Summary
Background
Cystic pancreatic lesions are more and more often found. Malignant risk ranges from nil to more than 60%. A precise diagnosis is required to adapt surveillance or therapeutic strategy.
Methods
We tried to identify the most difficult differential diagnoses encountered in a tertiary center of pancreatology and to guide the reader as how to reach the correct strategy and diagnosis in these situations.
Results
We identified eight clinically difficult situations: i) chronic pancreatitis versus intraductal papillary mucinous neoplasms, ii) serous versus mucinous cystic neoplasms, iii) serous cystic neoplasms versus branch-duct intraductal papillary mucinous neoplasms, iv) intraductal papillary mucinous neoplasms versus acinar cell cystadenoma, v) (pseudo-) solid serous cystic neoplasm versus neuroendocrine tumor, vi) pancreatic neuroendocrine tumors versus solid pseudopapillary tumors, vii) cystic forms of a solid tumor, and viii) rare pancreatic or peripancreatic cystic lesions. The work-up should rely on computed tomography scan, pancreatic magnetic resonance imaging, and, only if necessary, endoscopic ultrasound with or without fine needle aspiration.
Conclusion
An expert analysis of imaging data allows a precise diagnosis in most of the cases. Pancreatic resection should no longer be performed in case of diagnostic doubt.
Keywords: Serous cystic neoplasm, Mucinous cystic neoplasm, Intraductal papillary mucinous neoplasm, Acinar cell cystadenoma, Chronic pancreatitis, Pseudocyst, Solid pseudopapillary tumors, Neuroendocrine tumors, Pancreas
Introduction
Cystic pancreatic lesions are more and more frequently found due to the large use of imaging procedures during systematic checkup, workup of abdominal pain, or follow-up of a patient with a non-pancreatic malignancy.
The frequency of cystic pancreatic lesions was 20% in 1,444 American people who had a systematic magnetic resonance imaging (MRI). In 80% of these cases, the lesion was smaller than 10 mm. The frequency of cysts as well as multiple cysts increased with age [1]. In a French series of MRI performed for a non-pancreatic reason, the frequency of branched cystic lesions was 7% (Laurent et al., unpublished data). In a post-mortem series, Kimura et al. [2] found cystic pancreatic lesions in 25% of the ‘patients’.
Therefore, cystic pancreatic lesions pose a public health problem, and differential diagnosis is essential to adapt the workup procedure, the follow-up, and, eventually, the decision for resection or surveillance.
Rather than writing an ‘n + 1’ catalog on cystic pancreatic tumors, we will try to describe clinical situations in which differential diagnosis is difficult according to an intuitive order of decreasing frequency.
How Should Imaging Workup Be Performed?
The decision tree is essential since it has consequences of paramount importance. Three choices are in our hands:
– to perform a released follow-up (one visit every 2–3 years),
– to perform a more tightened follow-up (every 6 or 12 months)
– to propose a pancreatectomy whose short- and long-term morbidity rate is high and which is associated with a non-negligible risk of perioperative mortality, whatever the quality of the surgical team.
Two pitfalls have to be avoided, i.e. i) to resect all lesions including those with very low (if any) risk of malignancy or of new onset symptoms or complication; ii) to leave in place a lesion with a high malignant potential.
Three main imaging procedures are easily available and required for a complete reasoning. Computed tomography (CT) scan is the most accurate means for detection of pancreatic calcifications, vascular cartography, pancreatic parenchyma study, and analysis of iodine contrast agent intake. In order to be useful, the technical parameters of the CT scan should include three helical series (without iodine contrast, with iodine contrast at arterial and portal phases) and slices with millimetric width.
Pancreato-MRI offers an excellent visualization of the pancreatic ductal system without an invasive procedure. Thick, heavily T2-weighted slices centered on the main pancreatic duct are necessary. Whether secretin injection increases the performance in this field needs to be proven. Resolution for pancreatic parenchyma is inferior to that of the CT scan. Diffusion-weighted MRI appears as a very promising technique to detect malignant parts of a cystic lesion [3].
Endoscopic ultrasound (EUS) is the best imaging procedure although invasive with general anesthesia. If doubt persists (and only in this case), it offers the possibility of performing fine needle aspiration (FNA) for cytology, measurement of tumor markers, and pancreatic enzymes in cyst fluid [4, 5, 6, 7]. EUS offers the best resolution and allows a precise examination of mural nodules. Contrast EUS may add to the diagnostic performance in this field [8].
Other imaging techniques such as positron emission tomography scan and Octreoscan® (Covidien Imaging, Élancourt, France) could help in specific situations, while some other techniques are under development (i.e. confocal microendoscopy) [9].
The Most Common Pancreatic Cystic Lesion Is the Pseudocyst!
This simple adage has to be kept in mind since pseudocysts represent approximatively 90% of all cystic pancreatic lesions. A second important adage is nearly always true: ‘There is no pseudocyst without pancreatitis’. In other words, if the patient has no personal history of previous acute (usually severe) or chronic pancreatitis, the diagnosis of pseudocyst should be challenged. The clinical context (i.e. heavy and long-term alcoholism, abdominal traumatism, family history of pancreatitis, etc.) may help in this regard.
The diagnostic difficulty increases when both acute or chronic pancreatitis and cystic well-organized lesion are discovered during the first imaging procedure. In these cases, the diagnostic alternative is a cystic tumor which is the cause rather than the consequence of pancreatitis.
Chronic Pancreatitis versus Intraductal Papillary Mucinous Neoplasms
The first step is the clinical and epidemiological context. Patients with intraductal papillary mucinous neoplasms (IPMNs) are often female (53 vs. 15%), older (63 vs. 43 years), and drink (19 vs. 107 g/day) and smoke less (8 vs. 21 cigarettes/day) than those with chronic pancreatitis [10].
This differential diagnosis remains difficult for several reasons:
– IPMN might be the cause of recurrent pancreatitis.
– Pancreatic calcifications, a paramount sign of chronic pancreatitis, might be present in patients with IPMN due to long-standing chronic obstructive pancreatitis [11, 12].
– Pancreatic lesions involve both main and branch duct in both diseases.
– Histological correlations are not always available to validate imaging criteria, which help in terms of differentiation.
The most specific signs in favor of IPMN diagnosis are:
– duct dilatation without stricture;
– bulging ampulla (figs. 1, 2);
Fig. 1.

Thick-slice, T2-weighted MRI showing a dilated main pancreatic duct with smooth aspect of main pancreatic duct wall and bulging ampulla (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 2.

Axial CT scan of the same patient showing a dilated main pancreatic duct with smooth aspect of main pancreatic duct wall and bulging ampulla (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
– nodule in a duct;
– grape-like cyst shape (fig. 3);
Fig. 3.
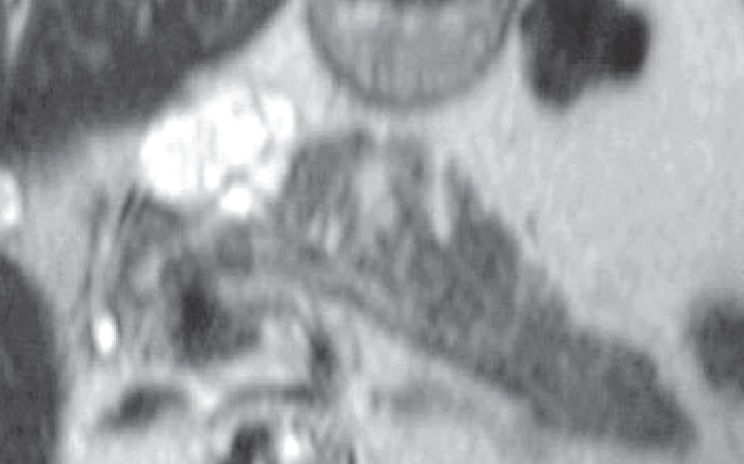
Axial, T2-weighted MRI showing a grape-like cyst of the pancreatic head (image courtesy of PR Hammel, Hôpital Beaujon).
– nodule in a cyst [13].
Furthermore, we add smooth appearance of the main pancreatic duct walls as well as branch ducts with a round shape (sparkshaped in chronic pancreatitis) to those criteria.
Serous versus Mucinous Cystic Neoplasms
Both tumors are incidentally discovered in the vast majority of cases. Related symptoms are exceptional. There is no communication with the pancreatic ductal system.
Serous cystic neoplasms (SCNs) are diagnosed more frequently in women (75%) approximately 60 years of age. Size ranges between some millimeters to >20 cm. SCNs are evenly located inside the gland. Extrapancreatic lesions and family history usually lead to the diagnosis.
In most of the cases, SCN diagnosis is easy if multiple microcysts are seen on CT scan (honeycomb aspect) (fig. 4), MRI, or EUS. Some SCNs contain macrocysts; however, the visualization of a microcystic area is sufficient for diagnosis. Central calcifications may be present. The peripheral wall is thin (<2 mm) and nearly invisible on imaging procedures. SCN is a unique tumor except in patients with von Hippel-Lindau disease in which there are numerous SCNs, pancreatic neuroendocrine tumors, and simple cysts within the gland [14] (fig. 5).
Fig. 4.
CT scan showing four typical SCNs (red arrows) with (both left panels) or without central calcifications (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 5.

Axial, T2-weighted MRI showing huge multiple SCNs in a patient with von Hippel-Lindau disease (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
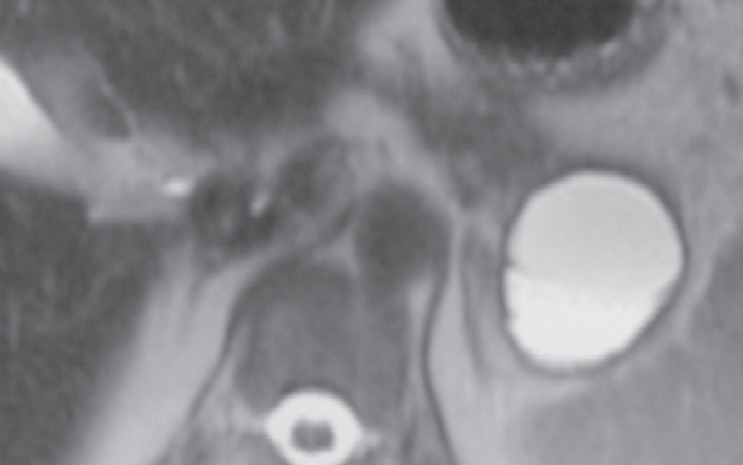
Mucinous cystic neoplasm (MCN) is always unique and is mostly encountered in women (sex ratio 20:1) with a malignant potential (20% at diagnosis). Its longitudinal natural history remains unknown. MCN is located at the posterior part of the pancreas body or tail in nearly all of the cases. The cyst wall is thicker than for SCNs (>2 mm width) and is therefore visible on all imaging procedures (figs. 6, 7). Peripheral calcifications are possible [15, 16].
Fig. 6.

CT scan showing a cystic lesion, round-shaped with a thick wall located in the tail, typical of an MCN (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 7.
Axial, T2-weighted MRI of the same patient as in fig. 6 (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
The diagnostic difficulty between SCN and MCN increases in the case of macro-unicystic SCN. In these cases, four criteria are in favor of SCN diagnosis: location in the head, polycyclic shape, thin (<2 mm) and non-enhancing peripheral wall (figs. 8, 9). If these three or four criteria are present, the specificity for SCN diagnosis is 100% [17]. Another important criterion is to show an area with microcysts inside the wall of a macrocystic lesion by using EUS [18] (fig. 10).
Fig. 8.

CT scan of a macrocystic SCN: polycyclic shape, thin and non-enhancing peripheral wall (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 9.

CT scan of a macrocystic SCN located in the pancreatic neck with thin and non-enhancing peripheral wall (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 10.
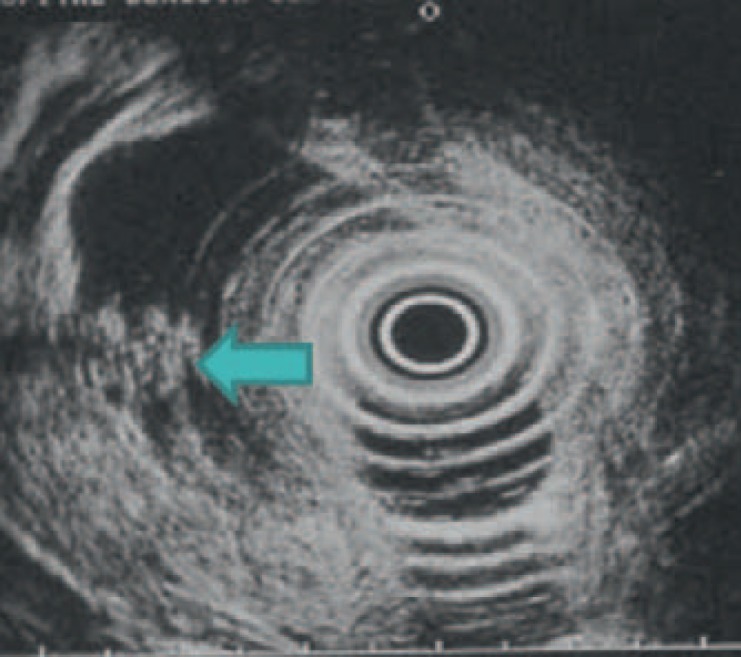
Endoscopic ultrasonography of the same patient showing a small area in which microcysts are visible (layered aspect) (arrow) (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
If doubt persists regarding a body cystic lesion, EUS and FNA may be helpful. Cytology rarely shows discriminating features; however, cyst fluid amylasis and lipase levels are low (<5,000 and <2,000, respectively). CEA is the best discriminant marker. Intracystic fluid CEA < 5 ng/ml has a sensitivity of 92% and a specificity of 87% to distinguish SCN from MCN [4, 5, 19].
However, it is important to note that:
– CEA threshold may vary in centers from 5 to more than 100 [7];
– cyst fluid analysis has a great value in distinguishing SCN from MCN (and pseudocyst) but not in the case of other cystic tumors like IPMN [20] or other, rarer lesions such as solid pseudopapillary tumors (SPPT) or cystic neuroendocrine tumors. For example, it has been shown that cyst fluid analysis is not helpful in IPMN diagnosis due to a wide range of results because of the communication with pancreatic ducts. In other words, interpretation of cyst fluid markers should be done very cautiously in other cases than suspected SCN or MCN.
In the future, needle-based confocal laser endomicroscopy inside the cyst [9] or VEGF (vascular endothelial growth factor) dosage in the cyst fluid may help regarding the diagnosis [21].
Serous Cystic Neoplasms versus Branch-Duct Intraductal Papillary Mucinous Neoplasms
Both tumors might appear as a grape-like structure. Strong arguments for IPMN are:
– the presence of several lesions along the gland (multiple SCNs are extremely rare except in von Hippel-Lindau disease);
– the presence of a tubular configuration indicating a ductal structure;
– evidence of a communication between the cyst and the ductal system. In this case, the diagnosis of IPMN should be considered first.
In order to see these aspects, an excellent pancreato-MRI with thin and thick slices centered on pancreatic ducts is required (fig. 11).
Fig. 11.

Thick-slice, T2-weighted pancreato-MRI showing a very thin communicating duct between a cystic lesion and the pancreatic duct. This communicating duct was visible only on MRI and not on CT scan (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Intraductal Papillary Mucinous Neoplasms versus Acinar Cell Cystadenoma
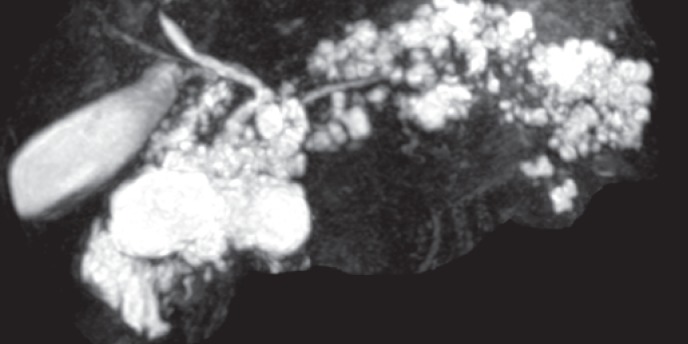
Acinar cell cystadenoma is a recently described entity. It is not clear whether it is a tumor or a congenital malformation. Less than 50 cases (more often in women) have been described so far. Incidental discovery is the most frequent circumstance although abdominal pain or acute pancreatitis are also possible. No malignant or dysplasia cases have been described yet. Cystic lesions are usually multiple, peripheral, and clustered without any visible communication with the pancreatic duct at imaging level. Hyperdense material or small calcifications might be visible (figs. 12–14). The main pancreatic duct is never involved [22, 23].
Fig. 12.
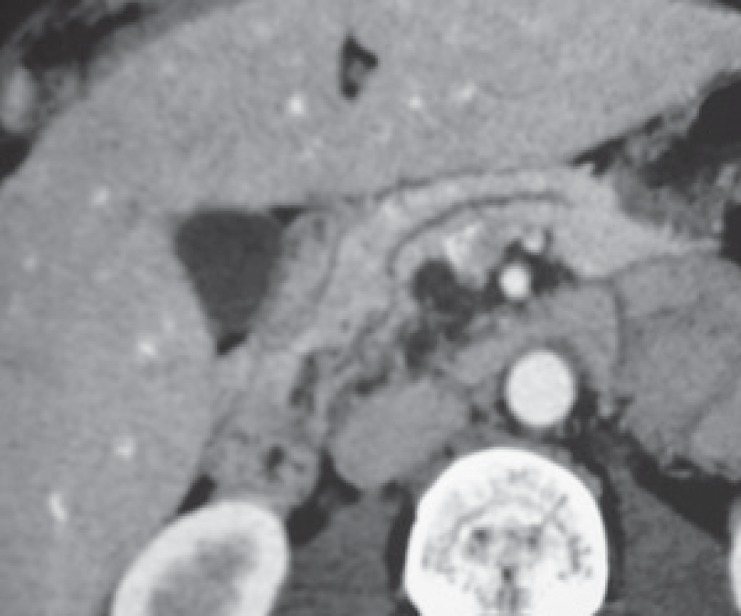
Multiplanar reconstructed CT scan showing multiple small cysts in pancreatic uncus corresponding to acinar cell cystadenoma (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 14.
Thick-slice, T2-weighted MRI of a patient with a huge acinar cell cystadenoma (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
(Pseudo-)Solid Serous Cystic Neoplasm versus Neuroendocrine Tumor
In a low percentage (5%), SCN appears as a more or less homogeneous solid tumor with a high uptake of iodine contrast in the arterial phase and is difficult to distinguish from a non-functioning, well-differentiated pancreatic neuroendocrine tumor. Octreoscan might not be helpful since some SCNs are rich in S2-somatostatin receptors; therefore, a clear uptake of radio-labelled somatostatin is possible in these cases, increasing the confusion (Pote et al., unpublished data). In these cases, two imaging techniques are available to distinguish these two tumors requiring a dramatically different therapeutic approach. In SCN, EUS shows a multilayered aspect which is completely different from a solid tumor. The simplest procedure is to use axial, T2-weighted MRI showing that the ‘solid’ tumor is in fact mainly cystic and therefore an SCN (figs. 15, 16).
Fig. 15.

CT scan showing a hyperintense pancreatic head tumor suggesting a neuroendocrine tumor (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Fig. 16.
Thick-slice, T2-weighted MRI of the same patient showing that the tumor is not solid but cystic, corresponding to a pseudosolid SCN (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Pancreatic Neuroendocrine Tumors versus Solid Pseudopapillary Tumors
SPPTs are rare tumors which are typically seen in young women (85–90%). Incidental diagnosis is most frequent but compression of regional organs is possible. The diameter ranges from 2 to more than 20 cm. It usually appears as a large tumor without biliary or pancreatic duct upstream dilatation. Its content is heterogeneous with cystic, necrotic, or hemorrhagic components and a thick peripheral capsule [24].
In small SPPTs (<3 cm), however, the imaging features might be different from those of larger ones and difficult to distinguish from pancreatic neuroendocrine tumors. The best discriminant features are:
– completely well-defined margin,
– pure solid consistency,
– low signal intensity on unenhanced T1-weighted images,
– high signal intensity on T2-weighted images, and
– early heterogeneous and slowly progressive enhancement [25].
Small SPPTs rarely have a capsule or hemorrhage. A characteristic qualitative feature of SPPTs that is significantly different from pancreatic neuroendocrine tumors is a very high signal intensity on T2-weighted images.
In contrast, endocrine tumors are homogeneous with a high intake of iodine contrast in the arterial phase. In case of doubt, Octreoscan might help in differential diagnosis. As far as possible, EUS-FNA should be avoided since there is a risk of tumor seeding especially for SPPTs [26, 27].
Cystic Forms of a Solid Tumor
All solid tumors can present as a cystic or partly cystic one. In case of pancreatic cystic adenocarcinoma, the diagnosis is usually easy when solid component, thick and irregular wall, and sign of local or distant spread are present.
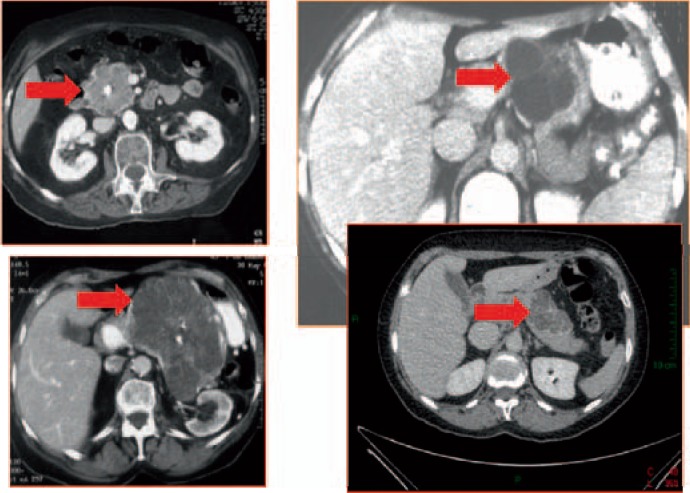
Cystic neuroendocrine tumors represent 12% of the pancreatic neuroendocrine tumors [28]. The diagnosis is not easy since nearly all are non-functioning and well-differentiated tumors. Peripheral calcifications are frequent. Major help in diagnosis is a well-delineated wall highly enhanced in the arterial phase of iodine injection (fig. 17). Chromogranin A dosage is not sensitive enough since the tumor mass is low. The same applies for nuclear imaging techniques which are rarely positive for cystic endocrine tumors. EUS-FNA gave a specific diagnosis in 71% of the cases [29]. Cyst fluid markers such as CEA are of no help in this setting and should not be used.
Fig. 17.

CT scan at arterial phase showing a cystic tumor with well-delineated wall highly enhanced at arterial phase of iodine injection (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Rare Pancreatic or Peripancreatic Cystic Lesions
Duodenal diverticulum can appear as a cystic pancreatic head lesion. However, it is always in the ampulla area (usually below), and it may contain gas bubbles (fig. 18). Oral contrast CT scan and duodenoscopy may help in the diagnosis.
Fig. 18.

CT scan showing a cystic lesion in the pancreatic head containing gas bubble corresponding to a duodenal diverticulum (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
Lymphangioma are a rare cystic lesion, mostly seen in the periportal area of liver pedicle. Most of the lesion is extrapancreatic.
Lymphoepithelial cyst is an unusual and benign cystic tumor. Accurate preoperative diagnosis is difficult. Most of the cysts are resected. The patients are primarily men (78%). The discovery is generally fortuitous. No specific radiological features are identified. FNA and cytologic analysis allow a correct preoperative diagnosis in 21% of the patients, showing presence of squamous cells, lymphocytes, and keratinous debris [30].
Conclusion
A correct differential diagnosis of cystic pancreatic tumors requires a consideration of the clinical context and an excellent radiological work-up including CT scan, pancreato-MRI and, if necessary, EUS with or without FNA. In most of the cases, an expert analysis of radiological data results in the correct diagnosis. In case of doubt, cyst fluid cytology as well as dosage of markers and enzymes are helpful. Other techniques such as nuclear imaging should only be used when necessary. New cyst fluid markers are under development.
At the beginning of the 21st century, it is no longer acceptable to propose a pancreatic resection in the case of diagnostic doubt concerning a cystic lesion.
Disclosure Statement
PL and VR have no disclosure to declare in relation with this paper.
Fig. 13.

Axial, T2-weighted MRI of the same patient. No communicating ducts are visible (image courtesy of Dr. Marie Pierre Vullierme, Hôpital Beaujon).
References
- 1.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 2.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 3.Jang KM, Kim SH, Min JH, et al. Value of diffusion-weighted MRI for differentiating malignant from benign intraductal papillary mucinous neoplasms of the pancreas. AJR Am J Roentgenol. 2014;203:992–1000. doi: 10.2214/AJR.13.11980. [DOI] [PubMed] [Google Scholar]
- 4.Hammel P, Levy P, Voitot H, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230–1235. doi: 10.1016/0016-5085(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 5.Hammel P, Voitot H, Vilgrain V, Levy P, Ruszniewski P, Bernades P. Diagnostic value of CA 72-4 and carcinoembryonic antigen determination in the fluid of pancreatic cystic lesions. Eur J Gastroenterol Hepatol. 1998;10:345–348. doi: 10.1097/00042737-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–47. doi: 10.1097/00000658-199301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 8.Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video) Gastrointest Endosc. 2011;73:52–63. doi: 10.1016/j.gie.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Napoleon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2014;47:26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 10.Talamini G, Zamboni G, Salvia R, et al. Intraductal papillary mucinous neoplasms and chronic pancreatitis. Pancreatology. 2006;6:626–634. doi: 10.1159/000097605. [DOI] [PubMed] [Google Scholar]
- 11.Campisi A, Brancatelli G, Vullierme MP, Levy P, Ruszniewski P, Vilgrain V. Are pancreatic calcifications specific for the diagnosis of chronic pancreatitis? A multidetector-row CT analysis. Clin Radiol. 2009;64:903–911. doi: 10.1016/j.crad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Zapiach M, Yadav D, Smyrk TC, et al. Calcifying obstructive pancreatitis: a study of intraductal papillary mucinous neoplasm associated with pancreatic calcification. Clin Gastroenterol Hepatol. 2004;2:57–63. doi: 10.1016/s1542-3565(03)00292-1. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Hong SS, Kim YJ, Kim JK, Eun HW. Intraductal papillary mucinous neoplasm of the pancreas: differentiate from chronic pancreatitis by MR imaging. Eur J Radiol. 2012;81:671–676. doi: 10.1016/j.ejrad.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 14.Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 15.Le Baleur Y, Couvelard A, Vullierme MP, et al. Mucinous cystic neoplasms of the pancreas: definition of preoperative imaging criteria for high-risk lesions. Pancreatology. 2011;11:495–499. doi: 10.1159/000332041. [DOI] [PubMed] [Google Scholar]
- 16.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen-Scali F, Vilgrain V, Brancatelli G, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003;228:727–733. doi: 10.1148/radiol.2283020973. [DOI] [PubMed] [Google Scholar]
- 18.Chatelain D, Hammel P, O'Toole D, et al. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol. 2002;97:2566–2571. doi: 10.1111/j.1572-0241.2002.06024.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole D, Palazzo L, Hammel P, et al. Macrocystic pancreatic cystadenoma: the role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59:823–829. doi: 10.1016/s0016-5107(04)00346-3. [DOI] [PubMed] [Google Scholar]
- 20.Maire F, Voitot H, Aubert A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–2877. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 21.Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg. 2014;218:608–617. doi: 10.1016/j.jamcollsurg.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Zamboni G, Terris B, Scarpa A, et al. Acinar cell cystadenoma of the pancreas: a new entity? Am J Surg Pathol. 2002;26:698–704. doi: 10.1097/00000478-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Delavaud C, d'Assignies G, Cros J, et al. CT and MR imaging of multilocular acinar cell cystadenoma: comparison with branch duct intraductal papillary mucinous neoplasia (IPMNs) Eur Radiol. 2014;24:2128–2136. doi: 10.1007/s00330-014-3248-0. [DOI] [PubMed] [Google Scholar]
- 24.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707–711. doi: 10.1148/radiology.199.3.8637992. [DOI] [PubMed] [Google Scholar]
- 25.Yu MH, Lee JY, Kim MA, et al. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol. 2010;195:1324–1332. doi: 10.2214/AJR.10.4452. [DOI] [PubMed] [Google Scholar]
- 26.Virgilio E, Mercantini P, Ferri M, et al. Is EUS-FNA of solid-pseudopapillary neoplasms of the pancreas as a preoperative procedure really necessary and free of acceptable risks? Pancreatology. 2014;14:536–538. doi: 10.1016/j.pan.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Law JK, Stoita A, Wever W, et al. Endoscopic ultrasound-guided fine needle aspiration improves the preoperative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: an international multicenter case series (with video) Surg Endosc. 2014;28:2592–2598. doi: 10.1007/s00464-014-3508-8. [DOI] [PubMed] [Google Scholar]
- 28.Gaujoux S, Tang L, Klimstra D, et al. The outcome of resected cystic pancreatic endocrine neoplasms: a case-matched analysis. Surgery. 2012;151:518–525. doi: 10.1016/j.surg.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 29.Morales-Oyarvide V, Yoon WJ, Ingkakul T, et al. Cystic pancreatic neuroendocrine tumors: the value of cytology in preoperative diagnosis. Cancer Cytopathol. 2014;122:435–444. doi: 10.1002/cncy.21403. [DOI] [PubMed] [Google Scholar]
- 30.Mege D, Gregoire E, Barbier L, Del Grande J, Le Treut YP. Lymphoepithelial cyst of the pancreas: an analysis of 117 patients. Pancreas. 2014;43:987–995. doi: 10.1097/MPA.0000000000000167. [DOI] [PubMed] [Google Scholar]