Summary
Background
Pancreatic cystic lesions (PCL) are common. They are increasingly detected as an incidental finding of transabdominal ultrasound or cross-sectional imaging. In contrast to other parenchymal organs, dysontogenetic pancreatic cysts are extremely rare. In symptomatic patients the most frequent PCL are acute and chronic pseudocysts. The majority of incidental cystic lesions, however, are neoplasias which have different risks of malignancy.
Methods
PubMed was searched for studies, reviews, meta-analyses, and guidelines using the following key words: (‘pancreatic cystic lesions’ OR ‘cystic pancreatic lesions’ OR ‘intraductal papillary mucinous neoplasia’ OR ‘mucinous cystic neoplasia’ OR ‘pancreatic cyst’ OR ‘pancreatic pseudocyst’) AND (management OR treatment OR outcome OR prognosis OR diagnosis OR imaging OR ‘endoscopic ultrasound’ EUS-FNA OR EUS OR ‘endoscopic ultrasonography’ OR CT OR MRI). Retrieved papers were reviewed with regard to the diagnostic and therapeutic management of incidental PCL.
Results
In addition to clinical criteria, transabdominal ultrasonography including contrast-enhanced ultrasonography, cross-sectional radiological imaging, and endoscopic ultrasound (EUS) are used for diagnostic characterization and risk assessment. EUS plays an outstanding role in differential diagnosis and prognostic characterization of incidental PCL. In a single examination it is possible to perform high-resolution morphological description, perfusion imaging, as well as fine-needle aspiration of cyst content, cyst wall, and solid components. An international consensus guideline has defined worrisome and high-risk criteria for the risk assessment of mucinous pancreatic cysts, which are mainly based on the results of EUS and cross-sectional imaging. Nevertheless, despite diagnostic progress and guideline recommendations, differential diagnosis and management decisions remain difficult. This review will discuss problems in and approaches to the diagnosis of incidental PCL.
Conclusion
An evidence-based algorithm for the diagnosis of incidental PCL is proposed.
Keywords: Pancreatic cystic lesion, Incidental finding, Intraductal papillary mucinous neoplasia, Mucinous cystic neoplasia, Serous cystadenoma, Endoscopic ultrasound, Cross-sectional imaging
Introduction: Chance and Challenge
Pancreatic cystic lesions (PCL) are common. Two Japanese autoptic studies detected small pancreatic cysts (>1–2 mm) in 73 of 300 (24.3%) and 378 of 1,374 (27.5%) consecutive autopsy cases, respectively [1, 2]. The incremental dissemination and technical development of modern imaging methods facilitates the detection of PCL by transabdominal ultrasonography (TUS), computed tomography (CT), or magnetic resonance imaging (MRI). A large retrospective Japanese study reviewed the TUS findings of 12,112 consecutive patients, among them 1,012 patients with and 11,100 patients without end-stage renal disease. The prevalence of PCL in both groups proved to be 9.3 and 1.3%, respectively, with a relatively high percentage of potentially malignant mucinous neoplasms among them (2.8 and 0.2%, respectively) [3]. CT and MRI studies in large cohorts of asymptomatic persons revealed unsuspected pancreatic cysts in 2.4–13.5% of the cases. There is a strong correlation of increasing age and prevalence of PCL [4, 5, 6]. A recent study compared follow-up data of 2,034 patients with PCL detected incidentally at CT or MRI with follow-up data of a matched control group (n = 6,018) without PCL, showing that the detection of a PCL is associated with a 3-fold increased risk to develop pancreatic adenocarcinoma [7]. However, not all incidentally detected PCL carry an elevated risk of malignant transformation. There is a broad spectrum of incidental PCL, comprising 25 different types defined by the World Health Organization, among them four types of primarily cystic neoplasias: serous cystic adenoma (SCA), mucinous cystic neoplasia (MCN), as well as main-duct (MD) and branch-duct (BD) intraductal papillary mucinous neoplasia (IPMN). Solid pseudopapillary neoplasia (SPN) and cystic variants of ductal adenocarcinoma, neuroendocrine tumor, and acinus cell cancer are the best known, though rare examples of PCL resulting from necrosis and cystic degeneration of solid tumors. Dysontogenetic cysts, which are common in the kidneys and the liver, are rare in the pancreas. Contrary to symptomatic patients, pseudocysts are a very rare diagnosis in asymptomatic patients. Other non-neoplastic PCL are very rare: lymphoepithelial cysts (LEC), dermoid cysts (DC), epidermoid cysts (ECIS), retention cysts, mucinous non-neoplastic cysts (MNC), duplication cysts of the foregut, cystic hamartoma, and cystic lymphangioma. MD-IPMN, BD-IPMN, and MCN are mucinous PCL and precursor lesions of pancreatic adenocarcinoma. Their premalignant risk varies according to the particular type of lesion, its size, and some further features like (in IPMN) their histological subtype [8, 9, 10]. IPMN are subclassified into neoplasias with intestinal, pancreatobiliary, oncocytic, or gastric cellular differentiation. Intestinal, pancreatobiliary, and oncocytic subtypes predominantly involve the main pancreatic duct within the pancreatic head and body, with the pancreatobiliary subtype being the most aggressive one and developing into tubular adenocarcinoma [11, 12]. A majority of these neoplasias is already malignant at the time of diagnosis or easily progresses to invasive adenocarcinoma. Nevertheless, prognosis of MD-IPMN in non-invasive and minimally invasive stages is more favorable compared to ductal adenocarcinoma, preferentially in the intestinal subtype, which develops into colloid (mucinous) adenocarcinoma [11, 13, 14, 15]. Gastric-type IPMN occur predominantly in the branch ducts, preferentially of the pancreatic head. They are frequently multifocal and have a distinctively lower risk (at the time of diagnosis approximately 15–20%) as well as slower course of progressing to invasive adenocarcinoma. In patients with gastric-type IPMN, however, simultaneous or metachronous development of ductal adenocarcinoma may occur, thus worsening the prognosis considerably [11, 12, 13, 16, 17, 18, 19]. Prospective data on the natural history and rate of malignant transformation of mucinous precursors of pancreatic cancer are rare, and epidemiological, surgical, and retrospective data show conflicting results [17, 18, 19, 20, 21, 22, 23].
Therefore, the incidental discovery of PCL at the same time is increasingly becoming a chance as well as a challenge for modern health care systems. On the one hand, the early detection of cystic precursor lesions in asymptomatic persons opens the window widely for the prevention of a substantial portion of pancreatic cancers. On the other hand, differential diagnosis is demanding, and natural history is not sufficiently understood. In older patients with significant comorbidity and slowly developing mucinous PCL, a low or moderate risk of malignancy may be outperformed by the risks of pancreatic surgery.
Methods
A systematic literature search was performed to identify studies, reviews, meta-analyses, and guidelines evaluating diagnosis, treatment, and prognosis of PCL. PubMed was searched using the following keywords: (‘pancreatic cystic lesions’ OR ‘cystic pancreatic lesions’ OR ‘intraductal papillary mucinous neoplasia’ OR ‘mucinous cystic neoplasia’ OR ‘pancreatic cyst’ OR ‘pancreatic pseudocyst’) AND (management OR treatment OR outcome OR prognosis OR diagnosis OR imaging OR ‘endoscopic ultrasound’ EUS-FNA OR EUS OR ‘endoscopic ultrasonography’ OR CT OR MRI). Retrieved papers were reviewed with regard to the diagnostic and therapeutic management of incidental PCL.
Diagnostic Tools
Asymptomatic PCL are most often initially detected on TUS or abdominal CT. The initial imaging modality gives relevant basic information about size, localization, and gross morphological appearance. For diagnostic characterization and risk assessment, however, detailed morphological information is necessary, in particular on ductal communication, cyst content, mural nodules, and septae.
Cross-Sectional Imaging
A prospective study proved a high accuracy and concordance of two readers of multidetector CT (MDCT) scans for the preoperative stratification of PCL into mucinous and non-mucinous types (82 and 85%) and the prediction of their biologic aggressiveness (85 and 86%). However, predictive values of MDCT were superior for lesions >30 mm and non-mucinous lesions [24]. Two retrospective studies suggested an equivalent accuracy of MDCT and MRI for characterizing small PCL as benign or malignant as well as mucinous or non-mucinous, and for suggesting a specific diagnosis. Whereas the accuracy for classification according to the risk of malignancy may be regarded as sufficient (75–86%), the accuracy for determining a specific diagnosis remained disappointing (40–84%) [25, 26]. In another retrospective study evaluating the accuracy of CT versus MRI and magnetic resonance cholangiopancreatography (MRCP) in the characterization of IPMN, pathologically proven ductal communication was detected by MRI in 73% of the cases, by CT, however, only in 18% of the cases. CT tended to overestimate an involvement of the pancreatic duct when compared with MRCP and surgical pathology. Moreover, CT was inferior to MRCP in identifying small-branch duct cysts and regarding the recognition of multifocality [27].
Ultrasonography
The diagnostic value of TUS has been evaluated only in preliminary studies. One study showed a high correlation of near-isovoxel ultrasound using matrix transducers and MRCP in evaluating the ductal communication of PCL [28]. Contrast-enhanced ultrasonography (CEUS) has been prospectively shown to discriminate between pseudocyst and cystic neoplasia with a very high diagnostic accuracy, outperforming TUS without contrast enhancement [29, 30]. A retrospective study suggested that CEUS compares favorably with MRI in displaying anatomic features of PCL (septae, nodules), and demonstrated a close correlation between CEUS findings and results of surgical pathology [31].
Endoscopic Ultrasound (EUS) and EUS-Guided Fine-Needle Aspiration
Due to its unsurpassed spatial resolution, feasibility of vascularity analysis, and opportunity to perform fine-needle aspiration (FNA), EUS is regarded to be the most valuable diagnostic tool for the classification and prognostic evaluation of PCL [32, 33, 34]. While one retrospective study showed a comparable accuracy of EUS and MRI in the characterization of PCL and prediction of malignancy [35], most other studies are in favor of EUS. Comparing the performance of CT, MRI, and EUS in characterizing PCL in 145 patients, EUS more frequently identified PCL to be multifocal and, even more important, their presence in different surgical fields. Communication with the pancreatic duct was detected significantly more frequently by EUS compared with CT and MRI. For the detection of mural nodules and septations, EUS performed significantly superior compared with CT but not with MRI [36]. In another large cohort of patients with PCL, in which the final diagnosis was established by surgical pathology, EUS(-FNA) was superior to cross-sectional imaging in accurately classifying a cyst as neoplastic. The incremental increase in diagnostic yield of EUS(-FNA) over CT and MRI for the prediction of a neoplastic cyst in this study was 36 and 54%. Again, EUS detected considerably more mural nodules than cross-sectional imaging. Nonetheless, the neoplastic nature and malignancy of a PCL were still underestimated by EUS(-FNA) in 23 and 16% of the cases, respectively [37]. To increase the yield of EUS-FNA of PCL, aspiration not only of the cyst content but also of the cyst wall and of possible solid components was suggested [38, 39]. EUS-FNA of PCL has a higher complication rate (bleeding, pancreatitis, infection, surgical complications: 5–6% in prospective studies) compared to EUS-FNA of solid pancreatic lesions (2.4% in prospective studies) [40, 41, 42, 43]. Two retrospective studies of large cohorts of patients undergoing resection of neoplastic pancreatic cysts did not observe an increased risk of peritoneal tumor seeding by performing EUS-FNA [40, 44]. To minimize the risk of cyst infection, complete aspiration of the cyst content and peri-interventional antibiotic treatment are recommended for EUS-FNA of PCL [41, 45].
Diagnostic Criteria
FLAG(S)
Following initial detection of a PCL, basic facts are known and should be considered: age and gender of the patient, symptoms, localization, and size of the lesion. These simple data (‘FLAGS – i.e. frequency, localization, age, gender, symptoms – criteria’) result in a relatively high pretest accuracy for various PCL and help to stratify the risk of malignancy (fig. 1, table 1). For example, it is virtually impossible that a PCL located within the pancreatic head of a 73-year-old man represents a MCN.
Fig. 1.
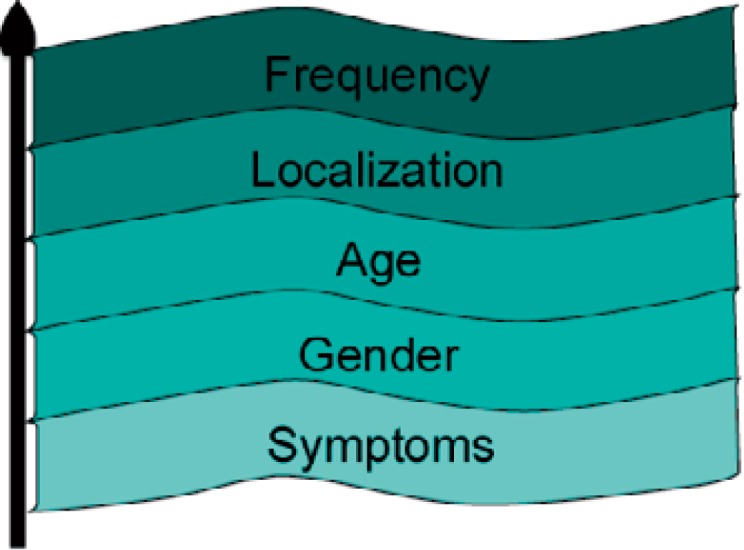
FLAG(S) criteria – high pretest probability in categorizing PCL by using simple data (modified from [34]).
Table 1.
| Pseudocyst | SPN | SCA | MCN | BD-IPMN | MD-IPMN | |
|---|---|---|---|---|---|---|
| Frequency | very common | very rare | moderate | moderate | common | rare |
| Localization | unifocal (predominantly head) | unifocal, variable | unifocal (microcystic: 70% left) | unifocal (70% left) | >60% multifocal, >70% head, branch ducts | main duct, predominantly head |
| Median age | variably | ≈20 years | ≈60 years | ≈45 years | ≈65 years | ≈65 years |
| Gender | m > f | f >> m (9:1) | microcystic: f > m (7:3); macrocystic: m > f (3:2) | f >>> m (>20:1) | m = f | m > f (3:2) |
| Symptoms | often, history of pancreatitis is obligatory | rare, only in large tumors, history of pancreatitis <10% in MCN | in up to one-third of cases mild (recurrent) pancreatitis | |||
Localization
Localization within the pancreas may give some clues for the diagnosis of PCL. Typically, BD-IPMNs are located within the pancreatic head, preferentially within the uncinate process. In up to two-thirds of the cases, however, smaller cysts can also be found in other parts of the pancreas [36, 46]. MD-IPMNs commonly develop from the pancreatic head region. Conversely, approximately 70% of microcystic SCA and most SPN are located outside the pancreatic head [33].
Age/Gender
Two types of cystic pancreatic neoplasias, i.e. SPN and MCN, almost exclusively occur in women. SPN is the least frequent cystic neoplasm, occurring with very rare exceptions only in girls and young women with a median age of 20 years. MCN is most commonly observed in middle-aged women.
Symptoms
Pseudocysts are associated with acute or chronic pancreatitis; therefore, they are only very rarely detected incidentally. Incidental PCL are smaller than symptomatic cysts and are found predominantly in older patients [47]. The majority of IPMNs are not accompanied by acute symptoms [48]. Due to transient mucinous ductal obstruction, however, IPMN of both types may cause mild and often recurrent flares of acute pancreatitis in 7–34.6% of the cases [49, 50, 51, 52]. Most other neoplastic PCL are asymptomatic unless accelerated growth causes compression of neighboring organs and structures.
Morphology
Some PCL have a very typical morphology and may thus be easily diagnosed by imaging. Relevant morphological criteria are in particular: unilocularity versus multilocularity, wall (thickness, nodules, vascularity), septae (thickness, vascularity), mural nodules/solid components, communications (cyst-cyst, cyst-duct), unifocal versus multifocal occurrence, central scar, calcifications, cyst content, and size [33, 34, 53, 54]. In addition to the cyst features, morphology of the pancreatic parenchyma as well as diameter and contour of the main pancreatic duct should be paid heed to (table 2) [33, 34].
Table 2.
Typical morphological features and average risk of malignancy of PCL (modified from [33])
| Pseudocyst | SPN | SCA | MCN | BD-IPMN | MD-IPMN | |
|---|---|---|---|---|---|---|
| Pancreatic parenchyma | often chronic pancreatitis | normal | normal | normal | normal, sometimes discrete features of chronic pancreatitis | commonly discrete features of chronic pancreatitis |
| Main pancreatic duct | variably dilated | normal | normal | normal | variably dilated, <5 mm; diameter <5 mm suggesting mixed-type | dilated, ‘fish mouth papilla’ (50%) |
| Ductal communication | common | never | never | very rarely | yes, to branch ducts | dilatation of the main pancreatic duct |
| Typical appearance | unilocular, thick wall | often large, solid-cystic | round/lobulated, thin septae, microcystic (‘honeycomb’, central scar, no distict wall), oligocystic (single cysts >20 mm), macrocystic (unilocular) | macrocystic (unilocular), orange-like: ‘cyst in cyst’, thick wall, septae | multilocular, grape-like: ‘cyst by cyst’, communication of cysts, mucin plugs, nodules; often multifocal; small BD-IPMN: unilocular | cystic dilatation of the main pancreatic duct, intraductal nodules, 50% fish mouth papilla |
| Vascularity | avascular | hypervascular | hypervascular (‘FNH of the pancreas’) | hypervascular wall, septae, nodules) | hypervascular (wall, septae, nodules) | hypervascular (duct wall, nodules) |
| Malignancy rate | never | <15% | never | =20% | 15–25% | >60% |
Microcystic SCA is characterized by multiple closely agglomerated microcysts (≤20 mm, often only 1–2 mm) separated by thin but highly vascularized septae (‘honeycombing’). Sometimes a central scar (rarely with calcification) may be found. Criteria of chronic pancreatitis are lacking, and the main pancreatic duct is not involved (fig. 2).
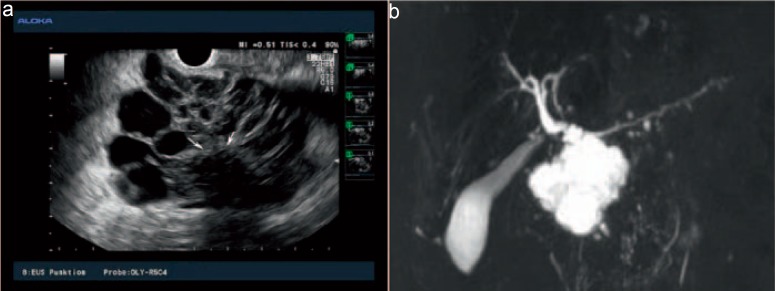
Fig. 2.
EUS (a) and MRC (b) images of a typical microcystic SCA: the large lesion of the pancreatic head consists of multiple small and middle-sized cysts, separated from each other by delicate septae. EUS finely delineates a calcified central scar (arrows). Septae are highly vascularized (not shown). The main pancreatic duct and common bile duct are not dilated (pictures courtesy of Dr. Bernd von Lampe, Berlin).
However, differential diagnosis of the oligocystic type of SCA (multilocular cyst > 2 cm with thin wall/septae without ductal communication) to BD-IPMN and of the macrocystic type (unilocular cyst, often lobulated, thin wall) to pseudocyst or MCN may be challenging. BD-IPMN is suspected in cases with grape-like agglomerations of cysts within the pancreatic head (‘cyst by cyst’), in particular if communication between neighboring cysts as well as between cysts and side branches of the main pancreatic duct may be displayed (fig. 3). MCN are unilocular cysts with a distinct, highly vascularized wall and septae or cysts within the cyst. MD-IPMN is characterized by complete or segmental cystic dilatation of the main pancreatic duct without underlying stricture. TUS or EUS may delineate hyperechoic luminal layering or mucinous plugs. In approximately 50% of MD-IPMN a dilated orifice of the papilla with mucinous secretion (patulous papilla, ‘fish-mouth papilla’) is observed (fig. 4) [55, 56]. Solid mural nodules are typical features and high-risk markers of mucinous cystic neoplasms (MCN, IPMN). However, the detection rate for small mural nodules is unsatisfactory when using cross-sectional radiological imaging, and discrimination from mucin plugs is challenging [57]. Contrast-enhanced EUS was suggested to increase the diagnostic accuracy to detect neoplastic PCL, to discriminate mural nodules from mucin plugs, and to determine growth patterns of mural nodules (fig. 4, 5) [58, 59, 60].
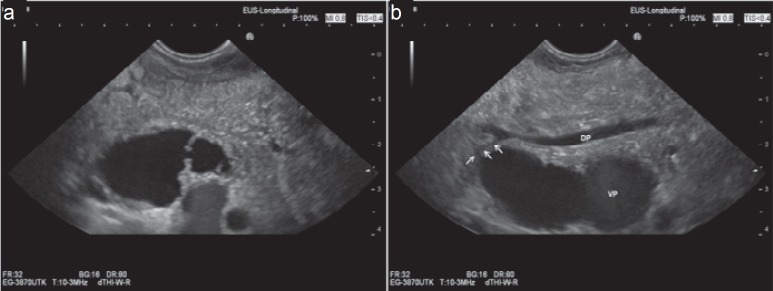
Fig. 3.
EUS images of a typical BD-IPMN: grape like agglomeration of cysts, communicating (a) with each other and (b) with a side branch of the main pancreatic duct (arrows). Main pancreatic duct is slightly dilated. Pancreatic parenchyma is homogeneous.
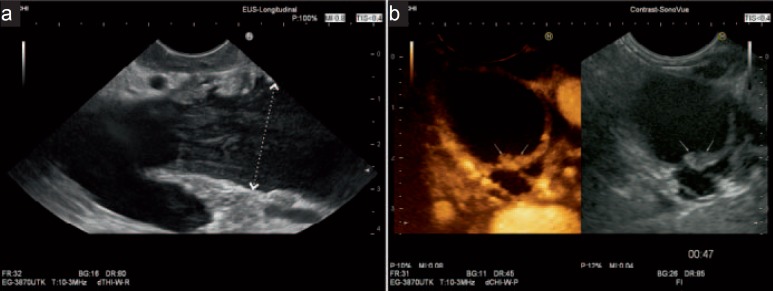
Fig. 4.
EUS images of a typical MD-IPMN: impressive dilatation of the main pancreatic duct (between markers: 30 mm). a Intraluminal layered echoes represent mucin (pancreatic body). b Contrast-enhanced EUS shows hyperechoic mural nodules (arrows; pancreatic tail).
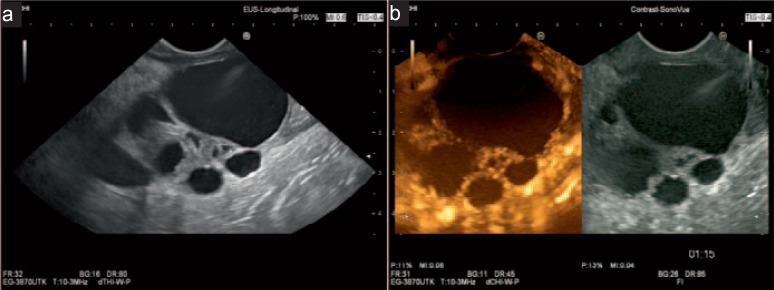
Fig. 5.
EUS images of a malignant BD-IPMN. a Large cystic lesions with solid parts and thick septae. b Contrast-enhanced EUS shows vascularization of solid parts and septae.
Cyst Fluid Analysis
EUS-FNA allows optical inspection, biochemical analysis (amylase or lipase; carcinoembryonic antigen (CEA)), and cytological examination of cyst fluid (table 3). Typically the pseudocysts’ content is a muddy brown fluid with low CEA concentration, containing neutrophils and/or histiocytes [61]. The gross appearance of aspirates of cystic lymphangioma (milky fluid) and of LEC, DC, and ECIS (thick milky, creamy, or frothy) may afford diagnosis [62]. Viscosity of fluid may be obvious if a string of fluid can be lifted with a needle from the slide (‘string sign’) [63]. For biochemical analysis only 0.5 ml of cyst fluid is necessary. Amylase or lipase are used as surrogate markers for the communication between PCL and pancreatic duct system. In a recent study, however, cyst fluid amylase was significantly higher in pseudocysts compared with MCN and IPMN but did not differ between IPMN and MCN [64]. CEA is a valid marker for mucinous pancreatic cysts but does not correlate with malignancy [65]. A high CEA concentration of cyst fluid was measured not only in MCN and IPMN but also in LEC and MNC. An international multicenter study figured out the optimal cut-off value of 192 ng/ml for the differentiation between mucinous and non-mucinous cysts (sensitivity 73%; specificity 84%; accuracy 79%) [66]. However, recent single-center studies reported cut-off values of 50, 67, and 110 ng/ml (accuracy 85, 84, and 86%, respectively) [64, 65, 67]. A recent meta-analysis including 18 studies with 1,438 patients revealed a pooled sensitivity and specificity of cyst fluid CEA levels of 63 and 88%, respectively [68]. CEA level in cyst fluid depends on the epithelial differentiation of IPMN. Recently, it was shown to be highest in the gastric subtype, followed by the pancreatobiliary and the intestinal subtype. CEA level in IPMN of the oncocytic subtype was found to be as comparably low as in non-mucinous cysts [69]. Preliminary data suggest that the CA 125 level may be helpful to differentiate between MCN (high) and IPMN (low) [67]. The incremental value of molecular (DNA) over biochemical analysis of cyst fluid is low. For most parameters (DNA content, mutations of kRAS and GNAS, loss of heterozygosity mutations), specificity is high, while sensitivity is reported to be only between 20 and 50%. The combination of molecular analysis and CEA or cytology results in a higher diagnostic performance for the diagnosis of neoplastic mucinous PCL than either of the individual tests [70, 71, 72]. The value of cytology is limited by the low cellularity of PCL. Specificity of diagnosis of a malignant PCL is sufficient but sensitivity is very low (in meta-analyses: 88–93% and 54–65%, respectively) [68, 73, 74]. High-grade atypia should be included in the definition of ‘positive cytology’ for a high risk of malignancy [75, 76]. Mucin expression has a limited accuracy for the diagnosis of mucinous neoplasia [77]. The mucin expression profile may be used to differentiate between the various epithelial subtypes of IPMN as well as for risk assessment (table 3). MUC5AC is expressed in MCN and in all epithelial subtypes of IPMN, and therefore may be an additional marker to distinguish MCN from non-mucinous PCL [78]. MUC6 is expressed in gastric- and oncocytic-subtype IPMN, whereas MUC2 and CDX2 are typical markers of intestinal-subtype IPMN. MUC1 has been detected nearly exclusively in IPMNs with an invasive component [79, 80, 81]. One study revealed MUC2 to be elevated in 75% of MD-IPMNs, in 36% of mixed-type IPMNs, but in no single BD-IPMN. In mixed-type IPMNs, expression of MUC2 had a high predictive value for high-grade dysplasia and invasive cancer [82].
Table 3.
Typical results of cyst fluid analysis of PCL (modified from [33]; adata from [69], bdata from [79, 80, 81], cperiodic acid-Schiff stains for detection of glycogen and mucin)
| Pseudocyst | SPN | SCA | MCN | BD-IPMN | MD-IPMN | |
|---|---|---|---|---|---|---|
| Gross appearance | non-viscous, muddy brown | non-viscous, old-bloody | non-viscous, water-clear, sometimes bloody | variably viscous, water-clear | variably viscous, water-clear | variably viscous, water-clear |
| Pancreatic enzymes | high | no data | low | low | variably high | variably high |
| CEA | low, <5 ng/ml | no data | low, <5 ng/ml | high | high, depending on histological subtype (high in gastric and pancreatobiliary subtype)a, no marker of malignancy! | |
| DNA | KRAS mutation absent | KRAS mutation highly specific | ||||
| Epithelium | no, amorphic yellow material | yes, branching papillae with myxoid stroma | only in 20–25%, glycogen-rich, cuboid, non-mucinous | yes, mucin-containing (PAS-positive)c columnar cells, variable atypia | ||
| Mucin phenotypeb | MUC5AC+; gastric differentiation: MUC6+, MUC5AC+; intestinal differentiation: CDX2+, MUC2+, MUC5AC+ | pancreatobiliary differentiation: MUC1+, MUC5AC+; oncocytic differentiation: MUC6+, MUC5AC+ | ||||
| MUC1 expression in MCN and IPMN is a marker of invasive growth. | ||||||
| Blood cells | histiocytes, leukocytes, erythrocytes | erythrocytes | hemosiderin-filled macrophages | rarely | rarely | rarely |
Reliability of Preoperative Diagnosis
Despite the diversity of high-resolution imaging tools and sophisticated cyst fluid markers, the diagnosis and risk assessment of PCL remains difficult. Even in tertiary referral centers with unquestionable experience, up to one-third of preoperative diagnoses of PCL proved to be incorrect [83, 84]. In one retrospective analysis of 136 patients with incidentally detected PCL which were operated on at a high-volume center due to preoperative diagnosis of mucinous PCL, 5% of resected cysts turned out not to be neoplastic. Even more worrying: when preoperative diagnosis was BD-IPMN or MCN, diagnosis failed in 40%. As many as 20% of presumed BD-IPMNs turned out to have main-duct involvement (‘mixed type’) and, therefore, carry a much higher risk of malignancy [83]. Accordingly, data from a German high-volume center showed histological main-duct extension in 67 out of 233 suspected BD-IPMNs (29%), which was not evident in preoperative imaging [85].
Interobserver agreement is disappointing in assessing morphological features, establishing a diagnosis, and estimating the risk of malignancy of PCL for MRI [86, 87] as well as for EUS [88, 89]. There is also substantial interobserver variability for the grading of cellular atypia in pancreatic cyst fluid [90, 91].
Risk Assessment
Several clinical, morphological, biochemical, and cytological criteria defining a high risk of malignancy of PCL have been evaluated (table 4). Combinations of different predictors in several studies have been shown to increase the accuracy of predicting malignancy [70, 77, 92]. However, the relative weight of predictive factors differs. The results of two meta-analyses of imaging features predicting the risk of malignancy of IPMN were not congruent [93, 94]. One meta-analysis included data from 41 studies on cyst features of both types of IPMN (MD and BD type). A cyst size > 30 mm was found to be most predictive of malignancy (odds ratio (OR) 62.4), followed by the presence of mural nodules (OR 7.3) and MD versus BD type (OR 4.7) [93]. The second meta-analysis focused on BD-IPMN and included 23 studies. Presence of mural nodules was the most important predictor of malignancy of BD-IPMN (OR 6.0), followed by dilatation of the main pancreatic duct (OR 3.4), thick septum/wall (OR 3.3), and cyst size > 30 mm (OR 2.3) [94]. For MCN, the absence of mural nodules and a cyst size < 40 mm are associated with no malignancy [95, 96, 97].
Table 4.
Predictors of malignancy of mucinous neoplastic cysts (data from [60, 68, 73, 74, 75, 80, 82, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117])
| Predictors of malignancy | |
|---|---|
| Epidemiological data | older age, male gender |
| Clinical data | symptoms, in particular jaundice and weight loss, past history of cancer |
| Laboratory findings | elevation of serum CA 19–9, serum MUC5AC, and serum pancreatic enzymes |
| Morphological features | solid components, mural nodules/protrusion (yes/no; height; morphological type), size ≥ 30 mm (BD-IPMN), size ≥ 60 mm (MCN), significant increase of size in follow-up, main duct dilatation (>5 mm), significant increase of main duct diameter in follow-up, typical features of MD-IPMN, fish mouth papilla, thick wall, thick septae, localization within the pancreatic head |
| Cyst fluid markers | MUC1, MUC2, interleukin-1 beta, mAb Das-1 |
| Cytology | criteria of malignancy and of high-grade epithelial atypia |
An international consensus guideline from 2006 (updated in 2012) recommended criteria (‘Sendai criteria’) as well as a diagnostic algorithm for the management of mucinous neoplastic cysts of the pancreas. Clinical and morphological features were defined to be ‘worrisome features’ or ‘high-risk stigmata’ (table 5) [115, 116].
Table 5.
Sendai criteria of the international consensus guidelines 2012 for the management of IPMN and MCN of the pancreas: worrisome features and high-risk stigmata [116]
| Worrisome features | |
| Clinical | pancreatitis |
| Imaging | cyst ≥ 30 mm; thickened/enhancing cyst walls; main duct size 5–9 mm; non-enhancing mural nodule; abrupt change in caliber of pancreatic duct with distal pancreatic atrophy |
| High-risk stigmata | |
| Clinical | obstructive jaundice in a patient with cystic lesions of the head of the pancreas |
| Imaging | enhancing solid component within cyst; main pancreatic duct > 10 mm in size |
| EUS | definite mural nodule; main duct features suspicious for involvement (thickened wall, intraductal mucin, mural nodules) |
| EUS-FNA | cytology suspicious or positive for malignancy |
The guideline recommends surgical treatment for all surgically fit patients with MD-IPMN and MCN. For patients with BD-IPMN, surgical treatment should be performed in the case of high-risk stigmata and should also be considered in patients without high-risk stigmata and a cyst size > 30 mm. Surveillance (cross-sectional imaging in lesions < 20 mm, EUS or MRI in lesions ≥ 20 mm) is proposed for BD-IPMN without high-risk stigmata, with the time interval depending on the size of the lesion and its growth [116]. Several cohort studies have been performed and initiated to evaluate the safety of these recommendations. Based on the guideline, surgery is indicated in less than 20% of BD-IPMNs [48]. Most published studies identified these recommendations to be reasonable and safe or suggested a more liberal management in the case of BD-IPMNs with small mural nodules [17, 60, 101, 109, 118, 119, 120]. One study demonstrated that absence of worrisome features, high-risk stigmata, and high-grade atypia or malignancy in EUS-FNA provided a predictive value of 99% for a safe non-surgical management [121]. Moreover, a management strategy based on a risk stratification using EUS-FNA and cyst fluid analysis proved to be most cost-effective in comparison with a conservative follow-up strategy or an aggressive surgical approach [122]. EUS proved to be the most effective method for the surveillance of BD-IPMNs and allows for an early detection of the majority of IPMN-derived or concomitantly developing pancreatic adenocarcinomas [22]. Nonetheless, weighting of the international consensus guideline criteria for the prediction of malignancy does not seem to be adequate. There is no stepwise increase in the rate of malignant or invasive IPMNs with the number of worrisome features [112]. Further research will be necessary to improve the risk stratification of the international consensus guidelines.
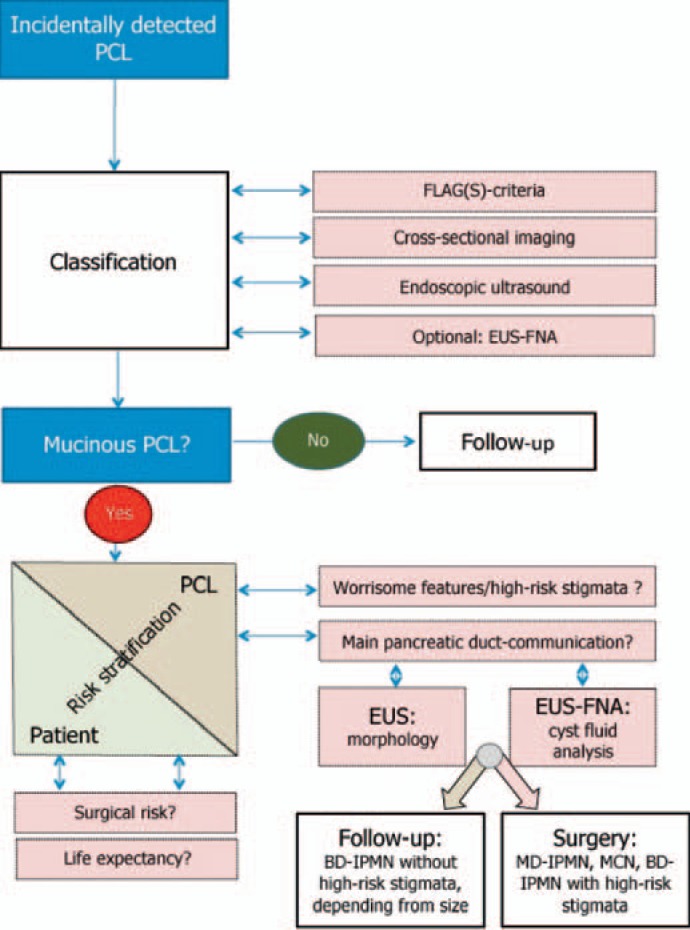
Conclusion and Proposal of a Diagnostic Algorithm
Despite the improvements of cross-sectional imaging and EUS(-FNA), differential diagnosis, risk stratification, and clinical management of PCL remain challenging. Morphological criteria and cyst fluid analysis are not sufficient in some cases to establish a definitive diagnosis and to differentiate between benign and (pre-) malignant PCL. Interobserver variability of imaging findings and cytology is considerably high. Therefore, the standardization of criteria for diagnosis and risk stratification of incidental PCL, training of examiners, and multidisciplinary management decisions is necessary. Diagnostic management should follow a multi-step algorithm. Diagnosis results from a synthesis of the patients’ history, clinical data, as well as findings of TUS, cross-sectional imaging, EUS, and EUS-FNA. EUS plays a pivotal role in the diagnosis and surveillance of patients with incidentally detected PCL (fig. 6).
Fig. 6.
Clinical algorithm for the diagnosis and treatment of incidentally detected PCL (modified from [34]).
Disclosure Statement
The authors have no conflict of interest do declare.
References
- 1.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 2.Kimura W. How many millimeters do atypical epithelia of the pancreas spread intraductally before beginning to infiltrate? Hepatogastroenterology. 2003;50:2218–2224. [PubMed] [Google Scholar]
- 3.Ishikawa T, Takeda K, Itoh M, et al. Prevalence of pancreatic cystic lesions including intraductal papillary mucinous neoplasms in patients with end-stage renal disease on hemodialysis. Pancreas. 2009;38:175–179. doi: 10.1097/MPA.0b013e31818786c9. [DOI] [PubMed] [Google Scholar]
- 4.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 6.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology. 2015;274:161–169. doi: 10.1148/radiol.14140796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–438. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 9.Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, Kloppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 10.Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007;20(suppl 1):S71–93. doi: 10.1038/modpathol.3800706. [DOI] [PubMed] [Google Scholar]
- 11.Distler M, Kersting S, Niedergethmann M, et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324–330. doi: 10.1097/SLA.0b013e318287ab73. [DOI] [PubMed] [Google Scholar]
- 12.Kloppel G, Basturk O, Schlitter AM, Konukiewitz B, Esposito I. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–466. doi: 10.1053/j.semdp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712–1720. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK, Kim YT, Yoon YB. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: comparison with pancreatic ductal adenocarcinoma. Pancreas. 2008;36:50–55. doi: 10.1097/MPA.0b013e31812575df. [DOI] [PubMed] [Google Scholar]
- 15.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–722. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ideno N, Ohtsuka T, Kono H, et al. Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg. 2013;258:141–151. doi: 10.1097/SLA.0b013e31828cd008. [DOI] [PubMed] [Google Scholar]
- 17.Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–1565. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 18.Tanno S, Nakano Y, Sugiyama Y, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology. 2010;10:173–178. doi: 10.1159/000231982. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–580. doi: 10.1097/MPA.0b013e318215010c. [DOI] [PubMed] [Google Scholar]
- 20.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256:313–320. doi: 10.1097/SLA.0b013e31825d355f. [DOI] [PubMed] [Google Scholar]
- 21.Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol. 2013;108:1546–1550. doi: 10.1038/ajg.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22–29. doi: 10.1055/s-0033-1353603. [DOI] [PubMed] [Google Scholar]
- 23.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265–1270. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M, Fernández-del Castillo C. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol. 2011;197:W53–61. doi: 10.2214/AJR.10.5866. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Kim MJ, Choi JY, Hong HS, Kim KA. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol. 2011;66:315–321. doi: 10.1016/j.crad.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Sainani NI, Saokar A, Deshpande V, Fernandez-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–731. doi: 10.2214/AJR.08.1253. [DOI] [PubMed] [Google Scholar]
- 27.Waters JA, Schmidt CM, Pinchot JW, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 28.Yu MH, Lee JY, Kim JH, Han JK, Choi BI. Value of near-isovoxel ultrasound for evaluation of ductal communications with pancreatic cystic lesions: correlation with magnetic resonance cholangiopancreatography. Ultrasound Med Biol. 2013;39:2279–2284. doi: 10.1016/j.ultrasmedbio.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Beyer-Enke SA, Hocke M, Ignee A, Braden B, Dietrich CF. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP. 2010;11:427–433. [PubMed] [Google Scholar]
- 30.D'Onofrio M, Barbi E, Dietrich CF, et al. Pancreatic multicenter ultrasound study (PAMUS) Eur J Radiol. 2012;81:630–638. doi: 10.1016/j.ejrad.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 31.D'Onofrio M, Megibow AJ, Faccioli N, Malago R, Capelli P, Falconi M, Mucelli RP. Comparison of contrast-enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR Am J Roentgenol. 2007;189:1435–1442. doi: 10.2214/AJR.07.2032. [DOI] [PubMed] [Google Scholar]
- 32.Barresi L, Tarantino I, Granata A, Curcio G, Traina M. Pancreatic cystic lesions: how endoscopic ultrasound morphology and endoscopic ultrasound fine needle aspiration help unlock the diagnostic puzzle. World J Gastrointest Endosc. 2012;4:247–259. doi: 10.4253/wjge.v4.i6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenssen C, Möller K. Schwierige endosonographische Differenzialdiagnosen am Pankreas – zystische Läsionen. Endo heute. 2010;23:253–266. [Google Scholar]
- 34.Schachschal G, Jenssen C. Stuttgart: Thieme; 2014. Pankreas: zystische Läsionen; in Jenssen C, Gottschalk U, Schachschal G Dietrich CF (eds): Kursbuch Endosonografie; pp. 223–240. [Google Scholar]
- 35.Kim YC, Choi JY, Chung YE, Bang S, Kim MJ, Park MS, Kim KW. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR Am J Roentgenol. 2010;195:947–952. doi: 10.2214/AJR.09.3985. [DOI] [PubMed] [Google Scholar]
- 36.Adimoolam V, Sanchez MJ, Siddiqui UD, Yu S, Dzuira JD, Padda MS, Aslanian HR. Endoscopic ultrasound identifies synchronous pancreas cystic lesions not seen on initial cross-sectional imaging. Pancreas. 2011;40:1070–1072. doi: 10.1097/MPA.0b013e31821f65e3. [DOI] [PubMed] [Google Scholar]
- 37.Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717–721. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 38.Hong SK, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–782. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Rogart JN, Loren DE, Singu BS, Kowalski TE. Cyst wall puncture and aspiration during EUS-guided fine needle aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. J Clin Gastroenterol. 2011;45:164–169. doi: 10.1097/MCG.0b013e3181eed6d2. [DOI] [PubMed] [Google Scholar]
- 40.Beane JD, House MG, Cote GA, et al. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844–853. doi: 10.1016/j.surg.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 41.Jenssen C, Alvarez-Sanchez MV, Napoleon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659–4676. doi: 10.3748/wjg.v18.i34.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarantino I, Fabbri C, Di Mitri R, et al. Complications of endoscopic ultrasound fine needle aspiration on pancreatic cystic lesions: final results from a large prospective multicenter study. Dig Liver Dis. 2014;46:41–44. doi: 10.1016/j.dld.2013.08.134. [DOI] [PubMed] [Google Scholar]
- 43.Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 44.Yoon WJ, Daglilar ES, Fernandez-del Castillo C, Mino-Kenudson M, Pitman MB, Brugge WR. Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE Study. Endoscopy. 2014;46:382–387. doi: 10.1055/s-0034-1364937. [DOI] [PubMed] [Google Scholar]
- 45.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 46.Salvia R, Partelli S, Crippa S, et al. Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg. 2009;198:709–714. doi: 10.1016/j.amjsurg.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–433; discussion 433–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hata T, Sakata N, Okada T, et al. Dilated papilla with mucin extrusion is a potential predictor of acute pancreatitis associated with intraductal papillary mucinous neoplasms of pancreas. Pancreatology. 2013;13:615–620. doi: 10.1016/j.pan.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Jang JW, Kim MH, Jeong SU, et al. Clinical characteristics of intraductal papillary mucinous neoplasm manifesting as acute pancreatitis or acute recurrent pancreatitis. J Gastroenterol Hepatol. 2013;28:731–738. doi: 10.1111/jgh.12121. [DOI] [PubMed] [Google Scholar]
- 51.Pelletier AL, Hammel P, Rebours V, et al. Acute pancreatitis in patients operated on for intraductal papillary mucinous neoplasms of the pancreas: frequency, severity, and clinicopathologic correlations. Pancreas. 2010;39:658–661. doi: 10.1097/MPA.0b013e3181c81b74. [DOI] [PubMed] [Google Scholar]
- 52.Ringold DA, Shroff P, Sikka SK, et al. Pancreatitis is frequent among patients with side-branch intraductal papillary mucinous neoplasia diagnosed by EUS. Gastrointest Endosc. 2009;70:488–494. doi: 10.1016/j.gie.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 53.Manfredi R, Ventriglia A, Mantovani W, et al. Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol. 2014 doi: 10.1007/s00330-014-3493-2. DOI: 10.1007/s00330-014-3493-2. [DOI] [PubMed] [Google Scholar]
- 54.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 55.Aso T, Ohtsuka T, Ideno N, et al. Diagnostic significance of a dilated orifice of the duodenal papilla in intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2012;76:313–320. doi: 10.1016/j.gie.2012.03.682. [DOI] [PubMed] [Google Scholar]
- 56.Murakami Y, Uemura K, Ohge H, Hayashidani Y, Sudo T, Sueda T. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery. 2006;140:448–453. doi: 10.1016/j.surg.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192–198, 198.e191–192. doi: 10.1016/j.cgh.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Hocke M, Cui XW, Domagk D, Ignee A, Dietrich CF. Pancreatic cystic lesions: the value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123–130. doi: 10.4103/2303-9027.131040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita Y, Ueda K, Itonaga M, et al. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61–68. doi: 10.7863/jum.2013.32.1.61. [DOI] [PubMed] [Google Scholar]
- 60.Ohno E, Itoh A, Kawashima H, et al. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas. 2012;41:855–862. doi: 10.1097/MPA.0b013e3182480c44. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez Obeso E, Murphy E, Brugge W, Deshpande V. Pseudocyst of the pancreas: the role of cytology and special stains for mucin. Cancer. 2009;117:101–107. doi: 10.1002/cncy.20000. [DOI] [PubMed] [Google Scholar]
- 62.Nasr J, Sanders M, Fasanella K, Khalid A, McGrath K. Lymphoepithelial cysts of the pancreas: an EUS case series. Gastrointest Endosc. 2008;68:170–173. doi: 10.1016/j.gie.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 63.Leung KK, Ross WA, Evans D, Fleming J, Lin E, Tamm EP, Lee JH. Pancreatic cystic neoplasm: the role of cyst morphology, cyst fluid analysis, and expectant management. Ann Surg Oncol. 2009;16:2818–2824. doi: 10.1245/s10434-009-0502-9. [DOI] [PubMed] [Google Scholar]
- 64.Oh HC, Kang H, Brugge WR. Cyst fluid amylase and CEA levels in the differential diagnosis of pancreatic cysts: a single-center experience with histologically proven cysts. Dig Dis Sci. 2014;59:3111–3116. doi: 10.1007/s10620-014-3254-8. [DOI] [PubMed] [Google Scholar]
- 65.Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024–1028. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- 66.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Nagashio Y, Hijioka S, Mizuno N, et al. Combination of cyst fluid CEA and CA 125 is an accurate diagnostic tool for differentiating mucinous cystic neoplasms from intraductal papillary mucinous neoplasms. Pancreatology. 2014;14:503–509. doi: 10.1016/j.pan.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 69.Yoon WJ, Daglilar ES, Mino-Kenudson M, Morales-Oyarvide V, Pitman MB, Brugge WR. Characterization of epithelial subtypes of intraductal papillary mucinous neoplasm of the pancreas with endoscopic ultrasound and cyst fluid analysis. Endoscopy. 2014;46:1071–1077. doi: 10.1055/s-0034-1377629. [DOI] [PubMed] [Google Scholar]
- 70.Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 71.Gillis A, Cipollone I, Cousins G, Conlon K. Does EUS-FNA molecular analysis carry additional value when compared to cytology in the diagnosis of pancreatic cystic neoplasm? A systematic review. HPB (Oxford) 2014 doi: 10.1111/hpb.12364. DOI: 10.1111/hpb.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381–4389. doi: 10.1158/1078-0432.CCR-14-0513. [DOI] [PubMed] [Google Scholar]
- 73.Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–2766. doi: 10.1007/s10620-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki R, Thosani N, Annangi S, Guha S, Bhutani MS. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: a systematic review and meta-analysis. Pancreatology. 2014;14:380–384. doi: 10.1016/j.pan.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Pitman MB, Michaels PJ, Deshpande V, Brugge WR, Bounds BC. Cytological and cyst fluid analysis of small (≤3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology. 2008;8:277–284. doi: 10.1159/000134276. [DOI] [PubMed] [Google Scholar]
- 76.Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino-Kenudson M. Cytological criteria of high-grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathol. 2014;122:40–47. doi: 10.1002/cncy.21344. [DOI] [PubMed] [Google Scholar]
- 77.Morris-Stiff G, Lentz G, Chalikonda S, Johnson M, Biscotti C, Stevens T, Matthew Walsh R. Pancreatic cyst aspiration analysis for cystic neoplasms: mucin or carcinoembryonic antigen–which is better? Surgery. 2010;148:638–644. doi: 10.1016/j.surg.2010.07.023. discussion 644-635. [DOI] [PubMed] [Google Scholar]
- 78.Haab BB, Porter A, Yue T, Li L, Scheiman J, Anderson MA, Barnes D, et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann Surg. 2010;251:937–945. doi: 10.1097/SLA.0b013e3181d7738d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Distler M, Aust D, Weitz J, Pilarsky C, Grutzmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int. 2014;2014:474905. doi: 10.1155/2014/474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji Y, Lou WH, Jin DY, Kuang TT, Zeng MS, Tan YS, Zeng HY, et al. A series of 64 cases of pancreatic cystic neoplasia from an institutional study of China. World J Gastroenterol. 2006;12:7380–7387. doi: 10.3748/wjg.v12.i45.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maker AV, Katabi N, Gonen M, DeMatteo RP, D'Angelica MI, Fong Y, Jarnagin WR, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masuda A, Arisaka Y, Hara S, Matsumoto I, Takenaka M, Sakai A, Shiomi H, et al. MUC2 expression and prevalence of high-grade dysplasia and invasive carcinoma in mixed-type intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2013;13:583–588. doi: 10.1016/j.pan.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 83.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernandez-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144–150. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G, Winslow ER, Weber SM. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112–3119. doi: 10.1245/s10434-013-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fritz S, Klauss M, Bergmann F, Strobel O, Schneider L, Werner J, Hackert T, et al. Pancreatic Main-Duct Involvement in Branch-Duct IPMNs: An Underestimated Risk. Ann Surg. 2014;260:848–856. doi: 10.1097/SLA.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 86.de Jong K, van Hooft JE, Nio CY, Gouma DJ, Dijkgraaf MG, Bruno MJ, Fockens P. Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions. Scand J Gastroenterol. 2012;47:1056–1063. doi: 10.3109/00365521.2012.674970. [DOI] [PubMed] [Google Scholar]
- 87.de Jong K, Nio CY, Mearadji B, Phoa SS, Engelbrecht MR, Dijkgraaf MG, Bruno MJ, et al. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas. 2012;41:278–282. doi: 10.1097/MPA.0b013e31822899b6. [DOI] [PubMed] [Google Scholar]
- 88.Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, Kimmey MB, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 89.de Jong K, Verlaan T, Dijkgraaf MG, Poley JW, van Dullemen H, Bruno MJ, Fockens P. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy. 2011;43:579–584. doi: 10.1055/s-0030-1256434. [DOI] [PubMed] [Google Scholar]
- 90.Pitman MB, Centeno BA, Genevay M, Fonseca R, Mino-Kenudson M. Grading epithelial atypia in endoscopic ultrasound-guided fine-needle aspiration of intraductal papillary mucinous neoplasms: an international interobserver concordance study. Cancer Cytopathol. 2013;121:729–736. doi: 10.1002/cncy.21334. [DOI] [PubMed] [Google Scholar]
- 91.Sigel CS, Edelweiss M, Tong LC, Magda J, Oen H, Sigel KM, Zakowski MF. Low interobserver agreement in cytology grading of mucinous pancreatic neoplasms. Cancer Cytopathol. 2014 doi: 10.1002/cncy.21492. DOI: 10.1002/ cncy.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977–983. doi: 10.1097/SLA.0b013e3182383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:913–921; quiz e59–60. doi: 10.1016/j.cgh.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg. 2014;259:72–81. doi: 10.1097/SLA.0b013e31829385f7. [DOI] [PubMed] [Google Scholar]
- 95.Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131–136. doi: 10.1016/j.pan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le Baleur Y, Couvelard A, Vullierme MP, et al. Mucinous cystic neoplasms of the pancreas: definition of preoperative imaging criteria for high-risk lesions. Pancreatology. 2011;11:495–499. doi: 10.1159/000332041. [DOI] [PubMed] [Google Scholar]
- 98.Arlix A, Bournet B, Otal P, et al. Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41:295–301. doi: 10.1097/MPA.0b013e3182285cc8. [DOI] [PubMed] [Google Scholar]
- 99.Buscaglia JM, Giday SA, Kantsevoy SV, et al. Patient-and cyst-related factors for improved prediction of malignancy within cystic lesions of the pancreas. Pancreatology. 2009;9:631–638. doi: 10.1159/000181173. [DOI] [PubMed] [Google Scholar]
- 100.Das KK, Xiao H, Geng X, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN) Gut. 2014;63:1626–1634. doi: 10.1136/gutjnl-2013-306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kato Y, Takahashi S, Gotohda N, Konishi M. Risk factors for malignancy in branched-type intraductal papillary mucinous neoplasms of the pancreas during the follow-up period. World J Surg. 2015;39:244–250. doi: 10.1007/s00268-014-2789-3. [DOI] [PubMed] [Google Scholar]
- 102.Jang JY, Park T, Lee S, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014;101:686–692. doi: 10.1002/bjs.9491. [DOI] [PubMed] [Google Scholar]
- 103.Kim YI, Woo SM, Lee WJ, et al. Appropriate indications of initial endoscopic ultrasound evaluation for detecting mural nodules in branch duct intraductal papillary mucinous neoplasms of the pancreas. Scand J Gastroenterol. 2013;48:610–616. doi: 10.3109/00365521.2013.782065. [DOI] [PubMed] [Google Scholar]
- 104.Kobayashi N, Sugimori K, Shimamura T, et al. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:141–145. doi: 10.1016/j.pan.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Kobayashi G, Fujita N, Maguchi H, et al. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: a Japan Pancreas Society multicenter study. Pancreas. 2014;43:532–538. doi: 10.1097/MPA.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koshita S, Fujita N, Noda Y, et al. Invasive carcinoma derived from ‘flat type’ branch duct intraductal papillary mucinous neoplasms of the pancreas: impact of classification according to the height of mural nodule on endoscopic ultrasonography. J Hepatobiliary Pancreat Sci. 2014 doi: 10.1002/jhbp.199. DOI: 10.1002/jhbp.199. [DOI] [PubMed] [Google Scholar]
- 107.Lee KH, Lee SJ, Lee JK, et al. Prediction of malignancy with endoscopic ultrasonography in patients with branch duct-type intraductal papillary mucinous neoplasm. Pancreas. 2014;43:1306–1311. doi: 10.1097/MPA.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 108.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–1508. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagai K, Doi R, Ito T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16:353–358. doi: 10.1007/s00534-009-0068-8. [DOI] [PubMed] [Google Scholar]
- 110.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–634. doi: 10.1097/SLA.0b013e3181a189a8. [DOI] [PubMed] [Google Scholar]
- 111.Roch AM, Parikh JA, Al-Haddad MA, et al. Abnormal serum pancreatic enzymes, but not pancreatitis, are associated with an increased risk of malignancy in patients with intraductal papillary mucinous neoplasms. Surgery. 2014;156:923–929. doi: 10.1016/j.surg.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 112.Roch AM, Ceppa EP, DeWitt JM, Al-Haddad MA, House MG, Nakeeb A, Schmidt CM. International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB (Oxford) 2014;16:929–935. doi: 10.1111/hpb.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010;39:232–236. doi: 10.1097/MPA.0b013e3181bab60e. [DOI] [PubMed] [Google Scholar]
- 114.Takuma K, Kamisawa T, Anjiki H, et al. Predictors of malignancy and natural history of main-duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2011;40:371–375. doi: 10.1097/MPA.0b013e3182056a83. [DOI] [PubMed] [Google Scholar]
- 115.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 116.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Correa-Gallego C, Do R, Lafemina J, et al. Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: development of a preoperative nomogram. Ann Surg Oncol. 2013;20:4348–4355. doi: 10.1245/s10434-013-3207-z. [DOI] [PubMed] [Google Scholar]
- 118.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas ≤3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 119.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 120.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 121.Wu RI, Yoon WJ, Brugge WR, Mino-Kenudson M, Pitman MB. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) contributes to a triple-negative test in preoperative screening of pancreatic cysts. Cancer Cytopathol. 2014;122:412–419. doi: 10.1002/cncy.21385. [DOI] [PubMed] [Google Scholar]
- 122.Das A, Ngamruengphong S, Nagendra S, Chak A. Asymptomatic pancreatic cystic neoplasm: a cost-effectiveness analysis of different strategies of management. Gastrointest Endosc. 2009;70:690–699.e6. doi: 10.1016/j.gie.2009.02.013. [DOI] [PubMed] [Google Scholar]