Summary
Background
Filiform polyposis (FP) is an uncommon cause of non-neoplastic and non-syndromic polyposis. Several hypotheses concerning its pathogenesis have been published. FP is most frequently associated with a post-inflammatory reparative process; indeed, the most frequent association is with inflammatory bowel disease (IBD). FP is characterized by one to hundreds of uniform, slender, arborizing, vermiform projections of the large bowel mucosa and submucosa lined by normal or inflamed colonic mucosa. The most common sites for these polyps are the transverse and descending colon.
Case Report
In this report we present a case of giant FP associated with locally invasive adenocarcinoma of the right colon in a 73-year-old man with no past medical history of IBD.
Conclusion
Few of these cases have been reported in the literature, and out of the approximately 20 of such case reports only one other was associated with colorectal adenocarcinoma.
Keywords: Filiform polyposis, Large bowel, Non-neoplastic
Introduction
Filiform polyposis (FP) is a rare cause of non-neoplastic, non-syndromic polyposis of uncertain pathogenesis. FP is most frequently secondary to a post-inflammatory reparative process and may occur in 10–20% of the cases of inflammatory bowel disease (IBD) [1, 2], in which chronic inflammation of the large bowel mucosa with repeated ulceration and healing may lead to the formation of worm-like polypoid projections. FP typically presents as one to hundreds of uniform, slender, arborizing, vermiform projections of the bowel mucosa and submucosa lined by normal or inflamed mucosa. In rare cases, polyps coalesce and a large tumor mass which may measure over 15 mm, known as giant filiform polyposis (GFP), is found [3]. The mucosa surrounding the polyps may be normal or show acute or chronic inflammatory changes depending on the context in which it arises. Non-IBD-associated GFP is extremely rare; in this report we present a case of GFP associated with locally invasive adenocarcinoma of the right colon in a 73-year-old man with no past medical history of colitis.
Case Report
A 73-year-old man was admitted to the emergency ward with fever, abdominal pain, and acute abdomen due to bowel occlusion. A computed tomography (CT) scan showed cecal bowel perforation and a distal, ascending colon stricture which was deemed to be neoplastic. Adjacent to this stricture, a hyperdense elevated and irregular area was also identified (fig. 1). The patient underwent emergency right hemicolectomy. He had no personal or family history of colon polyps, colon cancer, or IBD. Prior surgery consisted of a cholecystectomy 25 years previously.
Fig. 1.
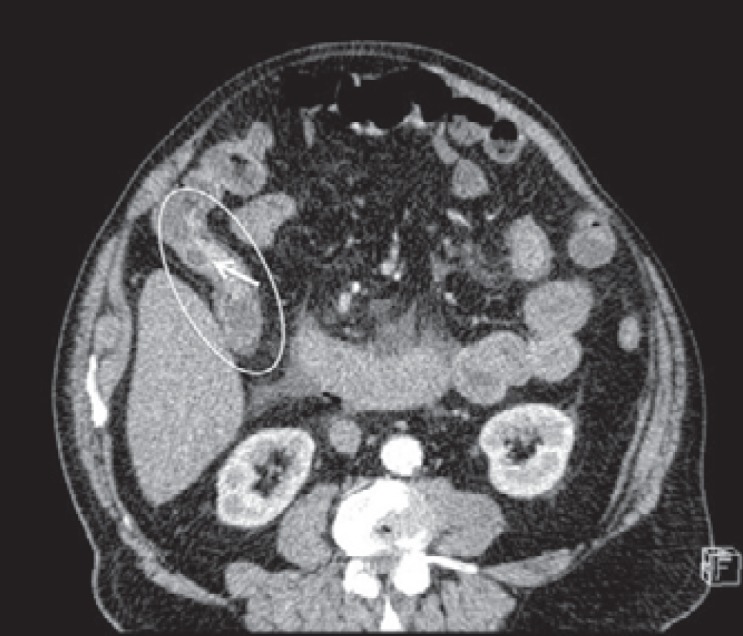
Preoperative emergency CT scan showing a cecal tract (oval) with stricture and adjacent hyperdense, elevated, and irregular area (arrow).
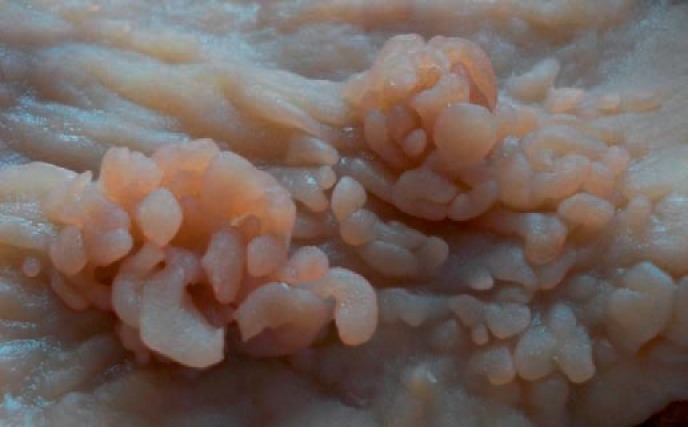
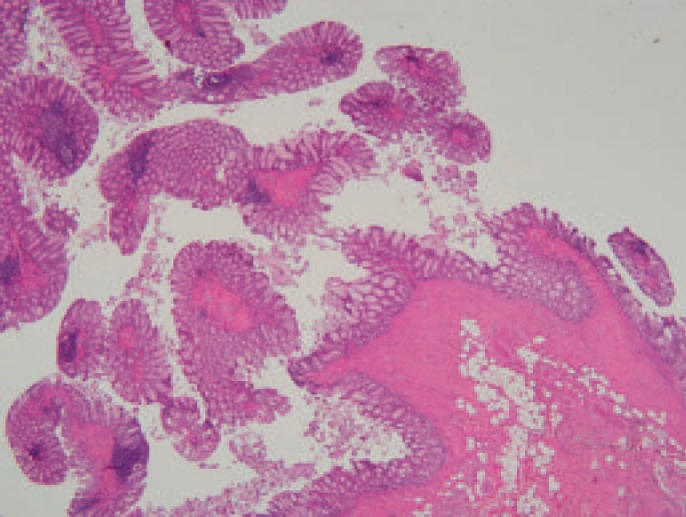
Examination of the right hemicolectomy specimen revealed a large cecal perforation and an ulcerated neoplasm in the ascending colon measuring approximately 5 cm. Proximal to the neoplasm, separated from it by 4 cm of normal-looking mucosa, numerous slender, worm-like mucosal projections were identified ranging in size and covering about 11 cm in length (fig. 2). Apart from a few erosions at the tips of the polyps, all the filiform polyps were histologically covered by normal colonic mucosa (fig. 3). The appendix and ileum were normal.
Fig. 2.
Macroscopic findings of the resected colon. Numerous finger-like polyps aggregating in the right colon are evident.
Fig. 3.
Hematoxylin- and eosin-stained histological section of filiform polyps showing little inflammation in the overlying and surrounding mucosa.
Histological examination of the colorectal carcinoma showed a poorly differentiated invasive adenocarcinoma. The carcinoma invaded the full thickness of the bowel wall with invasion of the pericolic adipose tissue and focal involvement of the serosal lining. Vascular and perineural invasion as well as lymph node metastases (16 metastatic nodes out of 45 isolated nodes) were seen. Immunostaining for mismatch repair protein expression (MLH1, MSH2, MSH6, and PMS2) showed preservation of nuclear staining.
A histological examination of the sampled polyps showed finger-like projections covered with architecturally normal colonic mucosa with non-specific inflammation and focal erosions (mostly at the tips of the polyps). The central core was composed of submucosal tissue containing vessels, nerves, and lymphoid aggregates. No dysplasia was observed in the mucosa covering the polyps or surrounding them. The uninvolved colonic and ileal mucosa showed no evidence of chronic inflammation. No evidence of other associated disease was seen; particularly histiocytosis X was excluded morphologically and by immunohistochemistry (negative reactions for S-100 protein and CD1a).
Discussion
We report a case of GFP, not associated with IBD, incidentally found in a patient with invasive colorectal carcinoma. Few of these cases have been described in the literature, and out of approximately 20 such case reports only one other was associated with colorectal adenocarcinoma [4].
The term FP was originally described by Appelman et al. [3] and consisted of numerous worm-like, filiform defects in the colon at imaging. Polyps may vary greatly in number from one to hundreds and are composed of a central core of submucosal connective tissue with overlying normal, non-inflamed mucosa. They are most often slender and long, and the unattached portion is free to move within the fecal stream. Size is variable and may be considerable (up to 9 cm long polyps have been described); some polyps may show bridging between adjacent polyps [3] while others may coalesce to form a mass-like lesion. The transverse, descending, and sigmoid colon are the most frequent locations, although the polyps can be seen in any portion of the large bowel [5], except for the rectum. They may rarely be identified in the stomach and the small bowel.
The pathogenesis of FP is uncertain. Polyps may result from repeated mucosal injury (such as in IBD) [6, 7], or they may be secondary to peristalsis and fecal stream traction on the mucosa. Oakley et al. [1] suggested that pathogenesis may be more hamartomatous in nature because neuromuscular and fibrovascular hyperplasia/disarray within the stalk were observed in their case series. Other authors have proposed that a localized response to cytokines and growth factors generated by inflamed mucosa may lead to polyp formation [8].
The most frequent association is with IBD; however, other conditions have been described (such as histiocytosis X, inflammatory processes such as diverticulitis, perforation, necrotizing enterocolitis, enema-induced colitis, stercoral ulcer, and colonic tuberculosis [9, 10, 11, 12, 13]).
FP is mostly asymptomatic and incidentally diagnosed at colonoscopy. A variety of symptoms have been described, such as anemia (probably due to ulceration and hemorrhage secondary to trauma caused by fecal flow and peristalsis), weight loss, cramping abdominal pain, and diarrhea [2, 5] as well as possible obstruction and intussusception [14].
While FP in IBD is a well-recognized entity, sporadic FP is unusual and may pose diagnostic challenges to both clinicians and pathologists, with differential diagnosis including adenoma or carcinoma. Proposed treatment has always been conservative (biopsy/polypectomy) [1]; however, a recent report [4] raises the possibility of malignant potential in these lesions as adenomas and an invasive adenocarcinoma developed on FP in a patient without known IBD.
In our present case, a stenosing invasive adenocarcinoma developed close to FP. Two possibilities exist to explain this association: Either FP is somehow related to the cancerogenetic process, as suggested by Boulagnon et al. [4], or the presence of stenosis and peristalsis is important in FP pathogenesis. In support of the second rather than the first hypothesis is that in our case, FP developed proximally to the neoplastic stenosis where peristaltic movements were probably the greatest. Furthermore, 4 cm of normal mucosa separated the polypoid length from the adenocarcinoma, making direct derivation improbable. If increased peristalsis is indeed one of the mechanisms, it is highly likely that a certain predisposition or concurrent cause must be present; after all, not all patients with increased peristalsis/stenosis develop FP.
Conclusions
In conclusion, this is the second case in which FP has been described in a patient with invasive colorectal carcinoma but without evidence of IBD or other causes of chronic colitis. FP is important as it may be a diagnostic challenge in patients who have massforming polypoid lesions which may be confused with cancer, and surgeons, endoscopists, and pathologists must be made aware of this entity in non-IBD patients.
Disclosure Statement
All authors declare that they have no conflict of interest.
References
- 1.Oakley G, Schraut WH, Peel R, Krasinskas A. Diffuse filiform polyposis with unique histology mimicking familial adenomatous polyposis in a patient without inflammatory bowel disease. Arch Pathol Lab Med. 2007;131:1821–1824. doi: 10.5858/2007-131-1821-DFPWUH. [DOI] [PubMed] [Google Scholar]
- 2.Sheikholeslami MR, Schaefer RF, Mukunyadzi P. Diffuse giant inflammatory polyposis: a challenging clinicopathologic diagnosis. Arch Pathol Lab Med. 2004;128:1286–1288. doi: 10.5858/2004-128-1286-DGIPAC. [DOI] [PubMed] [Google Scholar]
- 3.Appelman HD, Threatt BA, Ernst C, Lindenauer SM, Blamey W. Filiform polyposis of the colon: an unusual sequel of ulcerative colitis. Am J Clin Pathol. 1974;62:145–146. [Google Scholar]
- 4.Boulagnon C, Jarezon JF, Diaz-Cives A, Ehrhard F, Bouche O, Diebold MD. Filiform polyposis: a benign entity? Case report and literature review. Pathol Res Pract. 2014;210:189–193. doi: 10.1016/j.prp.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Lee CG, Lim YJ, Choi JS, Lee JH. Filiform polyposis in the sigmoid colon: a case series. World J Gastroenterol. 2010;16:2443–2447. doi: 10.3748/wjg.v16.i19.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauknecht KJ, Grosse G, Kleinert J, Lachmann A, Niedobitek F. Filiform polyposis of the colon in chronic inflammatory bowel disease (so-called giant inflammatory polyps) Z Gastroenterol. 2000;38:845–859. doi: 10.1055/s-2000-9994. [DOI] [PubMed] [Google Scholar]
- 7.Okayama N, Itoh M, Yokoyama Y, Tsuchida K, Takeuchi T, Kobayashi K, Mizuno A, Manabe T. Total obliteration of colonic lumen by localized giant inflammatory polyposis in ulcerative colitis: report of a Japanese case. Intern Med. 1996;35:24–29. doi: 10.2169/internalmedicine.35.24. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Lee KY, Kim YW. Filiform polyposis associated with sigmoid diverticulitis in a patient without inflammatory bowel disease. J Crohns Colitis. 2010;4:671–673. doi: 10.1016/j.crohns.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Hew JM, Chandie Shaw P, Blickman G. Filiform polyposis: a manifestation of histiocytosis X. Rofo. 1985;143:474–476. doi: 10.1055/s-2008-1052851. [DOI] [PubMed] [Google Scholar]
- 10.Peh WC. Filiform polyposis in tuberculosis of the colon. Clin Radiol. 1988;39:534–536. doi: 10.1016/s0009-9260(88)80227-7. [DOI] [PubMed] [Google Scholar]
- 11.Nebel OT, el-Masry NA, Castell DO, Farid Z, Fornes MF, Sparks HA. Schistosomal disease of the colon: a reversible form of polyposis. Gastroenterology. 1974;67:939–943. [PubMed] [Google Scholar]
- 12.Berkowitz D, Bernstein LH. Colonic pseudopolyps in association with amebic colitis. Gastroenterology. 1975;68:786–789. [PubMed] [Google Scholar]
- 13.Segal I, Solomon A, Mirwis J. Radiological manifestations of ritual-enema-induced colitis. Clin Radiol. 1981;32:657–662. doi: 10.1016/s0009-9260(81)80334-0. [DOI] [PubMed] [Google Scholar]
- 14.Fitterer JD, Cromwell LG, Sims JE. Colonic obstruction by giant pseudopolyposis. Gastroenterology. 1977;72:153–156. [PubMed] [Google Scholar]