Abstract
Cardiovascular disease remains the number one cause of death in the US annually. The development in recent years of imaging strategies that can identify coronary endothelial dysfunction noninvasively provide new information about the early presence and local spatial heterogeneity of endothelial function in patients with, and those at risk for, coronary artery disease. In this summary, we will briefly review the mechanisms relating endothelial function and atherosclerosis, contemporary imaging strategies now able to quantify coronary endothelial function noninvasively, and recent insights on human coronary endothelial function.
Introduction
Coronary atherosclerotic disease is a spatially heterogeneous vascular disorder occurring at discrete locations with a chronic, staccato progression often punctuated by abrupt, unheralded plaque changes, erosion and rupture. Although systemic cardiovascular risk factor modification strategies, such as those directed at hyperlipidemia, hypertension, and tobacco abuse, reduce cardiovascular events and have been temporally associated with a 50% reduction in annual cardiovascular mortality rates over the last two decades, cardiovascular disease remains the number one cause of death in the US annually (Roger et al. 2011). Coronary endothelial dysfunction is both a consequence and cause of atherosclerosis and may be an important factor that contributes locally to coronary atherosclerosis and its progression. Historically coronary endothelial function could only be studied during invasive cardiac catheterization procedures, which sometimes precluded evaluation of healthy or low risk populations and often of serial studies in a given patient. The development in recent years of imaging strategies that can identify coronary endothelial dysfunction noninvasively provide new information about the early presence and local spatial heterogeneity of endothelial function in patients with, and those at risk for, coronary artery disease. Although prior reviews of endothelial function and atherosclerosis have been published, the literature on noninvasive coronary endothelial functional imaging approaches is relatively recent and the concept of heterogeneity of coronary endothelial function is rapidly evolving, with potential translational importance. Thus, we will briefly review here the mechanisms relating endothelial function and atherosclerosis, contemporary imaging strategies now able to quantify coronary endothelial function noninvasively, and recent insights on spatial heterogeneity of human coronary endothelial function.
The endothelium as a dynamic organ responding to signaling pathways critical in vascular physiology and pathology
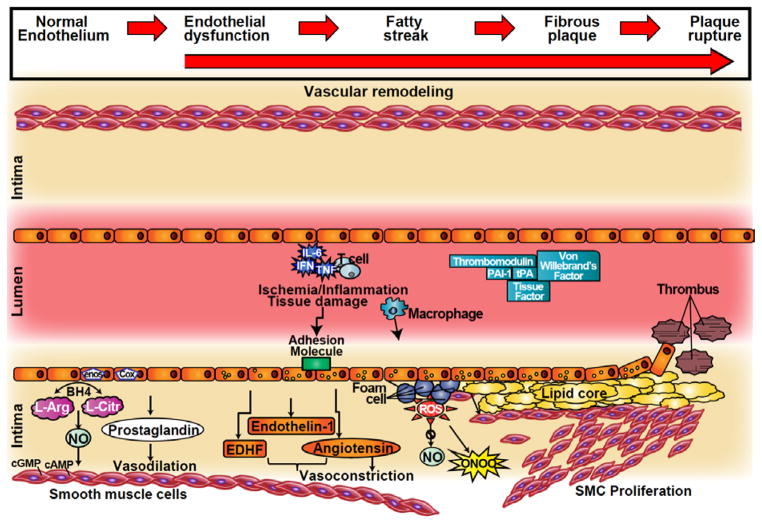
The vascular endothelium plays a central role in the maintenance of vascular health (Vita and Keaney 2002), and controls vasomotor tone, hemostatic balance, vascular permeability, innate and adaptive immunity and trafficking blood cells (Vita and Keaney 2002) while also inhibiting vascular smooth muscle cell proliferation and apoptosis (Herman and Moncada 2005). In addition to acting as a functional and structural barrier between blood and vessel wall preventing platelet and leukocyte adhesion and aggregation, the endothelium behaves as a receptor-effector structure sensing physical and chemical stimuli and/or releasing products to maintain homeostasis. The endothelium can be described as an endocrine organ, capable of producing a large variety of different molecules including vasodilators and vasoconstrictors, procoagulants (e.g., tissue factor, von Willebrand factor) and anticoagulants (e.g., thrombomodulin), inflammatory and anti-inflammatory, fibrinolytics (e.g., tissue plasminogen activator,) and antifibrinolytics, oxidizing and antioxidizing molecules (van Hinsbergh 2012). The endothelium also regulates vascular tone by releasing vasoconstrictors such as endothelin-1 (ET-1), and by converting angiotensin I to II. ET-1 and angiotensin II have roles not only in vasoconstriction, but also in the development of atherosclerosis, including stimulation of smooth muscle cell proliferation, vascular remodeling, and inflammatory cell adhesion (Figure 1), (Ferrario and Strawn 2006).
Figure 1.
Cross-sectional depiction of a coronary artery progressing from normal endothelial function to endothelial dysfunction and plaque formation (left to right). In the healthy state the endothelium is responsible for regulating vascular tone with balanced production of vasodilators and vasoconstrictors. In endothelial dysfunction this balance is disrupted in favor of the vasoconstrictors. An inflammatory and/or pro-thrombotic state promotes the formation of the plaque, plaque erosion and rupture.
cGMP = cyclic guanosine monophosphate; cAMP = cyclic adenosine monophosphate; NO = nitric oxide; NOS = nitric oxide synthase; COX = cyclooxygenase; BH4 = tetrahydrobiopterin; L-arg = L-arginine; L-cit = L-citrulline; EDHF= endothelium derived hyperpolarizing factor; IL = interleukin; TNF = tumor necrosis factor; ONOO = peroxinitrite; PAI1= plasminogen activator inhibitor 1; tPA = tissue plasminogen activator; ROS = reactive oxygen species; SMC = Smooth muscle cells
Nitric Oxide (NO), the predominant mediator of normal vascular function, is released by healthy endothelium and diffuses within the vessel wall, causing smooth muscle dilation in response to stimulation by endogenous factors such as bradykinin, acetylcholine, and catecholamines as well as ischemia, temperature change, and mechanical stimuli, including shear stress, as previously reviewed (Libby 2012). Because of the fundamental role of NO in mediating endothelial function, detection of impaired vasodilation in response to stressors that increase endothelium-derived NO is often used to measure abnormal endothelial function (Corretti et al. 2002). When the normal balance between vasodilators and vasoconstrictors cannot be maintained by the endothelium, the term “endothelial dysfunction” is often used and it refers to a maladapted endothelial phenotype characterized by reduced NO bioavailability, increased oxidative stress, elevated expression of pro-inflammatory and pro-thrombotic factors, and abnormal vasoreactivity to endothelial-dependent stressors (Drexler 1997). A growing number of interventions known to reduce cardiovascular risk improve endothelial function (Egashira et al. 1994) (Hornig et al. 1996) (Eguchi et al. 2012). In addition, endothelial dysfunction is an independent risk factor for cardiac death, myocardial infarction, and stroke (Bonetti et al. 2003). Thus, because established cardiovascular risk factors operate through the common pathway of impairment in endothelial function, endothelial function has been proposed as a “barometer” (Vita and Keaney 2002) for cardiovascular health that may be useful for guiding patient care and evaluating therapeutic strategies.
Endothelial function in peripheral arteries
Endothelial function was first studied in the peripheral arteries because they are more accessible than the coronary arteries (Flammer et al. 2012). Briefly, invasive and non-invasive techniques have been used to assess peripheral endothelial function. Venoocclusive strain-gauge plethysmography has, for more than 100 years, been used to measure the forearm vein impedance following intra-arterial infusion of vasoactive drugs at doses designed to limit systemic effects (Joyner et al. 2001). Intra-arterial infusion of vasodilators causes either endothelium-dependent (ie acetylcholine, bradykinin and substance P) or independent (sodium nitroprusside) vasodilation. Acetylcholine acts primarily via eNOS stimulation through endothelial muscarinic membrane receptor activation, which generates nitric oxide by metabolizing L-arginine (Flavahan 1992). The response to endothelium-independent vasodilatory agents, such as nitroglycerin or sodium nitroprusside, defines the vasodilatory properties of the blood vessels independent of the endothelium, and has been used as a control stimulus. The dose required to produce a local effect is much lower than the systemically effective dose. However, the need for arterial cannulation limits its use in large-scale studies. Ultrasound has been used to quantify the vasoactive shear stress related response following arterial occlusion with a blood pressure cuff that results in post-occlusion flow mediated dilatation (FMD) (Celermajer et al. 1992, Corretti et al. 2002). Because ultrasound quantification of FMD is non-invasive and easily repeated, it has become the most widely used noninvasive method for assessing endothelial function. However the method of evaluating endothelial response by FMD is rather technically challenging, time consuming and associated with a significant learning curve (Corretti et al. 2002). Although brachial FMD has been considered one of the most promising cardiovascular risk markers (Bonetti et al. 2003), a recent large prospective cohort study failed to demonstrate a significant correlation between brachial FMD and the incidence of coronary heart disease (Yeboah et al. 2012). Fingertip peripheral arterial tonometry (PAT) techniques evaluate finger arterial pulse wave amplitude before and during reactive hyperemia and have been proposed as noninvasive method to assess microvascular function in individuals (Kuvin et al. 2003). Although both FMD and PAT responses reflect aspects of vasoreactivity, conflicting results regarding their correlation have been reported (Lee et al. 2012) and it is not clear that they always reliably predict cardiovascular events (Anderson et al. 1995, Yeboah et al. 2012).
Invasive measures of coronary endothelial function
Seminal clinical studies of coronary endothelial dysfunction were performed invasively, measuring the vasomotor response of epicardial arteries with quantitative coronary angiography and coronary blood flow by Doppler in response to graded concentrations of acetylcholine (Ludmer et al. 1986). These approaches have been considered the “gold standard” for the evaluation of coronary endothelial function for the last 25 years. Interestingly, when used in the coronary circulation, acetylcholine causes dilatation of the epicardial vessels if endothelial function is preserved but constriction with endothelial dysfunction. The vasoconstriction is likely related to unopposed muscarinic receptor stimulation (Ludmer et al. 1986) and is in contrast to other vascular beds, such as the brachial or femoral arteries, where endothelial dysfunction is manifested as a diminished dilator response rather than constriction (Panza et al. 1993). Isometric handgrip exercise, cold pressor, and mental stress are considered predominant endothelial-dependent stressors because they dilate normal coronary arteries and paradoxically constrict atherosclerotic arteries (Brown et al. 1984, Nabel et al. 1988) (Yeung et al. 1991).
Noninvasive Detection of Coronary Endothelial Function
Although catheter-based studies of coronary endothelial function have provided critical insights over many years into the mechanisms, importance, and clinical relevance of coronary endothelial function and its relationship to atherosclerosis, the invasive nature is not well suited to studies in healthy and low risk populations or to longitudinal studies in the same individual over time. Advances in newer imaging technologies such as magnetic resonance imaging, positron emission tomography, and computed tomography promise noninvasive means to assess coronary vasomotor responses that could greatly extend our understanding of the role of coronary endothelial dysfunction in both early and later stage atherosclerosis as well as the impact of conventional and evolving strategies on endothelial function and atherosclerosis.
Positron Emission Tomography (PET) and Multi-detector Computed Tomography (MDCT)
The availability of PET scanners is growing and several positron-emitting tracers can be reliably used to quantify myocardial blood flow (MBF), including15O-labeled water, 13N-ammonia and 82Rubidium. Although the spatial resolution of PET is limited and does not permit direct visualization of the coronary arteries at this time, MBF can be quantified in absolute terms (ml/min/g) with PET and intervention-induced MBF changes used to infer coronary artery vasomotor responses (Yoshinaga et al. 2011). Cold pressor testing and mental stress are the most common interventions used to probe coronary endothelial function with PET and such studies have identified abnormal responses in subjects with CAD risk factors but angiographycally normal coronaries (Schindler et al. 2010) and in stenosed and in non-stenosed segments of CAD patients (Arrighi et al. 2000). Because MBF is affected by both epicardial coronary vasomotor tone and microvascular beds, it is not possible with PET to distinguish epicardial coronary responses from microvascular responses or to identify segmental arterial differences in coronary endothelial function. MDCT offers a much higher spatial resolution than PET and is currently the leading modality for noninvasively imaging coronary artery anatomy. Changes in coronary cross-sectional area in response to vasoactive stressors can be quantified with MDCT and, in theory, could be related to the presence of local coronary calcium and/or to local atherosclerosis determined by MDCT angiography. Unlike conventional cardiac catheterization with a Doppler flow probe, MDCT cannot currently quantify coronary blood flow, although it can be used to index relative MBF (Salerno and Beller 2009). Both PET and MDCT expose subjects to the risks of ionizing radiation which may be an important consideration in low risk populations and some research settings where imaging would be performed two times: in the absence and presence of an endothelial-dependent stressor.
Magnetic Resonance imaging (MRI)
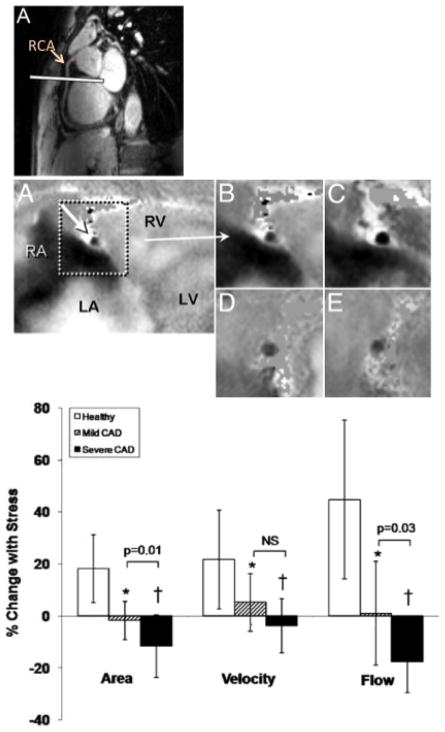
MRI is an appealing non-invasive imaging platform for quantifying coronary endothelial function because it is a tomographic technique offering high soft tissue contrast, excellent spatial resolution, the ability to quantify blood velocity and flow, as well as local atherosclerosis and vessel remodeling without exposing subjects to ionizing radiation. Specifically, coronary MRI has been validated as a noninvasive means to assess coronary cross-sectional area (Scheidegger et al. 1996) and flow velocity (Hundley et al. 1996). It has also been used to document endothelial-independent coronary artery vasomotor responses to nitroglycerin, which are altered in patients with calcified and severe coronary artery disease (Jin et al. 2012). Recently, our group developed an MRI approach to noninvasively quantify coronary endothelial function that exploits the increased signal available with high-field (3T) MRI systems and phased-array detector coils and combines that with isometric handgrip exercise, a well-tolerated endothelial-dependent stressor that is easily performed in a MRI scanner. “Bright blood” cine MR images are acquired orthogonal to the long-axis of coronary arteries during a single breathhold (15–20 sec) in order to obtain cross-sectional coronary artery dimensions (area and diameter) throughout the cardiac cycle with an approximate time resolution of 30–45ms (Figure 2) (Hays et al. 2010). Phase-contrast cine MR images are obtained in the same imaging plane and provide robust, validated measures of blood flow on a pixel-by-pixel basis. With measures of coronary cross-sectional area and blood velocity, local coronary blood flow can be calculated. In healthy subjects without CAD risk factors, isometric handgrip results in coronary vasodilation, an increase in coronary blood velocity, and coronary blood flow (Hays et al. 2010). In patients with documented CAD, the same stressor causes an identical hemodynamic response (i.e. increase in rate pressure product) but instead coronary vasoconstriction and reduced coronary blood flow. The response is rapid (within 30 seconds), resolves quickly (within 2 minutes), and is reproducible on repeat study (Hays et al. 2010). In patients with single-vessel critical CAD the vasomotor response in the severely diseased vessel (not at the site of stenosis but approximately 2 cm away) was more abnormal than that in a non-stenosed coronary artery (Figure 2B). To evaluate earlier stages of atherosclerosis, the MRI technique for evaluating local coronary endothelial function was combined with “black blood” MRI to quantify coronary wall thickness (CWT), an important index of arterial remodeling and early coronary atherosclerosis (Kim et al. 2007) (He et al. 2012). Using this combined approach, patients with non-critical CAD demonstrated abnormal coronary endothelial function (impaired coronary vasodilation and flow changes in response to isometric handgrip stress) and increased CWT and normalized wall index, a quantitative measure of vessel remodeling (Figure 3) (Hays et al. 2012). Importantly, in patients with mild coronary disease, CWT correlated inversely with the endothelial-dependent vasomotor response indicating that at the earliest stages of coronary atherosclerosis detected noninvasively there is local coronary endothelial dysfunction and it is related to the extent of early atherosclerosis (Figure 3I) (Hays et al. 2012). Taken together these findings demonstrate that endothelial dysfunction is heterogeneous in patients with CAD and suggests that a single measure of peripheral endothelial function is unlikely to represent the spectrum of endothelial function present in the coronary arteries of a CAD patient (Hays et al. 2010) (Hays et al. 2012). This is also consistent with the variable correlation between peripheral and coronary endothelial function noted in the literature (Anderson et al. 1995).
Figure 2.
Noninvasive detection of coronary endothelial dysfunction and regional differences in coronary endothelial function. (Upper Panel) “White blood” scout MRI of the right coronary artery (RCA) in long axis with white line representing orientation of subsequent cross-sectional images shown in middle panel. (Middle Panel) Flow velocity-encoded MRI phase images showing a cross section of the RCA (A) that was selected for analysis for coronary flow velocity measurements in a healthy volunteer. The signal intensity is proportional to flow velocity, with a black signal indicating high velocity down through the imaging plane. In the magnified view of the RCA at baseline (B) and during isometric handgrip stress (C), the decrease in luminal coronary signal intensity (increased blackness) illustrates an increase in through-plane coronary flow velocity. The cross section of the RCA in a coronary artery disease patient at rest (D) and during handgrip stress (E) indicates no increase in through-plane coronary flow velocity during stress. (Bottom Panel) Summary data showing relative change in coronary artery area, peak diastolic coronary flow velocity and blood flow during isometric handgrip stress for healthy subjects (n = 20 [open bars]) and patients with single-vessel coronary artery disease (CAD) (n = 10). Brackets show comparisons between arteries with mild CAD (hatched bars) versus severe CAD (solid bars) within the same patients. *p < 0.005, †p < 0.001 versus healthy subjects. Reproduced with permission from Hays et al (Hays et al. 2010)
Figure 3.
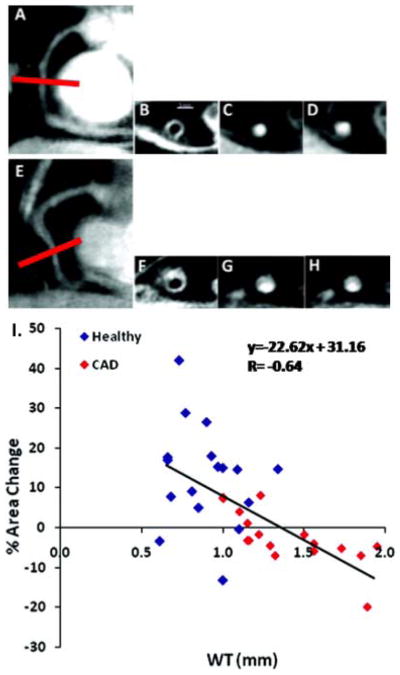
Noninvasive assessment of early coronary atherosclerosis, coronary endothelial function, and their relationship with MRI. (Upper Panel) Typical anatomic and functional coronary images using MRI at rest and with isometric handgrip stress. In a healthy subject (A), a scout scan obtained along the right coronary artery (RCA) in a healthy adult subject is shown together with the location for cross-sectional imaging (red line). B, A view perpendicular to the RCA in a healthy adult subject is shown, illustrating a black blood vessel wall cross-section. C, A white blood view for endothelial function in a healthy subject is shown at rest (C) and during stress (D). In a patient with CAD (E–H), a scout scan obtained parallel to the RCA is shown (E) together with the location for cross-sectional imaging (red line). F, RCA black blood vessel wall cross-sectional image in the same patient with CAD. G, A view perpendicular to the RCA in the patient with CAD is shown at rest (G) and during stress (H). (Lower Panel). Relationship between early atherosclerosis and endothelial function is shown in the summary data (I). MRI measures of coronary wall thickness (mm) versus percent coronary cross-sectional area changes with isometric handgrip stress in healthy subjects and patients with coronary artery disease (combined) while individual data points are shown for healthy subjects (blue diamonds) and patients with coronary artery disease (red diamonds). Reproduced with permission from Hays et al (Hays et al. 2012)
Heterogeneity of endothelial function
As noted above the endothelium is not an inert layer of cells but a dynamic, responsive cellular lining that may display structural and functional heterogeneity to meet the diverse and varying demands of the local tissues (Aird 2007a, Aird 2007b). With the introduction of the electron microscope in the 1950s, the endothelium was described as a monolayer of cells with variable characteristics in different vascular beds (Tse and Stan 2010). The discovery of the variable morphology of endothelial cells led to the understanding that these cells differ from each other also in their function and response to certain stimuli. In fact, it is striking to note that vascular beds respond differently to pro-inflammatory stimuli. For example, TNF-alpha receptor expression differs among vascular territories (Al-Lamki et al. 2009) and intravenous injection of TNF-alpha in mice, induces E- and P-selectin expression in the heart, lung, kidney and liver but not in the spleen (Yao et al. 1999). At a genetic level, mapping of the endothelial cells in specific territories has identified the presence of “vascular zip-codes” referring to specific expression profiles for each vascular bed (Ruoslahti 2004). Interestingly, the plasticity of the endothelium is demonstrated by the ability to modify gene expression depending on the environment to which the endothelial cells are exposed. A recent study showed that the differences observed in vivo between endothelial cells harvested from porcine coronary artery when compared to the ones from an iliac artery were lost when those cells were cultured in vitro (Burridge and Friedman 2010), implying an active modification of the expression of certain genes depending on the environmental exposure. Differences in epigenetic regulatory mechanisms, affecting methylation status of gene promoters and posttranslational histone modifications such as acetylation, methylation, phosphorylation, may also partially explain endothelial subset preference of gene expression (Regan and Aird 2012). These epigenetic modifications are not a static characteristic of endothelial cells but can dynamically vary in time as has been recently shown for eNOS, whose histone markers were reset due to hypoxia, thus affecting gene expression (Fish et al. 2010).
Vasoreactivity varies among vascular beds in a given individual for several reasons including the intrinsic mechanical properties of the vascular bed, non-endothelial dependent factors and endothelial-dependent differences. In apo-E deficient mice, local endothelial function was associated with the local presence and extent of atherosclerosis (Crauwels et al. 2003) while in pigs, segmental coronary endothelial dysfunction was related to the presence of local markers of inflammation and oxidative stress (Pendyala et al. 2009). Spatially heterogeneous or segmental coronary endothelial function has been observed in patients with coronary artery disease (Suwaidi et al. 2000) and in patients following cardiac transplantation (Davis et al. 1996). With the use of spectral analysis of intravascular ultrasound (IVUS) radiofrequency data to characterize predominant plaque components, local coronary endothelial dysfunction in patients with early or minimal coronary atherosclerosis was closely associated with high-risk plaque characteristics such as the presence of a necrotic core (Lavi et al. 2009). More recently, local epicardial coronary endothelial dysfunction closely correlated with the presence and extent of a lipid core plaque detected by near-infrared spectroscopy (NIRS) and typically associated with high-risk lesions (Choi et al. 2013), The most recent non-invasive coronary MRI studies, mentioned earlier, built on this line of evidence for segmental coronary endothelial dysfunction and identified a greater degree of endothelial dysfunction in coronary arteries with more severe stenotic disease than in minimally diseased vessels in the same patients (Hays et al. 2010). All of these findings are consistent with a hypothesis that endothelial dysfunction is a heterogeneous process within the same patient that may predispose to segmental disease progression and events (Suwaidi et al. 2000). It should be noted that although both peripheral and coronary endothelial function have been considered predictors of future cardiovascular events (Schachinger et al. 2000), the two do not always closely correlate and vasoreactivity is not always uniform across vascular regions within the same individual (Silber et al. 2007). One study, using MRI compared brachial versus femoral vasoreactivity during postocclusion hyperemia and found that femoral but not brachial reactivity was impaired in patients with increased cardiovascular risk (Silber et al. 2007). Other data suggest that peripheral and coronary endothelial function measures may be weakly related (Anderson et al. 1995), possibly due to differences in vascular properties (Hirooka et al. 1994). The difference in local vascular properties between coronary and peripheral vessels is consistent with the clinical experience that acute coronary plaque rupture is common while brachial arterial plaque rupture rarely, if ever, occurs. Similarly, a recent study did not find any correlation between carotid intima media thickness (IMT) and coronary circulatory function using PET-measured MBF, again suggesting different features of early atherosclerosis in the peripheral and coronary circulations (Schindler et al. 2009). Taken together, there is an evolving body of evidence that endothelial function varies among vascular beds and varies segmentally within a vascular territory, in part related to local disease and suggests that measures of local endothelial function may offer a valuable window into the local vascular pathophysiology.
Many important questions remain unanswered and thus offer fertile areas for investigation in terms of the distribution and significance of endothelial function heterogeneity in atherosclerosis. Because noninvasive approaches offer the most readily applicable means to study vasoreactivity in larger populations and low-risk individuals, the type of endothelial-dependent stressor becomes an important consideration. Intra-arterial acetylcholine cannot be readily administered in noninvasive settings and post-occlusion FMD, used extensively for peripheral endothelial function, cannot be directly applied to studies of coronary, pulmonary or renal vascular function. Thus it will be important to compare the responses and mechanisms of different currently used “less-invasive” endothelial-dependent stressors including isometric handgrip exercise, cold pressor testing, and mental stress.
Implications
More important than the technical advances are the important biologic questions that can now be addressed with these new insights and tools. First, if endothelial function is indeed a barometer of local (or even segmental) vascular health, can noninvasive coronary endothelial function measures be used to predict local coronary atherosclerotic progression, plaque rupture and events? It is clear that anatomic indices of atherosclerosis alone are poor individual predictors of plaque progression and events (Stone et al. 2011) and it may be time to consider a new paradigm whereby endothelial cell physiology, rather than vessel anatomy alone, is used to gauge risk and likelihood of future events. Second, coronary endothelial function should be evaluated as a tool to assess the vascular impact of new and emerging treatment strategies and to guide drug development. New molecules and approaches designed to limit oxidative stress, local inflammation, apoptosis, or improve regenerative capacity could be evaluated repeatedly and noninvasively over time in the same subjects and same arterial segments with these approaches. Third, noninvasive assessment of coronary endothelial function should be investigated as a complementary tool in the development of individualized medicine.–In summary, coronary endothelial dysfunction is both a consequence and cause of atherosclerosis and the development in recent years of imaging strategies that can identify coronary endothelial dysfunction noninvasively may provide new information about the early presence and local spatial heterogeneity of endothelial function in patients with, and those at risk for, coronary artery disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007a;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007b;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- Al-Lamki RS, Brookes AP, Wang J, Reid MJ, Parameshwar J, Goddard MJ, et al. TNF receptors differentially signal and are differentially expressed and regulated in the human heart. Am J Transplant. 2009;9(12):2679–2696. doi: 10.1111/j.1600-6143.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, et al. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356(9226):310–311. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation. 1984;70(1):18–24. doi: 10.1161/01.cir.70.1.18. [DOI] [PubMed] [Google Scholar]
- Burridge KA, Friedman MH. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am J Physiol Heart Circ Physiol. 2010;299(3):H837–846. doi: 10.1152/ajpheart.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Choi BJ, Prasad A, Gulati R, Best PJ, Lennon RJ, Barsness GW, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59(1):189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Davis SF, Yeung AC, Meredith IT, Charbonneau F, Ganz P, Selwyn AP, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93(3):457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- Drexler H. Endothelial dysfunction: clinical implications. Prog Cardiovasc Dis. 1997;39(4):287–324. doi: 10.1016/s0033-0620(97)80030-8. [DOI] [PubMed] [Google Scholar]
- Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, et al. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89(6):2519–2524. doi: 10.1161/01.cir.89.6.2519. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Hoshide S, Ishikawa S, Shimada K, Kario K. Aggressive blood pressure-lowering therapy guided by home blood pressure monitoring improves target organ damage in hypertensive patients with type 2 diabetes/prediabetes. J Clin Hypertens (Greenwich) 2012;14(7):422–428. doi: 10.1111/j.1751-7176.2012.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Fish JE, Yan MS, Matouk CC, St Bernard R, Ho JJ, Gavryushova A, et al. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J Biol Chem. 2010;285(2):810–826. doi: 10.1074/jbc.M109.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85(5):1927–1938. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56(20):1657–1665. doi: 10.1016/j.jacc.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Hays AG, Kelle S, Hirsch GA, Soleimanifard S, Yu J, Agarwal HK, et al. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging. 2012;5(3):341–348. doi: 10.1161/CIRCIMAGING.111.969691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang Z, Dai Q, Zhou Y, Yang Y, Yu W, et al. Accuracy of MRI to identify the coronary artery plaque: a comparative study with intravascular ultrasound. J Magn Reson Imaging. 2012;35(1):72–78. doi: 10.1002/jmri.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AG, Moncada S. Therapeutic potential of nitric oxide donors in the prevention and treatment of atherosclerosis. Eur Heart J. 2005;26(19):1945–1955. doi: 10.1093/eurheartj/ehi333. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Egashira K, Imaizumi T, Tagawa T, Kai H, Sugimachi M, et al. Effect of L-arginine on acetylcholine-induced endothelium-dependent vasodilation differs between the coronary and forearm vasculatures in humans. J Am Coll Cardiol. 1994;24(4):948–955. doi: 10.1016/0735-1097(94)90854-0. [DOI] [PubMed] [Google Scholar]
- Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93(2):210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- Hundley WG, Lange RA, Clarke GD, Meshack BM, Payne J, Landau C, et al. Assessment of coronary arterial flow and flow reserve in humans with magnetic resonance imaging. Circulation. 1996;93(8):1502–1508. doi: 10.1161/01.cir.93.8.1502. [DOI] [PubMed] [Google Scholar]
- Jin H, Zeng MS, Yun H, Ge MY, Ma JY, Yang S. Noninvasive test of nitrate-induced coronary vasomotion by 1.5-T whole-heart 3D magnetic resonance angiography using a T2-prepared SSFP sequence. Int J Cardiovasc Imaging. 2012;28(7):1707–1716. doi: 10.1007/s10554-011-9999-7. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91(6):2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kim WY, Astrup AS, Stuber M, Tarnow L, Falk E, Botnar RM, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115(2):228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95(18):1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Bass A, Ellis K, Tran B, Steele S, Caughey M, et al. Relation between digital peripheral arterial tonometry and brachial artery ultrasound measures of vascular function in patients with coronary artery disease and in healthy volunteers. Am J Cardiol. 2012;109(5):651–657. doi: 10.1016/j.amjcard.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77(1):43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87(5):1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- Pendyala LK, Li J, Shinke T, Geva S, Yin X, Chen JP, et al. Endothelium-dependent vasomotor dysfunction in pig coronary arteries with Paclitaxel-eluting stents is associated with inflammation and oxidative stress. JACC Cardiovasc Interv. 2009;2(3):253–262. doi: 10.1016/j.jcin.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Regan ER, Aird WC. Dynamical systems approach to endothelial heterogeneity. Circ Res. 2012;111(1):110–130. doi: 10.1161/CIRCRESAHA.111.261701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans. 2004;32(Pt3):397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2(5):412–424. doi: 10.1161/CIRCIMAGING.109.854893. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Scheidegger MB, Stuber M, Boesiger P, Hess OM. Coronary artery imaging by magnetic resonance. Herz. 1996;21(2):90–96. [PubMed] [Google Scholar]
- Schindler TH, Facta AD, Prior JO, Cadenas J, Zhang XL, Li Y, et al. Structural alterations of the coronary arterial wall are associated with myocardial flow heterogeneity in type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36(2):219–229. doi: 10.1007/s00259-008-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Silber HA, Lima JA, Bluemke DA, Astor BC, Gupta SN, Foo TK, et al. Arterial reactivity in lower extremities is progressively reduced as cardiovascular risk factors increase: comparison with upper extremities using magnetic resonance imaging. J Am Coll Cardiol. 2007;49(9):939–945. doi: 10.1016/j.jacc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Tse D, Stan RV. Morphological heterogeneity of endothelium. Semin Thromb Hemost. 2010;36(3):236–245. doi: 10.1055/s-0030-1253447. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34(1):93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood. 1999;94(11):3820–3828. [PubMed] [Google Scholar]
- Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Manabe O, Tamaki N. Assessment of coronary endothelial function using PET. J Nucl Cardiol. 2011;18(3):486–500. doi: 10.1007/s12350-011-9370-3. [DOI] [PubMed] [Google Scholar]