Abstract
Purpose of review
Here we present fetal genotoxicity and mitochondrial toxicity, induced by NRTIs, in HIV-1-infected pregnant women treated to prevent mother-to-child HIV-1 transmission (MTCT), and in virus-free pregnant patas monkeys.
Recent findings
In offspring of pregnant patas monkeys given human-equivalent NRTI protocols, aneuploidy was found in cultured bone marrow cells taken at birth, 1 year and 3 years of age. In some newborn human infants, offspring of HIV-1-infected mothers given zidovudine (AZT) therapy, aneuploidy, mitochondrial DNA (mtDNA) depletion, morphologically-damaged mitochondria, and reduction in cardiac left ventricular muscle were observed. NRTI-exposed human and patas umbilical cords had similar levels of mtDNA depletion and mitochondrial morphological damage. NRTI-exposed patas offspring showed a compensatory increase in heart mtDNA, and a 50% loss of brain mtDNA at 1 yr of age. Mitochondrial morphological damage and mtDNA loss were persistent in blood cells of NRTI-exposed infants up to 2 yrs of age, and in heart and brain from NRTI-exposed patas up to 3 yrs of age (human equivalent of 15 yr).
Summary
Whereas use of NRTIs in human pregnancy protects many thousands of children worldwide, some HIV-1-uninfected infants born to HIV-1-infected mothers receiving antiretroviral drug therapy sustain toxicities that may have adverse consequences later in life.
Keywords: AZT, aneuploidy, genotoxicity, mitochondrial DNA, electron microscopy
INTRODUCTION
Antiretroviral (ARV) drug combinations, which include 2-3 nucleoside reverse transcriptase inhibitors (NRTIs) and drugs of other classes, constitute standard of care therapy for individuals infected with the human immunodeficiency virus-1 (HIV-1) [1,2]. Each year in the United States ~10,000 HIV-1-infected pregnant women receive ARV drug combinations to prevent mother-to-child HIV-1 transmission (MTCT). This approach has saved many thousands of children worldwide from HIV-1 infection [2]. However, the HIV-1-uninfected children born to HIV-1-infected mothers (HEU children) are susceptible to manifestations of genotoxicity and mitochondrial toxicity likely due to ARV drug exposures occurring while in utero. This is not surprising since, in adult patients, mitochondrial toxicities may limit NRTI use [3], and the NRTI zidovudine (AZT) is a transplacental carcinogen in mice [4].
Like normal nucleosides, NRTIs become phosphorylated and incorporated into host nuclear and mitochondrial DNA, as well as cDNA of the virus [5]. However, the lack of a 3’-ribose-OH results in arrest of DNA replication, which has been documented in many species and cell types. The consequences to the cell include shortened DNA, mutagenesis, shortened telomeres, centrosome/spindle abnormalities, and aneuploidy as well as transplacentally-induced tumorigenesis in mice [4,6,7]. Whereas most HEU children, now in their teenage years, show no evidence of increased tumor risk, one study reported an elevated risk (SIR 3.1, p=0.05) of central nervous tumors in a few infants born to HIV-1-infected mothers receiving the NRTI combination AZT/didanosine (ddI) during pregnancy [8]. Transplacental AZT exposure has produced similar levels of AZT-DNA incorporation in newborn fetal tissues from mice and monkeys, and blood and placenta from humans [5]. Because the mice exposed to AZT in utero developed liver and lung tumors in middle age [4] there is a concern that NRTIs might be human transplacental carcinogens.
Mitochondrial dysfunction induced by NRTIs is considered to result from direct inhibition of the mitochondrial polymerase γ, as well as drug-DNA incorporation and arrest of DNA replication [9,10]. Clinical manifestations of NRTI use in human pregnancy [11], in HEU children, include neurological and cardiac effects evident in a subset of children. However, morphological and molecular manifestations of mitochondrial compromise, which have been frequently observed in children exposed to NRTIs in utero [12-14], include aberrant levels of mtDNA, mitochondrial morphological damage by electron microscopy (EM), and altered oxidative phosphorylation (OXPHOS).
This review will focus on genotoxicity and mitochondrial toxicity evident in HEU children born to HIV-1 infected mothers given ARV drugs for MCTC, and in parallel in Erythrocebus patas (patas) monkey offspring given human-equivalent ARV drug protocols. The patas NRTI metabolism and placentation are similar to humans [15], and because these monkeys do not become infected with an immunodeficiency virus, evaluation of drug toxicity can take place with no confounding retroviral effects.
GENOTOXICITY IN PATAS MONKEYS
Incorporation of NRTIs into host nuclear DNA has been shown to occur in many species and cultured cells [5]. With transplacental exposures, DNA incorporation of the most commonly-used NRTs, AZT and lamivudine (3TC), has been found in human blood cell and placental DNA, as well as in DNA from many organs of monkey and mouse offspring [5]. Incorporation of NRTIs into DNA has been shown to produce various manifestations of chromosomal distress, including centrosomal amplification (CA, >2 centrosomes/cell), micronucleus (MN) formation, and micronuclei containing whole chromosomes (MN+C), indicative of aneuploidy in the parent cell [7].
To examine these end points in the patas, pregnant dams were given 10 weeks of daily human-equivalent NRTI dosing that included AZT/3TC, AZT/3TC and Abacavir (ABC), or AZT/3TC and the non-NRTI Nevirapine (NVP), and the results were published previously. Offspring were evaluated at birth, 1 or 3 years of age (equivalent to 5 and 15 year old humans). To model human clinical exposures, the 1 and 3 year old patas received 6 wk of NRTI dosing post-birth. Mesenchymal cells were taken from bone marrow of patas at birth, 1 year and 3 years of age, cultured and scored. Compared to unexposed controls, significant increases in CA and MN were found in offspring exposed in utero to AZT/3TC when examined at birth, offspring exposed in utero to AZT/3TC/ABC when examined at 1 and 3 years, and offspring exposed to AZT/3TC/NVP when examined at 3 years [16].
Of particular importance is the MN+C end point (Figure 1 A), because aneuploidy or chromosomal loss may lead to cancer. Therefore, Figure 1A shows MN+C data from the patas study described above [16]. Offspring in all of the NRTI-treated groups, AZT/3TC at birth, AZT/3TC/ABC at 1 year, and AZT/3TC/ABC and AZT/3TC/NVP at 3 years, showed increased MN+C compared to the unexposed controls (p<0.05). Therefore, these drugs damage DNA indirectly by altering chromosome integrity and producing centrosomal post-mitosis aneuploidy. The long-term persistence of all these genotoxic events is consistent with a potential increased cancer risk in primates, and the transplacental tumorigenicity observed in offspring of mice exposed to AZT during gestation [4].
Figure 1.
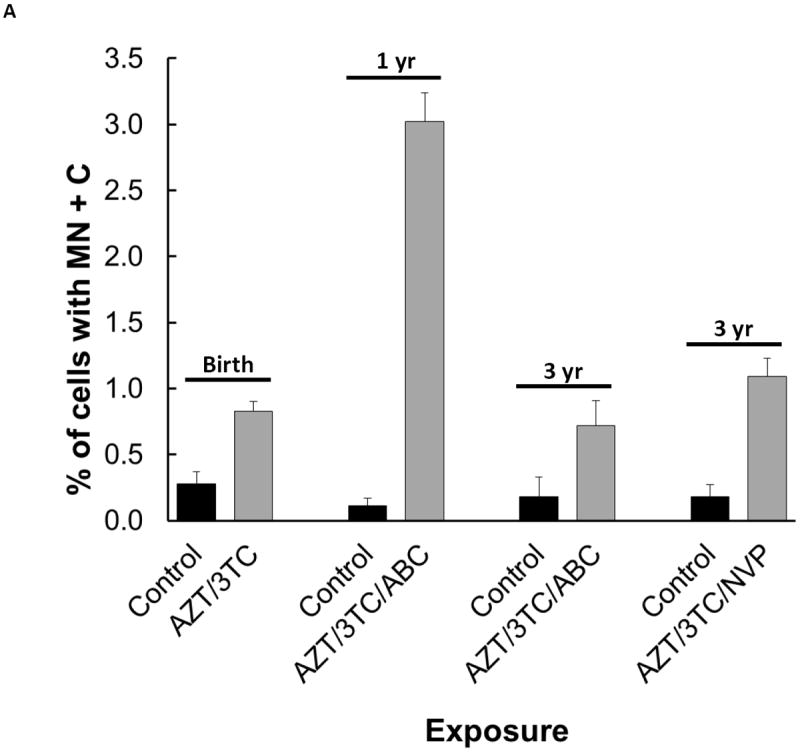
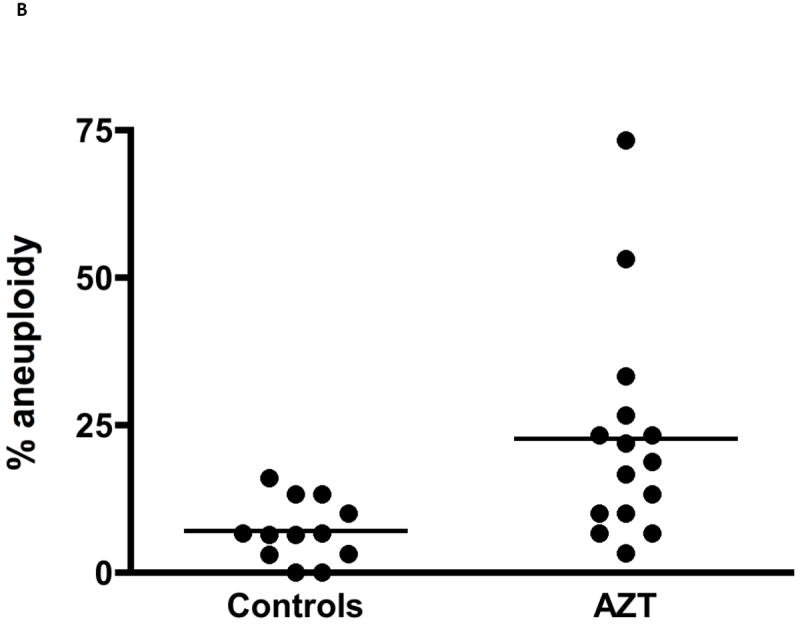
Aneuploidy observed as: (A) MN+C in cultured bone marrow cells from patas monkey offspring; and (B) percentage of human cord blood CD34(-) cells with aneuploidy by karyotype analysis of metaphase spreads. For (A), a graph using the data in [16], pregnant patas dams were given human-equivalent daily doses of ARV drugs, or no drugs, for the last 50% (10 wk) of gestation. Bone marrow cells, obtained from the offspring at birth (AZT/3TC), 1 yr of age (AZT/3TC/ABC) and 3 yr of age (AZT/3TC/ABC or AZT/3TC/NVP) were cultured and stained with: DAPI to reveal MN; and CREST antiserum to reveal centromeres within the MN. The Figure shows % of cells with MN+C (2-4 monkeys/group, 5000 cells/monkey), where control vs. NRTI-treated p values were ≤ 0.05 for each treatment. For (B), taken from [17], cord blood CD34(-) cells were obtained from 15 AZT-exposed pregnancies and 12 uninfected pregnancies, PHA-stimulated, cultured for 72 hr and karyotyped. The % of cells with aneuploidy, based on 30 metaphases from each individual, was significantly higher in the AZT-exposed group than in the unexposed group (p≤ 0.001).
GENOTOXICITY IN CHILDREN
The question of chromosomal aberration/aneuploidy in children born to HIV-infected mothers was addressed by Andre-Schmutz et al. [17]. Cord blood CD34(-) cells were collected from 15 HIV-1-infected mothers receiving AZT-based combinations, and 12 uninfected controls. Chromosomes were scored in 30 metaphases from each infant, to determine individual aneuploidy rate. A significant (p<0.001) increase in aneuploidy rate was seen in the HIV-1-infected pregnancies compared to the controls (Figure 1B), again supporting the hypothesis that aneuploidy is a consequence of transplacental NRTI exposure in primates, and an indicator of genotoxicity that may be associated with increased cancer risk.
MITOCHONDRIAL TOXICITY
Mitochondrial toxicity in children born to HIV-1-infected mothers receiving NRTI therapy has been hypothesized and observed for some time [11]. The cardiac insufficiency, skeletal muscle weakness and neurological effects seen in adult HIV-1-infected patients were recognized to be the result of mitochondrial compromise partially caused by chronic NRTI therapy. Early on it was considered that the duration of pregnancy was too short a time for NRTI-induced mitochondrial dysfunction to appear in the fetus. However, reports of mitochondrial morphological damage in umbilical cord [13], depletion of infant mtDNA [12], reduction in heart left ventricular (LV) muscle mass [18,19], and neurological compromise [14], all indicated that transplacental use of ARV drugs is not without consequences for the developing fetus.
CLINICAL MITOCHONDRIAL DYSFUNCTION
Clinically-evident mitochondrial dysfunction is seen infrequently in HEU children. However cardiac effects have been reported, including reduced cardiac growth in HIV-1-infected [19] and HEU children [18,20]. In HEU children, transplacental ARV drug exposure was associated with significantly-reduced LV mass and LV septal thickness, compared to children from uninfected mothers, and girls were more frequently affected than boys. In addition, when biomarkers of cardiac integrity were examined in 338 HEU children (median age 4.3 yr), 51% had at least one elevated biomarker [21]. Other clinical manifestations of mitochondrial dysfunction include blood lactate elevation, which is infrequent in HEU children [22], small reductions in neurodevelopmental and cognitive outcomes in HEU children [23], and increased susceptibility to infections [24], which, along with altered innate immune development in the first few months of life [25], suggest a compromised immune response in HEU children.
BIOMARKERS OF MITOCHONDRIAL COMPROMISE
Biomarkers indicative of mitochondrial and molecular compromise have been observed in human infants and in patas monkey offspring exposed in utero to NRTIs. Unlike the clinical mitochondrial dysfunction, which is seen infrequently, these manifestations have been found frequently in the human and patas monkey infants examined. Mitochondrial morphology, observed by EM, typically shows compromise of the mitochondrial membrane, resulting in rounding of the organelle, influx of proteolytic enzymes, and gradual erosion of the cristae [26]. Quantitative real time PCR (qRT-PCR)-based methods are most-commonly used to measure mtDNA [27], and whereas frequently NRTI-exposure induces mtDNA depletion [13,28], compensatory mtDNA amplification has also been observed [29]. Both damaged mitochondria and depletion of mtDNA have been observed in heart, brain, liver and placenta of NRTI-exposed patas monkey offspring at birth and 1 year of age [26,30,31], and there is persistence of mitochondrial morphological damage in brain and heart of 3 year old patas.
SOURCE OF MITOCHONDRIAL DYSFUNCTON
In children born to HIV-1-infected mothers it has been difficult to ascertain whether the presence of maternal HIV-1 infection, or the ARV drugs given to prevent MTCT, or both, are major causative events. It has been possible to address this issue using umbilical cord and cord blood, which are available from both human and monkey pregnancies [26]. Combinations of AZT/3TC and AZT/ddI were compared in HIV-1-infected pregnant women and in uninfected patas dams. Daily dosing was equivalent in both species, but the patas received drug for the last 50% of gestation, whereas the human recommendation is for drug to be given for the last 6 months of pregnancy. At birth, the degree of NRTI-induced mitochondrial morphological damage, by EM, was similar in the umbilical cord endothelial cells of both species, and similar reductions in cord blood mtDNA quantity were found in the NRTI-exposed human and monkey offspring, compared to the unexposed controls [26]. These studies show that, in the absence of virus, NRTIs may produce significant mitochondrial compromise.
mtDNA QUANTITATION
As mentioned previously, mtDNA quantity is often depleted in the presence of NRTI exposures, but it can also be abnormally increased in HEU children. The actual values obtained may vary based on the population, the assay conditions, the tissue used, and the region of mtDNA amplified. Figure 2 shows previously unpublished data demonstrating depletion in placental mtDNA from HIV-1 infected women (n=41), compared to placental DNA from uninfected women (n=48). These samples were collected in Medellin, Colombia, and all of the HIV-1-infected women received antiretroviral drug therapy during pregnancy and/or at the time of delivery. The placental mtDNA was quantified by a Hybrid Capture Chemiluminescence Assay [31]. There was no difference between the two groups by Wilcoxon rank-sum test (p=0.57). However, taking into consideration the fact that all of the samples from infected mothers had less mtDNA than the blood bank control sample (considered 100%), and 13 of the 48 samples from uninfected women had higher values than any of those from the 41 infected subjects, the groups can be compared by dichotomizing values above and below the control, which is set at 100%. This analysis could be biased because the control value was a blood bank sample, and the comparison determined post-hoc. However, the adjusted p value by Fisher’s Exact test was p<0.01, indicating significantly lower levels of mtDNA in the placentas from HIV-1-infected women, compared to uninfected women.
Figure 2.
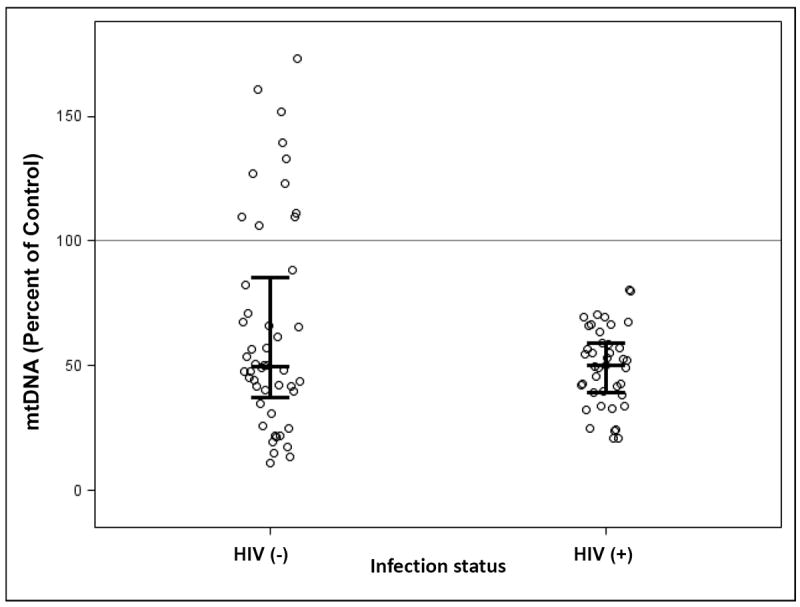
Placental DNA was prepared from 48 uninfected pregnancies, and 41 pregnancies in which the mother was HIV-1-infected and received ARV therapy. mtDNA quantity was determined by HCCA [31] in the 89 samples of placenta DNA and in blood bank DNA, which was designated 100% and used as control. All of the mtDNA values from HIV-1-infected/ARV-exposed pregnancies were lower than the control, and 13 (27%) of those from uninfected pregnancies were higher than the control. Although these results may be biased because dichotomizing the data at 100% was done post-hoc, the comparison (Fisher’s Exact test p < 0.01) showed that placentas from uninfected mothers had more mtDNA than placentas from HIV-1-infected mothers.
In another study [12], mtDNA was examined in cord blood taken at birth and peripheral blood taken at 1 and 2 years of age, from infants born to uninfected mothers, infants born to HIV-1-infected mothers taking no antiretroviral drugs, and infants born to HIV-1-infected mothers taking AZT during pregnancy. Compared to the unexposed controls, there was mtDNA depletion in the infants exposed in utero to HIV-1 alone. However even lower mtDNA levels were observed in the infants born to HIV-1-infected mothers who received AZT. Therefore, both the maternal HIV-1 infection and NRTI exposures contributed to the observed reduction in mtDNA levels in cord and peripheral blood [12].
LONG-TERM PERSISTENCE OF ARV-INDUCED TRANSPLACENTAL EFFECTS
The issue of long-term persistence of the NRTI-induced transplacental effects cannot be readily addressed in children, but can be examined in the patas monkey model. A patas monkey at 3 years of age is developmentally-equivalent to a 15 year old human adolescent, and a 1 year old patas is similar to a 5 year old child. The human-equivalent daily doses of ARV drug given to patas dams for the last 10 weeks of gestation were designed to model the recommended 6 months of therapy for prevention of human MTCT. As mentioned previously, the long-term persistence of aneuploidy and other genotoxic effects in the patas infants extended to at least 3 years of age [16]. In human children aneuploidy was observed birth [17] but persistence at later ages has not been investigated.
For analysis of mitochondrial toxicity, echocardiographic analysis revealed reduced LV muscle mass in some HEU children up to 5 years of age [20], but the long-term cardiac consequences will only become apparent as the children age. The monkey studies may be more informative as the offspring organs are available for study at any age. Using combinations of two NRTIs, we showed depletion of mtDNA at birth in patas heart, skeletal muscle, brain and liver [30,31]. Furthermore, at 1 year of age the brain mtDNA levels were only 50% of those found in the unexposed controls, while the heart mtDNA had increased in a compensatory fashion, largely due to the abnormal proliferation of damaged mitochondria [30,31]. Finally, ongoing EM studies with the patas show no reduction in the degree of damage induced by AZT/3TC/ABC or AZT/3TC/NVP in heart and brain at 1 and 3 years of age, indicating that the damage is not readily removed.
CONCLUSIONS
Use of ARV drugs in human pregnancy constitutes one of the most successful strategies in the war on HIV-1/AIDS, as many thousands of children worldwide are protected from HIV-1 infection. There are, however, increasing numbers of HEU children, who have developed in utero under the influence of these drugs. HEU children typically function without problem, though some clinical studies, and the biomarker and monkey evidence presented here (Table 1), suggest that there may be progressive changes that will compromise important organs, such as heart and brain, as aging occurs.
Table 1.
Comparison of biomarkers of genotoxicity and mitochondrial toxicity, between human infants exposed in utero to ARV drugs, and patas offspring exposed transplacentally for the last half of gestation to human-equivalent ARV-drug protocols.
| Biomarker | Patas infants (Dam virus free) | Human infants (Mother HIV-1-infected) |
|---|---|---|
| AZT-DNA & 3TC-DNA incorporation | Yes (DNA of all organs) | Yes (cord and peripheral blood DNA) |
| Centrosomal amplification | Yes | ? |
| Micronuclei (MN) | Yes | ? |
| Aneuploidy | Yes (to age 3) (MN+C, bone marrow) | Yes (birth) (blood karyotyping) |
| Abnormal Mitochondrial Morphology | ||
| Umbilical Cord | Yes | Yes |
| Heart | Yes (to age 3) | ? |
| Brain | Yes (to age 3) | ? |
| Abnormal mtDNA Quantity | ||
| Umbilical Cord (depletion) | Yes | Yes |
| Heart (depletion & compensation) | Yes (to age 3) | ? |
| Brain (depletion) | Yes (to age 3) | ? |
| Cord and peripheral blood (depletion) | Yes | Yes (to age 2) |
| Placenta (depletion) | Yes | Yes |
| Heart LV muscle loss | ? | Yes |
Key Points.
The NRTIs AZT and 3TC become incorporated into offspring nuclear and mitochondrial DNA when women and patas monkeys are treated with similar protocols during pregnancy.
Offspring of both species have blood cell aneuploidy at birth, and this persists in the patas for 3 years (equivalent to a 15 yr old human).
Mitochondrial morphological damage and mtDNA abnormalities are similar in NRTI-exposed patas and humans at birth, and persist in patas heart and brain up to 3 years of age.
Depletion of mtDNA (50%) was found in brains of 1 year old patas exposed in utero to mixtures containing 2 NRTIs.
The data suggest that some HIV-1-uninfected infants born to HIV-1-infected mothers receiving ARV drug therapy during pregnancy sustain toxicities that may produce adverse consequences later in life.
Acknowledgments
We wish to thank Seth M. Steinberg and David J. Liewehr, of the NCI Biostatistics and Data Management Section, for statistical analysis of the human mtDNA data.
Funding was provided by: the intramural research program of the Center for Cancer Research, National Cancer Institute, NIH; the French Agence Nationale de Recherche sur le SIDA; and, the Sustainability Program 2014-2015, Immunovirology Group, Universidad de Antioquia.
Footnotes
Conflicts of Interest: No conflicts of interest were declared.
References
- 1**.Heidari S, Mofenson LM, Bekker LG. Realization of an aids-free generation: Ensuring sustainable treatment for children. JAMA. 2014;312(4):339–340. doi: 10.1001/jama.2014.5806. Good description of UNAIDS goals with current references. [DOI] [PubMed] [Google Scholar]
- 2*.Govender T, Coovadia H. Eliminating mother to child transmission of hiv-1 and keeping mothers alive: Recent progress. The Journal of infection. 2014;68(Suppl 1):S57–62. doi: 10.1016/j.jinf.2013.09.015. Describes progress and challenges of eliminating MTCT in resource-poor countries. [DOI] [PubMed] [Google Scholar]
- 3.Feeney ER, Mallon PW. Impact of mitochondrial toxicity of hiv-1 antiretroviral drugs on lipodystrophy and metabolic dysregulation. Current pharmaceutical design. 2010;16(30):3339–3351. doi: 10.2174/138161210793563482. [DOI] [PubMed] [Google Scholar]
- 4.Olivero OA, Anderson LM, Diwan BA, Haines DC, Harbaugh SW, Moskal TJ, Jones AB, Rice JM, Riggs CW, Logsdon D, Yuspa SH, et al. Transplacental effects of 3’-azido-2’,3’-dideoxythymidine (azt): Tumorigenicity in mice and genotoxicity in mice and monkeys. Journal of the National Cancer Institute. 1997;89(21):1602–1608. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 5.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicology and applied pharmacology. 2004;199(2):151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 6.IARC. Some antiviral and antineoplastic drugs, and other pharmaceutical agents. Vol. 76. World Health Organization, International Agency for Research On Cancer; Lyon FR: 2000. [Google Scholar]
- 7.Borojerdi JP, Ming J, Cooch C, Ward Y, Semino-Mora C, Yu M, Braun HM, Taylor BJ, Poirier MC, Olivero OA. Centrosomal amplification and aneuploidy induced by the antiretroviral drug azt in hamster and human cells. Mutation research. 2009;665(1-2):67–74. doi: 10.1016/j.mrfmmm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benhammou V, Warszawski J, Bellec S, Doz F, Andre N, Lacour B, Levine M, Bavoux F, Tubiana R, Mandelbrot L, Clavel J, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22(16):2165–2177. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- 9.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. NatMed. 1995;1(5):417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 10.Copeland WC. Defects in mitochondrial DNA replication and human disease. Critical reviews in biochemistry and molecular biology. 2012;47(1):64–74. doi: 10.3109/10409238.2011.632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster C, Lyall H. Hiv and mitochondrial toxicity in children. The Journal of antimicrobial chemotherapy. 2008;61(1):8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 12.Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, Landay AL, Walker VE, Charurat M, Blattner WA. Long-term mitochondrial toxicity in hiv-uninfected infants born to hiv-infected mothers. J Acquir Immune Defic Syndr. 2003;33(2):175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Divi RL, Walker VE, Wade NA, Nagashima K, Seilkop SK, Adams ME, Nesel CJ, O’Neill JP, Abrams EJ, Poirier MC. Mitochondrial damage and DNA depletion in cord blood and umbilical cord from infants exposed in utero to combivir. AIDS. 2004;18(7):1013–1021. doi: 10.1097/00002030-200404300-00009. [DOI] [PubMed] [Google Scholar]
- 14.Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, Abzug MJ, Brady M, Jean-Philippe P, Hughes MD, Seage GR., 3rd In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in hiv-uninfected children. AIDS. 2007;21(8):929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 15.Divi RL, Doerge DR, Twaddle NC, Shockley ME, St Claire MC, Harbaugh JW, Harbaugh SW, Poirier MC. Metabolism and pharmacokinetics of the combination zidovudine plus lamivudine in the adult erythrocebus patas monkey determined by liquid chromatography-tandem mass spectrometric analysis. Toxicology and applied pharmacology. 2008;226(2):206–211. doi: 10.1016/j.taap.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 16**.Olivero OA, Torres LR, Gorjifard S, Momot D, Marrogi E, Divi RL, Liu Y, Woodward RA, Sowers MJ, Poirier MC. Perinatal exposure of patas monkeys to antiretroviral nucleoside reverse-transcriptase inhibitors induces genotoxicity persistent for up to 3 years of age. The Journal of infectious diseases. 2013;208(2):244–248. doi: 10.1093/infdis/jit146. Demonstrates aneuploidy in bone marrow cells of monkey infants up to 3 yrs of age, after in utero exposure to human-equivalent ARV drug protocols. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Andre-Schmutz I, Dal-Cortivo L, Six E, Kaltenbach S, Cocchiarella F, Le Chenadec J, Cagnard N, Cordier AG, Benachi A, Mandelbrot L, Azria E, et al. Genotoxic signature in cord blood cells of newborns exposed in utero to a zidovudine-based antiretroviral combination. The Journal of infectious diseases. 2013;208(2):235–243. doi: 10.1093/infdis/jit149. Demonstrates aneuploidy in cord blood cells of human infants exposed in utero to AZT and other ARV drugs for inhibition of MTCT. [DOI] [PubMed] [Google Scholar]
- 18.Lipshultz SE, Shearer WT, Thompson B, Rich KC, Cheng I, Orav EJ, Kumar S, Pignatelli RH, Bezold LI, LaRussa P, Starc TJ, et al. Cardiac effects of antiretroviral therapy in hiv-negative infants born to hiv-positive mothers: Nhlbi chaart-1 (national heart, lung, and blood institute cardiovascular status of haart therapy in hiv-exposed infants and children cohort study) J Am Coll Cardiol. 2011;57(1):76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipshultz SE, Mas CM, Henkel JM, Franco VI, Fisher SD, Miller TL. Haart to heart: Highly active antiretroviral therapy and the risk of cardiovascular disease in hiv-infected or exposed children and adults. Expert review of anti-infective therapy. 2012;10(6):661–674. doi: 10.1586/eri.12.53. [DOI] [PubMed] [Google Scholar]
- 20**.Lipshultz SE, Miller TL, Wilkinson JD, Scott GB, Somarriba G, Cochran TR, Fisher SD. Cardiac effects in perinatally hiv-infected and hiv-exposed but uninfected children and adolescents: A view from the united states of america. Journal of the International AIDS Society. 2013;16:18597. doi: 10.7448/IAS.16.1.18597. Recommends comprehensive cardiac evaluation in HIV-1-infected and HEU children born to HIV-1-infected mothers, since both groups are subject to heart LV aberration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Wilkinson JD, Williams PL, Leister E, Zeldow B, Shearer WT, Colan SD, Siberry GK, Dooley LB, Scott GB, Rich KC, Lipshultz SE. Cardiac biomarkers in hiv-exposed uninfected children. AIDS. 2013;27(7):1099–1108. doi: 10.1097/QAD.0b013e32835cf21c. 51% of HEU children (mean age 4.3 years) had at least one abnormal cardiac biomarker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crain MJ, Williams PL, Griner R, Tassiopoulos K, Read JS, Mofenson LM, Rich KC. Point-of-care capillary blood lactate measurements in human immunodeficiency virus-uninfected children with in utero exposure to human immunodeficiency virus and antiretroviral medications. The Pediatric infectious disease journal. 2011;30(12):1069–1074. doi: 10.1097/INF.0b013e318234c886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, Kanjanavanit S, Hansudewechakul R, Wongsawat J, Luesomboon W, Ratanadilok K, et al. Neurodevelopmental outcomes in hiv-exposed-uninfected children versus those not exposed to hiv. AIDS care. 2014;26(11):1327–1335. doi: 10.1080/09540121.2014.920949. HEU children have mild reductions in neurodevelopment compared to unexposed children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. Hiv-exposed uninfected children: A growing population with a vulnerable immune system? Clinical and experimental immunology. 2014;176(1):11–22. doi: 10.1111/cei.12251. HEU children have increased morbidity and mortality, compared to unexposed children, largely due to increased infectious disease incidences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Reikie BA, Adams RC, Leligdowicz A, Ho K, Naidoo S, Ruck CE, de Beer C, Preiser W, Cotton MF, Speert DP, Esser M, et al. Altered innate immune development in hiv-exposed uninfected infants. J Acquir Immune Defic Syndr. 2014;66(3):245–255. doi: 10.1097/QAI.0000000000000161. Innate immune response in HEU children is reversibly altered at 2-6 wk of age, corresponding to the timing of increased morbidity and mortality, and returns to normal after 6 wk of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divi RL, Leonard SL, Kuo MM, Nagashima K, Thamire C, St Claire MC, Wade NA, Walker VE, Poirier MC. Transplacentally exposed human and monkey newborn infants show similar evidence of nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity. Environmental and molecular mutagenesis. 2007;48(3-4):201–209. doi: 10.1002/em.20201. [DOI] [PubMed] [Google Scholar]
- 27.Cote HC, Gerschenson M, Walker UA, Miro O, Garrabou G, Hammond E, Villarroya J, Giralt M, Villarroya F, Cinque P, Garcia-Arumi E, et al. Quality assessment of human mitochondrial DNA quantification: Mitonauts, an international multicentre survey. Mitochondrion. 2011;11(3):520–527. doi: 10.1016/j.mito.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gingelmaier A, Grubert TA, Kost BP, Setzer B, Lebrecht D, Mylonas I, Mueller-Hoecker J, Jeschke U, Hiedl S, Friese K, Walker UA. Mitochondrial toxicity in hiv type-1-exposed pregnancies in the era of highly active antiretroviral therapy. Antiviral therapy. 2009;14(3):331–338. [PubMed] [Google Scholar]
- 29.Ross AC, Leong T, Avery A, Castillo-Duran M, Bonilla H, Lebrecht D, Walker UA, Storer N, Labbato D, Khaitan A, Tomanova-Soltys I, et al. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV medicine. 2012;13(2):98–106. doi: 10.1111/j.1468-1293.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divi RL, Einem TL, Fletcher SL, Shockley ME, Kuo MM, St Claire MC, Cook A, Nagashima K, Harbaugh SW, Harbaugh JW, Poirier MC. Progressive mitochondrial compromise in brains and livers of primates exposed in utero to nucleoside reverse transcriptase inhibitors (nrtis) Toxicological sciences : an official journal of the Society of Toxicology. 2010;118(1):191–201. doi: 10.1093/toxsci/kfq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Divi RL, Leonard SL, Kuo MM, Walker BL, Orozco CC, St Claire MC, Nagashima K, Harbaugh SW, Harbaugh JW, Thamire C, Sable CA, et al. Cardiac mitochondrial compromise in 1-yr-old erythrocebus patas monkeys perinatally-exposed to nucleoside reverse transcriptase inhibitors. Cardiovascular toxicology. 2005;5(3):333–346. doi: 10.1385/ct:5:3:333. [DOI] [PubMed] [Google Scholar]


