were either found to suffer from invasive cancer or had high-grade dysplasia. In comparison, only 40 out of 367 patients had nodules in cysts smaller than 3 cm. However, 58% of those were found to be malignant on resection, proving the concept that mural nodules are the strongest predictors of malignancy in BD-IPMNs [42]. Recent studies suggest that an increasing height of nodules predicts the specificity and accuracy of malignancy with a cut-off of 10 mm [43, 44]. Recent studies comparing imaging modalities showed that EUS is superior in the detection of worrisome features in BD-IPMNs (fig. 4B and C); therefore, it was suggested that follow-up should be performed by EUS [42]. Contrast-enhanced endosonography can aid to the differentiation between mucus and mural nodules and helps in predicting malignant transformation [45]. If it comes to the diagnosis of BD-IPMN, a major focus is put on FNA of cystic fluid as well as cytology from mural nodules and the cyst wall. The initial study by Brugge et al. [46] proposed a cut-off of 192 ng/ml for carcinoembryonic antigen (CEA) in cyst fluid to confirm the diagnosis of a mucinous lesion. Meta-analyses studying the value of CEA in cyst fluid calculated a positive predictive value of 96% for CEA levels greater than 400 ng/ml and a negative predictive value of 98% for CEA levels below 5 ng/ml. Of note, neither does the level of CEA in cyst fluid correlate with malignancy nor does carbohydrate antigen (CA) 19–9 have any significant predictive value in the diagnosis of a mucinous lesion. Yoon et al. [47] recently correlated the level of CEA in cyst fluids to the histological subtypes of BD-IPMNs described above. While gastric-type IPMNs were smaller in diameter and less likely to develop mural nodules or mass lesions, CEA levels were with a median of 619 ng/ml highest in comparison to pancreatobiliary-type (270 ng/ml), intestinal-type (83 ng/ml), or oncocytic-type (5.1 ng/ml) IPMNs [47]. Kanda et al. [48] added a new dimension to the differential diagnosis of cystic pancreatic lesions.
Fig. 4.
A BD-IPMN – note the mural nodule associated with the cyst wall. B BD-IPMN on EUS – note the mural associated to the cyst wall with an approximate size of 10 mm (arrow). C The nodule was found to harbor high-grade dysplasia on FNA (Papanicolaou staining × 600).
By next-generation sequencing of DNA extracted from secretin-stimulated pancreatic juice or cyst fluid aspirate, mutations at codon 202 of a small g-protein GNAS were detected. 64.1% of the patients with histologically proven IPMN displayed GNAS mutations while neither SCN nor MCN, nor pseudocysts showed these mutations. If KRAS and GNAS mutations were studied, up to 96% of the patients harbored these mutations [39, 48, 49, 50, 51]. Therefore, GNAS and KRAS mutations might represent the new armamentarium for differential diagnosis of IPMNs.
MPD Dilation
The diameter of the MPD is an important independent feature in determining a malignant risk. A dilation of ≥10 mm should be regarded as highly suspicious (fig. 5). Furthermore, those with an MPD of 5–9 mm in diameter are also dubbed as lesions with worrisome features. However, a non-dilated pancreatic duct does not predict a benign lesion.
Fig. 5.
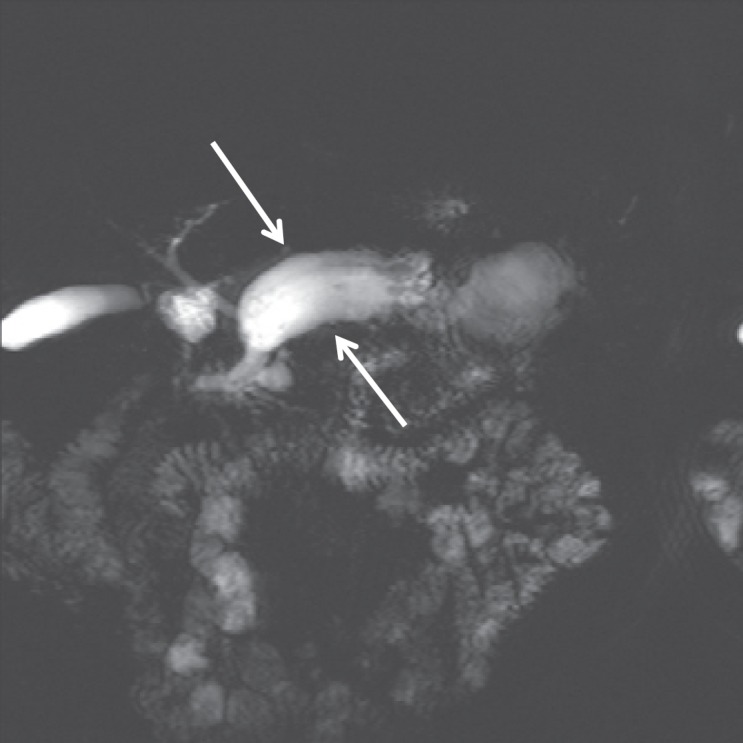
MD-IPMN – note the marked main duct dilation without visualization of a reason (no sudden break-off).
Associated Findings
There are other radiological findings of the pancreas that also imply malignancy, including distal pancreatic atrophy with an abrupt change in MPD diameter. Furthermore, the association of local lymphadenopathy may also help in conferring malignancy.
Non-Radiological Predictors of Malignancy
The use of tumor markers is important in the determination of malignancy of all tumors. A study by Fritz et al. [27] has shown that CA 19-9 has proven to be an independent predictor of malignancy and should be taken into account when determining malignancy preoperatively.
Conclusion
IPMNs are a distinct cystic neoplasm arising from the MPD or its branches. With an unknown etiology, associated with GNAS mutations and their ability to progress to malignant lesions, their presence poses a potential life-threatening risk to the patient. Correct preoperative diagnosis and risk stratification therefore need to be the pillars of clinical management [2, 4, 17, 52, 53]. Much of the difficulty comes from the inability to accurately distinguish malignant lesions from their benign precursors. And even if all imaging modalities are employed, the accuracy of diagnosis is given with 80%. Moreover, if patients with BD-IPMNs are resected for ‘worrisome features’, malignancy is only detected in 30%, and the final diagnosis is given with MCN, SCN, or non-neoplastic cyst (congenital or pseudocysts) in 20%. Furthermore, the decision for resection remains quite challenging as many of the radiological characteristics are controversial and contentious, even with the addition of pathologic markers. There is still a need for further studies looking into features associated with malignancy of these lesions, providing the clinicians in an elderly, multimorbid population with better tools to decide which lesions to resect and which to follow up.
Disclosure Statement
The authors declare that there is no conflict of interest in the manuscript. This disclosure includes direct or indirect financial or personal relationships relevant to the subject matter of the manuscript.
References
- 1.Bai XL, Zhang Q, Masood N, Masood W, Zhang Y, Liang TB. Pancreatic cystic neoplasms: a review of preoperative diagnosis and management. J Zhejiang Univ Sci B. 2013;14:185–194. doi: 10.1631/jzus.B1200283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Manfredi R, Graziani R, Motton M, Mantovani W, Baltieri S, Tognolini A, Crippa S, Capelli P, Salvia R, Mucelli RP. Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology. 2009;253:106–115. doi: 10.1148/radiol.2531080604. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton SR, Aaltonen LA., (eds) Lyon: IARC Press; 2000. WHO Classification of Tumours – Pathology and Genetics of Tumours of the Digestive System. [Google Scholar]
- 5.Xiao SY. Intraductal papillary mucinous neoplasm of the pancreas: an update. Scientifica (Cairo) 2012;2012:893632. doi: 10.6064/2012/893632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klöppel G, Offerhaus GJA, Pitman MB, Shimizu M, Zamboni G. Lyon: IARC; 2010. Intraductal neoplasms of the pancreas; in Bosman T, Carneiro F, Hruban RH, Theise ND (eds): WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 7.Sand J, Nordback I. The differentiation between pancreatic neoplastic cysts and pancreatic pseudocyst. Scand J Surg. 2005;94:161–164. doi: 10.1177/145749690509400213. [DOI] [PubMed] [Google Scholar]
- 8.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43:1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 9.de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Mensel B, Messner P, Mayerle J, Fluhr G, Völzke H, Lerch MM, Ittermann T, Kühn JP. Secretin-stimulated MRCP in volunteers: assessment of safety, duct visualization, and pancreatic exocrine function. AJR Am J Roentgenol. 2014;202:102–108. doi: 10.2214/AJR.12.10271. [DOI] [PubMed] [Google Scholar]
- 11.Marchegiani G, Fernández-del Castillo C. Is it safe to follow side branch IPMNs? Adv Surg. 2014;48:13–25. doi: 10.1016/j.yasu.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 14.Chetty R, Salahshor S, Bapat B, Berk T, Croitoru M, Gallinger S. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol. 2005;58:97–101. doi: 10.1136/jcp.2004.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maire F, Hammel P, Terris B, et al. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut. 2005;51:97–101. doi: 10.1136/gut.51.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macgregor-Das AM, Iacobuzio-Donahue CA. Molecular pathways in pancreatic carcinogenesis. J Surg Oncol. 2013;107:8–14. doi: 10.1002/jso.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubezky N, Ben-Haim M, Lahat G, Marmor S, Solar I, Brazowski E, Nackache R, Klausner JM. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151:70–75. doi: 10.1016/j.surg.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 18.Sparr JA, Bandipalliam P, Redston MS, Syngal S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am J Surg Pathol. 2009;33:309–312. doi: 10.1097/PAS.0b013e3181882c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chetty R, Serra S, Salahshor S, et al. Expression of Wntsignaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol. 2006;33:212–217. doi: 10.1016/j.humpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708–713, 713.e1–2. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Fritz S, Schirren M, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Grenacher L, Buchler MW, Werner J. Clinicopathologic characteristics of patients with resected multifocal intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2012;152(suppl 1):S74–80. doi: 10.1016/j.surg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Boot C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann Clin Biochem. 2014;51:151–166. doi: 10.1177/0004563213503819. [DOI] [PubMed] [Google Scholar]
- 24.Maker AV, Katabi N, Gonen M, DeMatteo RP, D'Angelica MI, Fong Y, Jarnagin WR, Brennan MF, Allen PJ. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvanescu A, Cros J, Ronot M, Hentic O, Grybek V, Couvelard A, Levy P, Chanson P, Ruszniewski P, Sauvanet A, Gaujoux S. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg. 2014;149:858–862. doi: 10.1001/jamasurg.2014.535. [DOI] [PubMed] [Google Scholar]
- 26.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–333. doi: 10.1097/SLA.0b013e3182378a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Bundy BD, Buchler MW, Werner J. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256:313–320. doi: 10.1097/SLA.0b013e31825d355f. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 29.Oku T, Maeda M, Wada Y. Intraductal oncocytic papillary neoplasm having clinical characteristics of mucinous cystic neoplasm and a benign histology. JOP 2007. 2007;8:206–213. [PubMed] [Google Scholar]
- 30.Longnecker DS, Adsay NV, Fernandez-del Castillo C, et al. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344–349. doi: 10.1097/01.mpa.0000186245.35716.18. [DOI] [PubMed] [Google Scholar]
- 31.Mino-Kenudson M, Fernández-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul T, Perez Johnston R, Turner BG, Androutsopoulos V, Deshpande V, McGrath D, Sahani DV, Brugge WR, Ogino S, Pitman MB, Warshaw AL, Thayer SP. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–1720. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 33.Yopp AC, Katabi N, Janakos M, Klimstra DS, D'Angelica MI, DeMatteo RP, Fong Y, Brennan MF, Jarnagin WR, Allen PJ. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–974. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 34.Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, Chen JH, Yiannoutsos CT, Lillemoe KD, Farnell MB, Sarr MG, Schmidt CM. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on Cancer Stage. J Am Coll Surg. 2011;213:275–283. doi: 10.1016/j.jamcollsurg.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aso T, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Ideno N, Osoegawa T, Takahata S, Shindo K, Ushijima Y, Aishima S, Oda Y, Ito T, Mizumoto K, Tanaka M. ‘High-risk stigmata’ of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014;43:1239–1243. doi: 10.1097/MPA.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 37.Cone MM, Rea JD, Diggs BS, Douthit MA, Billingsley KG, Sheppard BC. Predicting malignant intraductal papillary mucinous neoplasm: a single-center review. Am J Surg. 2011;201:575–579. doi: 10.1016/j.amjsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Grutzmann R, Post S, Saeger HD, Niedergethmann M. Intraductal papillary mucinous neoplasia (IPMN) of the pancreas: its diagnosis, treatment, and prognosis. Dtsch Arztebl Int. 2011;108:788–794. doi: 10.3238/arztebl.2011.0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakorafas GH, Sarr MG. Cystic neoplasms of the pancreas; what a clinician should know. Cancer Treat Rev. 2005;31:507–535. doi: 10.1016/j.ctrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Jones MJ, Buchanan AS, Neal CP, Dennison AR, Metcalfe MS, Garcea G. Imaging of indeterminate pancreatic cystic lesions: a systematic review. Pancreatology. 2013;13:436–442. doi: 10.1016/j.pan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:517–522. doi: 10.1097/SLA.0b013e3182444231. [DOI] [PubMed] [Google Scholar]
- 42.Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, Imai H, Maekawa K, Chikugo T, Kumano M, Hyodo T, Murakami T, Chiba Y, Takeyama Y. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22–29. doi: 10.1055/s-0033-1353603. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi G, Fujita N, Maguchi H, Tanno S, Mizuno N, Hanada K, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R, Yanagisawa A, Tanaka M. Working Group for the Natural History of IPMN of the Japan Pancreas Society: Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532–538. doi: 10.1097/MPA.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawada N, Uehara H, Nagata S, Tsuchishima M, Tsutsumi M, Tomita Y. Predictors of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas. JOP. 2014;15:459–464. doi: 10.6092/1590-8577/2805. [DOI] [PubMed] [Google Scholar]
- 45.Ohno E, Itoh A, Kawashima H, Ishikawa T, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R, Ohmiya N, Ishigami M, Katano Y, Goto H, Hirooka Y. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas. 2012;41:855–862. doi: 10.1097/MPA.0b013e3182480c44. [DOI] [PubMed] [Google Scholar]
- 46.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, Fernandez-del Castillo C, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Yoon WJ, Daglilar ES, Mino-Kenudson M, Morales-Oyarvide V, Pitman MB, Brugge WR. Characterization of epithelial subtypes of intraductal papillary mucinous neoplasm of the pancreas with endoscopic ultrasound and cyst fluid analysis. Endoscopy. 2014;46:1071–1077. doi: 10.1055/s-0034-1377629. [DOI] [PubMed] [Google Scholar]
- 48.Kanda M, Knight S, Topazian M, Syngal S, Farrell J, Lee J, Kamel I, Lennon AM, Borges M, Young A, Fujiwara S, Seike J, Eshleman J, Hruban RH, Canto MI, Goggins M. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–1033. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. doi: 10.1038/srep00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takano S, Fukasawa M, Maekawa S, Kadokura M, Miura M, Shindo H, Takahashi E, Sato T, Enomoto N. Deep sequencing of cancer-related genes revealed GNAS mutations to be associated with intraductal papillary mucinous neoplasms and its main pancreatic duct dilation. PLoS One. 2014;9:e98718. doi: 10.1371/journal.pone.0098718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2012;10:555–558. doi: 10.1016/j.cgh.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, Kendrick M, Farnell MB. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 54.Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]