Abstract
Background
Chronic alcoholism is associated with increased incidence and severity of cutaneous infection. Skin-resident T cells orchestrate numerous immunological functions that are critically involved in both tissue homeostasis and cutaneous immunity. The impact of chronic ethanol (EtOH) exposure on skin T cells has not previously been examined; given their important role in maintaining the immune barrier function of the skin further study is warranted.
Methods
Mice were administered EtOH in the drinking water for 12 to 16 weeks. Flow cytometry was used to evaluate impact of EtOH feeding on skin T cell numbers, rates of proliferation, and apoptosis as well as activation marker expression and cytokine production after ex vivo stimulation.
Results
Chronic EtOH feeding caused a baseline reduction in dendritic epidermal T cell (DETC) numbers that corresponded with reduced expression of the activation marker JAML following phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation. Chronic EtOH feeding did not alter total numbers of dermal T cells, but specific subset loss was observed in Foxp3+ regulatory T cells (Tregs) as well as CD3hi, Vγ3+ and CD3int, Vγ3− dermal γδ T cells. EtOH-induced dysfunction in the latter population, which represents prototypical interleukin-17 (IL-17)-producing dermal γδT17s, was made evident by diminished IL-17 production following anti-CD3 stimulation. Additionally, the capacity of lymph node γδ T cells to produce IL-17 following anti-CD3 and PMA/ionomycin stimulation was impaired by chronic EtOH feeding.
Conclusions
Chronic EtOH feeding induced defects in both numbers and function of multiple skin T cell subsets. The decreased density and poor responsiveness of DETCs and γδT17 cells in particular would be expected to compromise immune effector mechanisms necessary to maintain a protective barrier and restrict pathogen invasion. These findings demonstrate the sensitivity of skin T cells to EtOH and provide new mechanisms to help explain the propensity of alcoholics to suffer skin infection.
Keywords: Dendritic Epidermal T Cells, Langerhans Cells, IL-17, Chronic EtOH, JAML
The Immunosuppressive Effects of chronic alcohol abuse are profound, wide-ranging, and readily apparent at common sites of pathogen entry such as the skin. Alcoholics are predisposed to skin infections caused by an assortment of microbes that include but are not limited to: Group A Streptococcus (GAS), Staphylococcus aureus, Corynebacterium diptheriae, Escherichia coli, and Vibrio vulnificus (Mohan et al., 2011; Smith and Fenske, 2000). Underscoring the seriousness of these illnesses, septicemia-induced mortality originating from GAS and S. aureus skin infections are greatly increased among alcoholics (Forsblom et al., 2011; Kaech et al., 2006; Rantala et al., 2009; Skogberg et al., 1988). Impaired host defense in the skin is therefore a severe dermatological consequence of alcoholism, yet the specific immunologic lesions responsible remain poorly defined.
As a barrier tissue, the skin serves as a first line of defense against innumerable environmentally derived dangers (Di Meglio et al., 2011). T lymphocytes are key cellular components of the skin’s immune apparatus (Clark et al., 2006; Naik et al., 2012; Streilein, 1978; Sumaria et al., 2011). In the steady state, murine epidermal T cells are almost exclusively (Vγ3+) γδ T cells, termed dendritic epidermal T cells (DETCs). While DETCs have a broad range of functions, they make unique contributions to the skin’s barrier integrity by controlling keratinocyte (KC) homeostasis (Macleod and Havran, 2011). Their dendritic morphology, expression of stress surveillance receptors and capacity to secrete KC growth factors, such as insulin-like growth factor 1 (IGF-1), enables DETCs modulate KC survival and proliferation in accordance with the pathophysiological state of their microenvironment.
When compared to the epidermis, the dermal T cell compartment exhibits considerable heterogeneity. While the existence of both dermal αβ and γδ T cells has been known for some time, the roles played by specific subsets in host defense and tissue homeostasis are just beginning to be elucidated. Recently, a subset of dermal γδ T cells have been shown to be the skin’s principal immediate source of interleukin-17 (IL-17) following Toll-like receptor stimulation as well as cutaneous bacterial infection (Cai et al., 2011; Gray et al., 2011; Myles et al., 2013; Sumaria et al., 2011). Dubbed γδT17s, these cells can be phenotypically distinguished from DETCs on the basis of their intermediate expression of both CD3 and γδ T cell receptor (TCR) (Cai et al., 2011; Gray et al., 2011, 2013; Hu and Xiong, 2013; Myles et al., 2013; Sumaria et al., 2011). Dermal resident αβ T cells are predominantly CD4 T cells with phenotypic features of memory populations (Naik et al., 2012). CD4 T cells possess the ability to migrate between skin and lymphoid tissues at baseline and at increased rates following cutaneous inflammation (Bromley et al., 2013; Tomura et al., 2010). Among these CD4 T cells, Foxp3+ regulatory T cells (Tregs) are perhaps the most prodigious traffickers. While Tregs constitute between 5 and 20% of dermal CD4 T cells they make up the majority of CD4 T cells migrating from the skin to the lymph nodes (LNs) in both the steady and inflammatory states (Tomura et al., 2010). The importance of dermal Tregs to skin homeostasis has been demonstrated by the rapid and severe skin inflammation occurring in mice that are selectively deficient in skin Tregs (Dudda et al., 2008). An additional consequence of skin-restricted Treg deficiency is impaired antiviral immune responses suggesting that Tregs make important positive contributions to cutaneous immunity as well (Freyschmidt et al., 2010). Taken together, skin T cells are needed for tissue homeostasis and host defense responses; as such, any ethanol (EtOH)-induced dysfunction occurring within this compartment would have major implications for the overall immunological performance of the skin.
The goal of this study was to characterize the impact of chronic EtOH feeding upon the maintenance and functionality of skin T cells. To study the effects of long-term EtOH administration upon the cutaneous immune system without prompting a systemic elevation in corticosteroids, the Meadows–Cook model of alcoholism was employed. This model has been successfully used to reproduce EtOH-induced immune defects and study the underlying mechanisms (Cook, 1998; Cook et al., 2007; Gurung et al., 2009; Parlet and Schlueter, 2013; Zhang and Meadows, 2005). The results indicate that chronic EtOH feeding differentially impacts skin T cell subsets. While the total number of αβ T cells in the skin was not different in EtOH-consuming mice, subset loss among αβ T cells occurred selectively in Tregs. Unlike αβ T cells, EtOH induced a reduction in the total number of skin-resident γδ T cells, which corresponded with reduced epidermal and dermal subsets. Additionally, DETC and γδT17 were hyporesponsive following ex vivo stimulation. The EtOH-induced alterations in Tregs and γδ T cells would be anticipated to impact both the structural integrity and immune responsiveness of the skin, which are fundamental to its barrier function. These newly identified EtOH-induced lesions offer novel information about the impact of EtOH upon peripheral T cell populations and may facilitate a better understanding of why alcoholics are more susceptible to skin infection.
MATERIALS AND METHODS
Mice and EtOH Feeding Model
Six- to 7-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Fort Detrick, MD). B6.FoxP3-GFP mice were kindly provided by Dr. Alexander Rudensky (Fontenot et al., 2005). Mice were housed in specific pathogen-free facilities with all animal protocols approved by the Animal Care and Use Committee at the University of Iowa. At approximately 8 weeks of age, mice were randomly divided into control and EtOH-consuming groups. EtOH was administered as previously described (Parlet and Schlueter, 2013). Mice treated with the Meadows–Cook regimen have only EtOH-containing water as their fluid source and are allowed to drink ad libitum. EtOH-fed mice were maintained on 20% EtOH for 12 to 16 weeks. By averaging the total volume of EtOH consumed from cages housing 4 mice, the mean values for daily 20% EtOH intake per mouse over the following durations on the Meadows–Cook diet were calculated: 1 to 3 weeks (1.97 − 0.08 ml/d, n = 14), 5 to 7 weeks (2.14 − 0.06 ml/d, n = 12), 8 to 9 weeks (2.43 − 0.12 ml/day, n = 8), 11 to 30 weeks (2.41 − 0.11 ml/d, n = 11).
Antibodies and Reagents
Five color flow cytometric analyses were performed using the following antibodies: Anti-langerin (929F3.01) was purchased from Dendritics (Lyon, France). PE-anti-JAML (4E10) and FITC- or PerCP-Cy5.5-anti-γδ TCR (GL3) were purchased from Biolegend (San Diego, CA). FITC-anti-CD69 (H1.2F3), PE-Cy7-anti-CD4 (RM4-5) were purchased from BD Biosciences (San Jose, CA). PE-anti MHC CL II (M5/114.15.2), APC-anti-NKG2D (CX5), APC-anti-CD3 (145-2C11), FITC- or PE-anti-CD103 (2E7), APC- or PE-anti-IL-17A (ebio17B7), APC-anti CD45 (30-F11), PE-anti-Foxp3 (FJK-16s), and PE-anti-tumor necrosis factor alpha (TNFα) (TN3-19) were purchased from (eBioscience, San Diego, CA). Goat anti-IGF-1 was purchased from (Santa Cruz Biotechnology, Santa Cruz, CA). H57, a hamster anti-mouse αβ TCR (Kubo et al., 1989); 104, a mouse anti-mouse CD45.2 (Shen, 1981) and 145-2C11, a hamster anti-mouse CD3ε were derived from hybridomas in the laboratory and conjugated to FITC using standard procedures (H57 and 104) or used for coating tissue culture plates (145-2C11). For the detection of Foxp3 expression on wild-type (WT) mice a Foxp3 staining kit (eBioscience) was used according to manufacturer’s instructions. BrdU incorporation was measured using a BrdU Flow kit (BD Biosciences) according to the manufacturer’s instructions. Apoptosis in epidermal and dermal cell suspensions was assessed using FAM caspase 3/7 kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Cell Suspension Preparations
Split ear skin and epidermal cell suspensions were prepared as previously described (Parlet and Schlueter, 2013). For dermal cell suspensions, dermal sheets were incubated in RPMI (Invitrogen) with 10% fetal bovine serum, L-glutamine/penicillin/streptomycin and 2-mercaptoethanol (RPMIc) containing 500 μg/ml collagenase type II (Gibco BRL, Grand Island, NY) for 90 minutes at 37°C and dissociated by passing through 18 and 20 gauge needles. The resulting cell suspensions were incubated in RPMIc for an additional 90 minutes at 37°C. For LN suspensions, inguinal LNs were cut into small pieces and incubated in RPMIc and further dissociated by passing through 18 and 20 gauge needles.
Ex Vivo Stimulation of Skin and LN T Cells
Evaluation of NKG2D, JAML, and CD69 Expression
Epidermal cell suspensions were cultured in RPMIc − phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich, St. Louis, MO) for 4.5 hours. Cells were then fixed, permeabilized and stained.
Evaluation of IGF-1, TNFα, and IL-17A Production in Dermal and LN T Cells
In 48 well plates, dermal or LN cells were cultured at 5 × 105 in 500 μl RPMIc for 4 hours − plate-bound anti-CD3 (10 μg/ml), or PMA (50 ng/ml) and ionomycin (500 ng/ml). Cells were cultured in the presence of brefeldin A (eBioscience), fixed, permeabilized, and stained for IL-17A, IGF-1, or TNFα.
Flow Cytometric Analysis
Following surface staining, cells were fixed in FACS lysing solution (BD Biosciences) and permeabilized using 0.5% saponin (Sigma-Aldrich). To block nonspecific binding, cells were incubated with rat anti-mouse CD16/32 FcγRIII/II (2.4G2) and rat sera prior to staining. Samples were collected on a FACS Canto II (BD Biosciences) using Diva acquisition software and analyzed using FlowJo software (TreeStar, Stanford, CA). Dead cells were excluded by low forward and side light scatter. Spectral overlaps between fluorochrome channels were corrected by automated compensation on singly stained positive controls for each fluorochrome. In general, 100,000 cells were collected per tube.
Statistics
The p-values were calculated from 2-tailed Student’s t-test using InStat software (GraphPad Software, LaJolla, CA) to compare EtOH and control groups.
RESULTS
Loss of Epidermal T Cells Following Chronic EtOH Feeding
As the skin’s outermost anatomical compartment, the epidermis directly engages the outside world. The ability to protect internal tissues from potentially harmful consequences of this engagement (e.g., injury, infection, or malignancy) depends heavily upon the epidermal immune system. Langerhans cells (LCs) and DETCs are key components of this system and together constitute virtually all of the bone marrow–derived cells in the pre-immune murine epidermis (Di Meglio et al., 2011). Using immunofluorescence microscopy, we previously showed that chronic EtOH feeding reduces the number of epidermal resident LCs (Ness et al., 2008). To more comprehensively inspect EtOH’s impact upon the maintenance of hematopoietic lineage cells in the epidermis, flow cytometry was used to quantify baseline numbers of LCs and DETCs in epidermal cell suspensions as shown in Fig. 1A. Consistent with prior histological evidence and further proving that LC subset loss is a highly reliable cutaneous manifestation of chronic EtOH feeding, the number of LCs detected in epidermal preparations from EtOH-fed mice was dramatically reduced, compared with age-matched H2O-fed control mice (Fig. 1B). In these same preparations, EtOH induced a similar numeric loss of DETCs. Taken together, it is apparent that chronic EtOH feeding severely impairs the capacity of the epidermis to maintain its immune cell compartments. This is the first demonstration that chronic EtOH exposure impairs T cell maintenance in a barrier tissue.
Fig. 1.
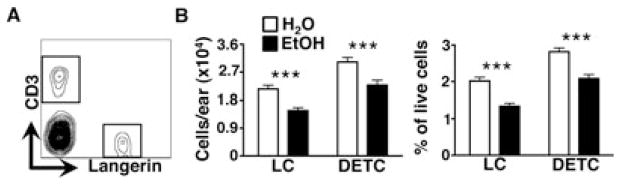
Chronic EtOH feeding reduces baseline numbers of dendritic epidermal T cells (DETCs). Fresh epidermal cell preparations from ear skin of mice fed EtOH for 12 to 16 weeks or age-matched water controls were counted and stained for flow cytometry. (A) Gating strategy for identifying epidermal LCs and DETCs from a representative H2O control mouse. (B) Numbers and frequencies of LCs and DETCs/ear. Error bars represent SEM. ***Post test p-value < 0.0005. n ≥ 22 mice/treatment group. EtOH, ethanol; LCs, Langerhans cells.
Loss of Dermal γδ T Cells Following Chronic EtOH Feeding
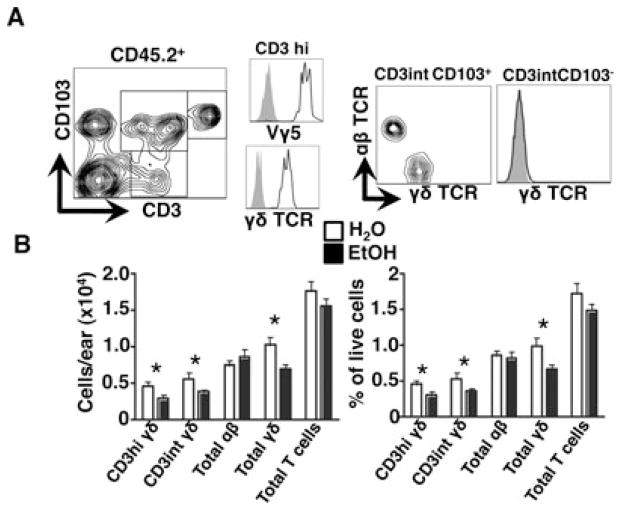
Unlike the epidermis, which is occupied almost exclusively by γδ TCR+ DETCs, both γδ and αβ TCR+ cells reside within the dermis. In light of the significant DETC subset loss observed in EtOH-fed mice, the capacity of EtOH to affect the maintenance of dermal T cells was next assessed. Flow cytometric analysis of dermal cell suspensions revealed multiple subsets of dermal T cells based on their expression of CD3 and the integrin CD103, a ligand for E-cadherin and key mediator of skin localization for DETCs and Tregs (Schön et al., 2002; Suffia et al., 2005). Consistent with previous reports, dermal γδ T cells were uniformly CD103+ and could be segregated into 2 populations on the basis of CD3 density, while 2 αβ T cell populations could be discriminated by differential expression of CD103 (Fig. 2A) (Sumaria et al., 2011). Among the dermal γδ T cells, the CD103+, CD3hi, Vγ3+ cells appear phenotypically similar to DETCs while the CD103+, CD3int, γδ TCRint cells exhibit phenotypic characteristics of dermal γδT17 (Gray et al., 2011; Hu and Xiong, 2013; Sumaria et al., 2011). Importantly, we found that EtOH-fed mice incurred a substantial loss in total dermal γδ T cells relative to H2O-fed controls, which corresponded with the depletion of both CD3hi and CD3int γδ T cell subsets. Unlike γδ T cells, dermal αβ T cell numbers and percentages were unaltered by chronic EtOH feeding (Fig. 2B). This indicates that the maintenance of γδ and αβ T lineage cells in the dermis is differentially susceptible to the effects of chronic EtOH feeding.
Fig. 2.
Chronic EtOH feeding reduces baseline numbers of dermal γδ T cells. Fresh dermal cell preparations from ear skin of mice fed EtOH for 12 to 16 weeks or age-matched water controls were counted and stained for flow cytometry. (A) Gating strategy for dermal T cell subsets for a representative H2O control mouse. (B) Numbers of dermal T cells/ear. Total γδ T cells were calculated as the sum of the CD3hi-gated cells and the γδ+ fraction within the CD3+ CD103+ gate. Likewise, total αβ T cells were calculated as the sum of CD3+ CD103− gated cells and the γδ-fraction within the CD3+ CD103+ gate. Error bars represent SEM. *Post test p-value < 0.05. n ≥ 8 mice/treatment group. EtOH, ethanol.
Loss of Dermal Tregs Following Chronic EtOH Feeding
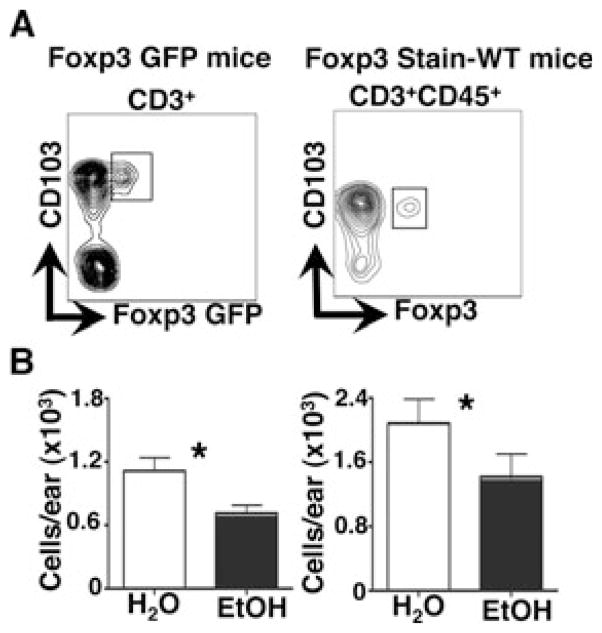
To determine whether chronic EtOH feeding impacts the capacity of Tregs to occupy the dermis Foxp3+ T cells were quantified in dermal cell suspensions prepared from EtOH-consuming or H2O control WT and Foxp3-GFP reporter mice (Fig. 3). Using 2 separate approaches to enumerate skin Tregs (i.e., Foxp3 staining in WT mice or GFP signal in Foxp3 reporter mice), we found that chronic EtOH feeding significantly reduced the number of dermal resident Tregs. The fact that this Treg depletion was not reflected by a change in the total size of the dermal αβ T cell pool (Fig. 2B) indicates that EtOH differentially impacts the maintenance of individual αβ T cell subsets in the dermis.
Fig. 3.
Chronic EtOH feeding reduces baseline numbers of dermal Tregs. Fresh dermal cell preparations from ear skin of mice fed EtOH for 12 to 16 weeks or age-matched water controls for both WT and FoxP3-GFP mice were counted and stained for flow cytometry. (A) Gating strategy for dermal Treg identification in both Foxp3-GFP and WT mice for representative H2O control mice. (B) Numbers of dermal Tregs/ear. Error bars represent SEM. *Post test p-value < 0.05. n ≥ 6 mice/treatment group. EtOH, ethanol; Treg, regulatory T cell; WT, wild-type.
EtOH-Induced Immune Cell Subset Loss Corresponds with Decreased Proliferation and Increased Apoptosis
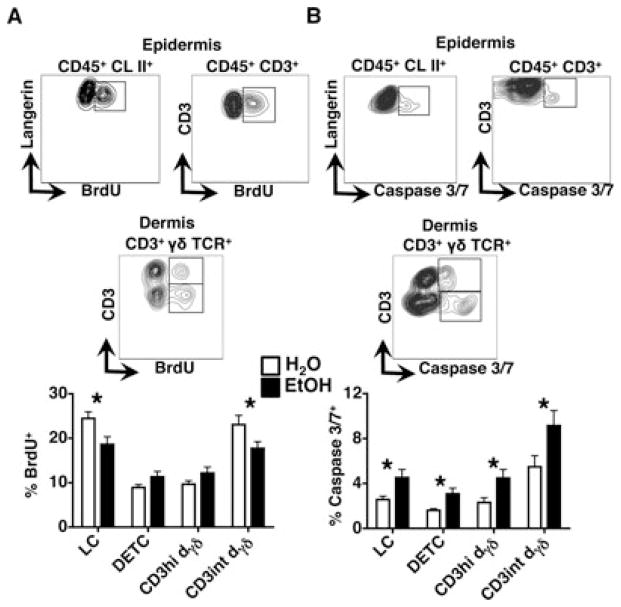
Given that in un-inflamed adult skin, normal numbers of LCs, DETCs, and dermal γδT cells are maintained through homeostatic repopulation, it is possible that the EtOH-induced depletion of these populations is due to diminished proliferation, or increased cell death (Chorro et al., 2009; Gray et al., 2011; Havran and Allison, 1988; Macleod and Havran, 2011). First, the impact of chronic EtOH feeding upon LC and DETC proliferation rates was assessed by administering BrdU to mice in the appropriate drinking liquid (i.e., EtOH solution or water), followed by the analysis of BrdU incorporation in epidermal cell suspensions. Importantly, multiple studies have used similar BrdU administration methods to assess the effects of chronic EtOH feeding upon the proliferation rates of other hematopoietic cells (Zhang and Meadows, 2005, 2009; Zhang et al., 2011). EtOH feeding differentially impacted BrdU incorporation rates of LCs and DETCs, with a significant reduction occurring in the former population but not the latter (Fig. 4A). Similar BrdU incorporation analysis of dermal T cells revealed that like DETCs, the proliferation rate of CD3hi dermal γδ T cells was not altered by EtOH feeding, while a significant reduction in proliferation was observed in CD3int γδ T cells (γδT17 cells). In light of the finding that the Vγ3+ T cell (DETCs and CD3hi dermal γδs) depletion occurred despite normal proliferation, investigation of EtOH’s impact upon additional cellular processes that could account for these losses was undertaken.
Fig. 4.
Decreased proliferation and increased apoptosis underlie the loss of skin T cells and LCs in chronic EtOH-fed mice. Fresh epidermal and dermal cell preparations from ear skin of mice fed EtOH for 12 to 16 weeks or age-matched water controls that received BrdU for the last 9 days were counted and stained for flow cytometry. (A) Gating strategy for BrdU incorporation in epidermal and dermal immune cell populations for a representative H2O control mouse (contour plots), and percentages of BrdU+ cells within the indicated populations. (B) Gating strategy for caspase 3/7 expression in epidermal and dermal immune cell populations for a representative EtOH mouse (contour plots), and percentages of caspase 3/7+ cells within the indicated populations. Error bars represent SEM. *Posttest p-value < 0.05. n ≥ 8 mice/treatment group. EtOH, ethanol; LCs, Langerhans cells.
Increased apoptosis could serve as the primary basis for the reduction in Vγ3+ T cells and could occur in combination with decreased proliferation to cause LC and CD3int γδ T cells. As shown in Fig. 4B, EtOH increased the number of caspase 3/7+ apoptotic cells in LC, DETC, CD3hi, and CD3int γδ T cell populations, indicating that poor survival serves as a commonmechanistic cause for immune cell subset loss in EtOH-exposed skin. Taken together, the data indicate that the numeric decrease in multiple self-renewing immune cell compartments (i.e., LCs, DETCs, and dermal γδT cells) corresponds with an EtOH-induced increase in basal levels of apoptosis. Whereas poor survival represents the major cause of DETC and CD3hi dermal γδ T cell depletion, elevated cell death together with decreased proliferation underlies the EtOH-induced loss of LCs and CD3int γδ T cells.
Hyporesponsive Phenotype in DETCs Following Chronic EtOH Feeding
Highly specialized for epidermal stress surveillance, DETCs have a unique ability to detect physiologic perturbations in their microenvironment. They express the immunoreceptors JAML and NKG2D, which confer the capacity to detect a myriad of antigens expressed by damaged or diseased KCs (Macleod and Havran, 2011). Within the context of local injury, DETCs mediate repair responses by secreting paracrine factors such as TNFα and IGF-1 that promote inflammatory cell recruitment and KC proliferation respectively (Macleod and Havran, 2011). To evaluate whether the EtOH-induced numeric loss of DETCs corresponds with impaired functionality of the remaining cells, the ability of DETCs to acquire phenotypic and functional attributes associated with activation and tissue repair was measured. To this end, expression of the activation markers CD69, NKG2D and JAML as well as production of IGF-1 and TNFα was measured on DETC in response to PMA/ionomycin stimulation. Figure 5 demonstrates that as expected, JAML expression and the number of cells producing TNFα and IGF-1 increased in the control groups following stimulation. (CD69 and NKG2D expression was not significantly higher following stimulation, likely because baseline expression of these molecules was quite high.) TNFα- and IGF-1-producing cells also increased in EtOH groups following stimulation, but JAML expression did not. In addition, the expression of JAML and CD69 by DETCs from EtOH mice in stimulated cultures was significantly lower than in stimulated cultures from water control mice. NKG2D and FGF-7 expression by DETCs as well as their capacity to produce IGF-1 and TNFα following stimulation was unaltered by EtOH, relative to stimulated controls (Fig. 5 and data not shown).
Fig. 5.
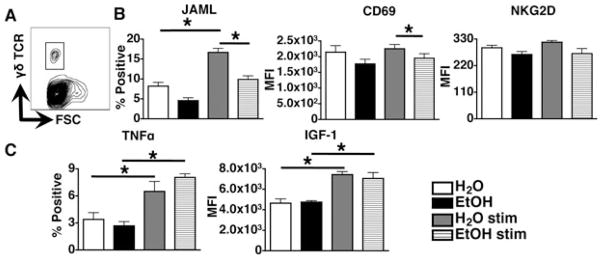
Chronic EtOH feeding induces hyporesponsiveness in dendritic epidermal T cells (DETCs). Epidermal cell suspensions prepared from mice fed EtOH for 12 to 16 weeks or age-matched water controls were cultured 4.5 hours − phorbol 12-myristate 13-acetate (PMA) and ionomycin. (A) Gating strategy for DETC identification in epidermal cultures for a representative H2O control mouse. (B) Percent-positive DETC values for JAML and MFI values for CD69 and NKG2D on gated DETCs for EtOH-fed or control mice. (MFIs rather than % positive cells are shown for CD69 and NKG2D because these molecules are constitutively expressed on DETC.) (C) MFI values and percent positive DETC for IGF-1 and TNFα respectively on gated DETCs. Error bars represent SEM. *Post test p-value < 0.05. n ≥ 8 mice/treatment group for assessment of JAML and CD69 expression and n ≥ 4 mice/treatment group for assessment NKG2D, TNFα and IGF-1 expression. EtOH, ethanol; IGF-1, insulin-like growth factor 1; MFI, mean fluorescent intensity; TNFα, tumor necrosis factor alpha.
Poor IL-17 Responses by Skin-Resident γδT17 Cells Following Chronic EtOH Feeding
IL-17 induction is critical for cutaneous host defense against numerous extracellular pathogens including S. aureus (Cho et al., 2010; Kagami et al., 2010). Recently, γδT17s were found to be the skin’s primary cellular source of IL-17 immediately after infection (Myles et al., 2013; Sumaria et al., 2011). Given the increased incidence and severity of S. aureus skin infection in alcoholics (Smith and Fenske, 2000), the impact of chronic EtOH feeding on IL-17 responses by γδT17s was investigated. Dermal cell suspensions from EtOH-fed and control mice were stimulated with anti-CD3 followed by cytoplasmic staining for IL-17A. IL-17 responses by γδTCRint γδT17s were significantly reduced in preparations from EtOH-fed mice, relative to water controls (Fig. 6). Therefore, like DETCs, γδT17s are both less abundant and functionally compromised as consequence of chronic EtOH exposure.
Fig. 6.
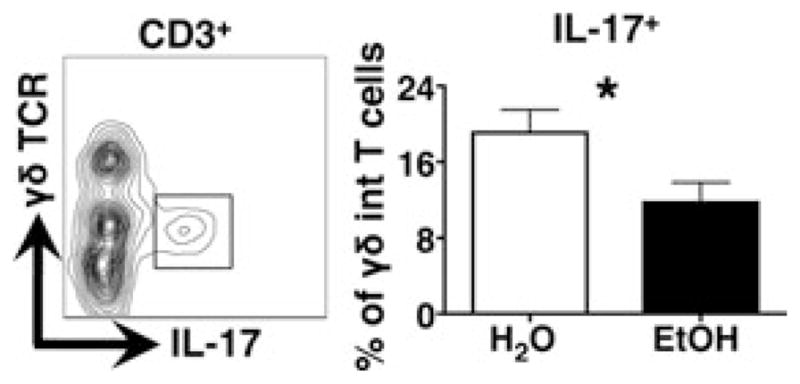
Chronic EtOH feeding reduces IL-17A production by dermal γδ T cells. Dermal cell suspensions prepared from mice fed EtOH for 12 to 16 weeks or age-matched water controls were cultured 4 hours − stimulation with plate-bound anti-CD3. IL-17A producing γδ T cells present in cultures prepared from EtOH-fed or control mice were identified according to gating strategy shown in the contour plot (from a representative H2O mouse). Error bars represent SEM. *Post test p-value < 0.05. n ≥ 8 mice/treatment group. EtOH, ethanol; IL-17A, interleukin 17A.
Poor IL-17 Responses by LN γδT17 Cells Following Chronic EtOH Feeding
The skin-draining LNs harbor both γδT17s and TH17 cells that possess the capability of producing IL-17 (Gray et al., 2011, 2013). To determine whether EtOH impacts IL-17 responses by skin-draining LN resident γδT17s and TH17 cells in a manner similar to that observed in the skin, inguinal LN cell suspensions were stimulated with anti-CD3 or PMA/ionomycin and assessed for IL-17 production. Similar to dermal resident γδT17s, EtOH feeding impaired the capacity of the LN γδ T cell population to produce IL-17 following ex vivo stimulation with either anti-CD3 or PMA/ionomycin. In contrast, IL-17 production by γδ TCR− T cells was unaltered by chronic EtOH feeding (Fig. 7).
Fig. 7.
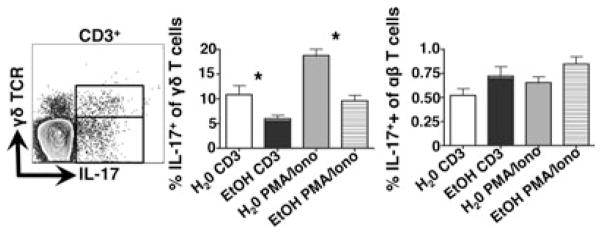
Chronic EtOH feeding reduces IL-17A production by lymph node (LN) γδ T cells, but not TH17 cells. LN cell suspensions prepared from mice fed EtOH for 12 to 16 weeks or age-matched water controls were cultured 4 hours − stimulation with plate-bound anti-CD3 or phorbol 12-myristate 13-acetate (PMA) and ionomycin. IL-17A-producing αβ or γδ T cells present in cultures prepared from EtOH-fed or control mice were identified according to gating strategy shown in the dot plot. Error bars represent SEM. *Post test p-value < 0.05. n ≥ 8 mice/treatment group. EtOH, ethanol; IL-17A, interleukin 17A.
DISCUSSION
The skin is an immune competent tissue that functions to prevent and remedy damage caused by harmful encounters with the environment. The capacity of chronic EtOH abuse to impair these functions is reflected by the heightened vulnerability of alcoholics to skin infection (Kaech et al., 2006; Rantala et al., 2009; Skogberg et al., 1988). Despite the well-documented clinical manifestations of alcohol-induced cutaneous immune deficiency, few studies have investigated its immunological basis. Using the Meadows–Cook murine model of alcoholism, we previously showed that chronic EtOH feeding causes subset loss and migratory dysfunction in skin DCs (Ness et al., 2008; Parlet and Schlueter, 2013). These results clearly demonstrate that chronic EtOH exposure alters numbers and function of cutaneous DCs and raise the question as to whether other components of the skin’s immune system are similarly affected.
Results from this investigation revealed that chronic EtOH feeding dramatically reduced the number of epidermal DETCs as well as LCs (Fig. 1). While the loss of the latter population is an established consequence of chronic EtOH feeding (Ness et al., 2008), the present study further clarifies the underlying mechanisms by showing that EtOH-induced LC depletion corresponds with decreased proliferation and increased apoptosis (Fig. 4). It is unlikely that altered trafficking of LCs or their precursors contribute to the loss of these cells in the steady-state epidermis. First, the infiltration of leukocytes (including BM precursors) into the postneonatal epidermis is rarely observed without severe inflammation (Chorro et al., 2009; Merad et al., 2002). Second, the number of dermal LCs (presumably en route to LNs) was previously found to be equivalent between EtOH-fed and control mice (Ness et al., 2008; Parlet and Schlueter, 2013). In aggregate, these results indicate that the loss of LC numbers is a consequence of EtOH-induced alterations that impair the proliferation and survival of LCs in the epidermis.
When compared to LCs, fewer mechanistic possibilities were found to explain DETC subset loss. In adult mice, Vγ3+ T cells are not detectable in extracutaneous tissues (e.g., thymus, draining LNs, other epithelia); therefore, alterations in DETC trafficking or thymic output of precursors could not account for the net loss of DETCs in EtOH-fed mice (Chorro et al., 2009; Havran and Allison, 1988; Macleod and Havran, 2011). Unlike LCs, chronic EtOH feeding did not impair the homeostatic proliferation of DETCs suggesting that their depletion in EtOH-fed mice is predominantly a function of increased cell death (Figs 1 and 4). These findings are reminiscent of the impact of chronic EtOH feeding on peripheral natural killer (NK) cells, which proliferate normally yet are unable to maintain appropriate numbers in mice chronically fed EtOH (Zhang and Meadows, 2009). Diminished trophic cytokine support was proposed to account for the loss of NK cells in this study (Zhang and Meadows, 2009), a mechanism that may likewise contribute to loss of LCs and DETCs after prolonged EtOH exposure. However, the fact that LC-deficient mice exhibit normal numbers of DETCs clearly disproves an essential role for LCs in DETC homeostasis (Taveirne et al., 2011).
Having established baseline numbers of DETCs in EtOH-fed and control mice, the impact of EtOH upon important functional attributes of these cells was evaluated. Chronic EtOH feeding was found to inhibit the up-regulation of JAML and a significant decrease in CD69 expression (Fig. 5). Given that cutaneous wound healing responses are greatly impaired in settings where JAML is unable to interact with cognate distress ligand (Witherden et al., 2010; Yoshida et al., 2012), the EtOH-induced decrease in the expression of this stress surveillance receptor would likely impair the ability of DETCs to detect and respond to tissue damage. Although EtOH did not impact NKG2D and FGF-7 expression, or the number of cells producing IGF-1 or TNFα, the reduced density of DETCs in EtOH-fed mice may compromise the immune surveillance and wound healing capabilities of the entire DETC network (Fig. 5 and data not shown).
The dermis is also highly enriched with T cells belonging to both γδ and αβ T lineages. While chronic EtOH feeding did not alter the total number of αβ T cells, a significant reduction in the number of dermal Foxp3+ Tregs was observed (Fig. 3). Given that peripheral DCs are involved in Treg induction/expansion pathways and chronic EtOH feeding causes defects in DC numbers and function, EtOH-induced alterations in DC/T cell interactions in the skin or skin-draining LNs may contribute to dermal Treg loss (Fan et al., 2011; Lau et al., 2009; Ness et al., 2008; Parlet and Schlueter, 2013). Additionally, Tregs are highly motile cells that routinely traffic into and out of the skin, raising the possibility that altered migration patterns underlie the EtOH-induced reduction in dermal Tregs (Tomura et al., 2010).
Among dermal γδ T cells, chronic EtOH feeding reduced the number of CD3hi and γδT17 T cell subsets. Interestingly, EtOH differentially impacted the dynamic cellular processes governing maintenance of these populations (i.e., proliferation and apoptosis). Measurements of BrdU incorporation revealed that decreased proliferation contributed to the loss of CD3int but not CD3hi γδT cells. However, analysis of caspase 3/7 staining showed EtOH increased basal levels of cell death in both populations (Fig. 4). Thymic generation of both Vγ3+ T cells and CD3int γδT cells occurs only during the fetal/neonatal period (Gray et al., 2011; Haas et al., 2012), and thus, thymic precursor output had long passed before adult EtOH feeding commenced in this study. Therefore, the EtOH-induced reduction in both subsets of dermal γδ T cells occurs independently of the thymus. Unlike γδT17s, which travel between the skin and draining LNs in both the steady and inflammatory states, CD3hi γδ T cells appear to be restricted to the skin indicating their depletion in EtOH-fed mice is primarily due to elevated apoptosis (Gray et al., 2013). It is unclear if altered trafficking behaviors of dermal γδT17s contribute to their net loss in EtOH-fed mice. However, no EtOH-induced alterations in either the total number of LN γδ T cells or subsets displaying phenotypic aspects associated with skin homing and IL-17 production (i.e., CD103, CCR6) were observed (Fig. S1).
For dermal CD3int γδT cells, EtOH-induced subset loss corresponded with impaired production of IL-17, which plays a critical role in host defense against extracellular pathogens such as S. aureus (Cho et al., 2010). IL-17 facilitates cutaneous immunity in part by triggering cytokine and chemokine production that promote neutrophil (PMN) recruitment, differentiation and survival. Thus, any EtOH-induced diminution of IL-17 production by dermal γδT17s would be predicted to correspond with poor PMN infiltration and pathogen eradication following certain skin infections. Inspection of IL-17 production in skin-draining LNs revealed that EtOH impaired IL-17 responses by γδ but not αβ T cells (Fig. 7). Poor IL-17 responses by γδ T cells may have serious implications for host defense. PMNs migrate from skin to interfollicular regions of the LN where γδ but not αβ T cells are concentrated (Kastenmüller et al., 2012). The diminished capacity of skin and LN resident γδT17s to produce IL-17 in EtOH-fed mice would be expected to impair both local clearance and systemic spread of invading pathogens, which is consistent with the heightened vulnerability of alcoholics to both skin infection and bacteremia (Kaech et al., 2006; Rantala et al., 2009; Skogberg et al., 1988).
In conclusion, the present study shows that chronic EtOH feeding negatively impacts the homeostasis and function of both epidermal and dermal T cell subsets. EtOH-induced defects within the skin T cell compartment include reduced numbers of skin-resident γδ T cell subsets including DETCs and CD3int γδT cells. Elevated basal levels of apoptosis and reduced proliferation are mechanistic causes of T cell subset loss in EtOH-fed mice. Additionally, EtOH-exposed DETCs and γδT17s exhibited impaired JAML up-regulation and IL-17 production, respectively. These findings provide the first information regarding the adverse effects of chronic EtOH feeding on skin T cells and build upon prior evidence of EtOH-induced immune cell alterations—subset loss and dysfunction of skin DCs (Ness et al., 2008; Parlet and Schlueter, 2013). When viewed together, these studies show that the predisposition of alcoholics to severe skin infection corresponds with a diminution of the cutaneous immune system that is characterized by an EtOH-induced inability to maintain appropriate cell numbers and function in key DC and T cell compartments.
Supplementary Material
Chronic EtOH feeding does not alter γδ T cell numbers in the skin-draining lymph nodes (LNs).
Acknowledgments
The authors thank Paul Naumann and the Department of Pathology Flow Cytometry Core for FACS Canto maintenance and support, and Ruth Coleman for laboratory administrative support. This work was supported by NIH R01 AA019568, and the Carver College of Medicine Department of Pathology.
Footnotes
Additional Supporting Information may be found in the online version of this article:
References
- Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang TZJ, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung ALCG, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system— a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion—absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Edsen-Moore MR, Turner LE, Cook RT, Legge KL, Waldschmidt TJ, Schlueter AJ. Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation. Alcohol Clin Exp Res. 2011;35:47–59. doi: 10.1111/j.1530-0277.2010.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Forsblom E, Ruotsalainen E, Mölkänen T, Ollgren J, Lyytikäinen O, Järvinen A. Predisposing factors, disease progression and outcome in 430 prospectively followed patients of healthcare- and community-associated Staphylococcus aureus bacteraemia. J Hosp Infect. 2011;78:102–107. doi: 10.1016/j.jhin.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Freyschmidt EJ, Mathias CB, Diaz N, MacArthur DH, Laouar A, Manjunath N, Hofer MD, Wurbel MA, Campbell J, Chatila TA, Oettgen HC. Skin inflammation arising from cutaneous regulatory T cell deficiency leads to impaired viral immune responses. J Immunol. 2010;185:1295–1302. doi: 10.4049/jimmunol.0903144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG. Deficiency in IL-17-committed Vγ4+ γδ T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J Leukoc Biol. 2009;85:34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E, Chennupati V, Föhse L, Naumann R, Weiss S, Krueger A, Förster R, Prinz I. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Hu S, Xiong N. Programmed downregulation of CCR6 is important for establishment of epidermal γδT cells by regulating their thymic egress and epidermal location. J Immunol. 2013;190:3267–3275. doi: 10.4049/jimmunol.1202261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech C, Elzi L, Sendi P, Frei R, Laifer G, Bassetti S, Fluckiger U. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006;12:345–352. doi: 10.1111/j.1469-0691.2005.01359.x. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009;30:13–22. doi: 10.1016/j.it.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci. 2011;68:2399–2408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan P, Ramu B, Bhaskar E, Venkataraman J. Prevalence and risk factors for bacterial skin infection and mortality in cirrhosis. Ann Hepatol. 2011;10:15–20. [PubMed] [Google Scholar]
- Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlet CP, Schlueter AJ. Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol Clin Exp Res. 2013;37:2098–2107. doi: 10.1111/acer.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J. Predictors of mortality in beta-hemolytic streptococcal bacteremia: a population-based study. J Infect. 2009;58:266–272. doi: 10.1016/j.jinf.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Schön MP, Schön M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol. 2002;119:190–193. doi: 10.1046/j.1523-1747.2002.17973.x. [DOI] [PubMed] [Google Scholar]
- Shen F-W. Monoclonal antibodies to mouse lymphocyte differentiation alloantigens. In: Hammerling GJ, Hammerling U, Kearney JF, editors. Monoclonal Antibodies and T-cell Hybridomas; Perspective and Technical Advances. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981. pp. 25–31. [Google Scholar]
- Skogberg K, Simonen H, Renkonen OV, Valtonen VV. Beta-haemolytic group A, B, C and G streptococcal septicaemia: a clinical study. Scand J Infect Dis. 1988;20:119–125. doi: 10.3109/00365548809032427. [DOI] [PubMed] [Google Scholar]
- Smith KE, Fenske NA. Cutaneous manifestations of alcohol abuse. J Am Acad Dermatol. 2000;43:1–16. doi: 10.1067/mjd.2000.104512. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol. 1978;71:167–171. doi: 10.1111/1523-1747.ep12547071. [DOI] [PubMed] [Google Scholar]
- Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+ CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J ExpMed. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveirne S, De Colvenaer V, Van Den Broeck T, Van Ammel E, Bennett C, Taghon T, Vandekerckhove B, Plum J, Clausen BE, Kaplan DH, Leclercq G. Langerhans cells are not required for epidermal Vgamma3 T cell homeostasis and function. J Leukoc Biol. 2011;90:61–68. doi: 10.1189/jlb.1010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, Waldmann H, Hori S, Cyster JG, Watanabe T, Miyachi Y, Kanagawa O, Kabashima K. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–893. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RETL, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Mohamed RH, Kajikawa M, Koizumi J, Tanaka M, Fugo K, Otsuka N, Maenaka K, Yagita H, Chiba H, Kasahara M. Involvement of an NKG2D ligand H60c in epidermal dendritic T cell-mediated wound repair. J Immunol. 2012;188:3972–3979. doi: 10.4049/jimmunol.1102886. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- Zhang H, Meadows GG. Exogenous IL-15 in combination with IL-15R alpha rescues natural killer cells from apoptosis induced by chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:419–427. doi: 10.1111/j.1530-0277.2008.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu Z, Meadows GG. Chronic alcohol consumption decreases the percentage and number of NK cells in the peripheral lymph nodes and exacerbates B16BL6 melanoma metastasis into the draining lymph nodes. Cell Immunol. 2011;266:172–179. doi: 10.1016/j.cellimm.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chronic EtOH feeding does not alter γδ T cell numbers in the skin-draining lymph nodes (LNs).