Abstract
Alarmins are a group of structurally diverse host defense antimicrobial peptides that are important immune activators. Here we present a novel role of two potent alarmins, human beta defensin 2 and 3 (HBD2 and 3) in promoting IFN-α production by human plasmacytoid DCs (pDCs). We demonstrate that HBD2 and 3 activate pDCs by enhancing the intracellular uptake of CpG and self DNA and promote DNA induced IFN-α production in a TLR9 dependent manner. Both CpG and host DNA form aggregates that resemble DNA nets when combined with HBD2 and 3. Isothermal Titration Calorimetry (ITC) studies to elucidate the nature of HBD3-CpG complexes demonstrates involvement of enthalpy driven interactions in addition to hydrophobic interactions with the formation of complexes at a molar ratio of 2:1 defensin/CpG. Intravenous administration of HBD3-CpG complexes induced proinflammatory cytokines like IL-12, IFN-γ, IL-6, IFN-α and IL-10 in serum associated with an increased recruitment of antigen presenting cells (APCs) in the spleen. Subcutaneous injections of these complexes showed enhanced infiltration of inflammatory cells at injection site indicating a potential pathophysiological role of alarmin/DNA complexes in contributing to inflammation. Intraperitoneal immunization of HBD3/CpG complexes with OVA enhanced both cellular and humoral responses in response to OVA as compared to OVA/HBD3 or OVA/CPG alone, indicative of a much more potent adjuvant effect of the HBD3/CpG complexes. Thus the ability of defensins to enhance cellular uptake of nucleic acids can lead to improved vaccine formulations by promoting their uptake by various cells resulting in an enhanced immune response.
Keywords: Defensins, interferon, plasmacytoid dendritic cells, inflammation, autoimmunity
Introduction
Human pDCs are a minor subset of peripheral blood dendritic cells (DCs) which display unique reactivity to bacterial, viral or self-nucleic acid sensing, resulting in copious IFN production. They are generally absent in peripheral tissues (in steady state conditions), but migrate to skin damaged by infection, injury, autoimmunity (lupus and psoriasis) and cancer. They recognize microbial and self-nucleic acids through endosomal receptors TLR 7 and 9 (1, 2). Activation of pDCs results in the indirect and/or direct activation of many other cell types, e.g., monocytes, myeloid DCs, B cells, NK cells, and T cells. Recent studies have shown that the functional interaction between pDCs and other immune cells may physiologically be critical in the regulation of both innate resistance and adaptive immunity to infections and host defense (3). Antimicrobial peptides like LL37 and beta defensins (BDs) are important host defense alarmins which recruit and activate a variety of cell types through multiple receptors. BDs are a subfamily of cationic antimicrobial peptides (AMPs), expressed by various epithelial cells and certain leukocytes. In addition to their antimicrobial abilities, these peptides are potent mediators of inflammation with stimulatory effects on epithelial and inflammatory cells, influencing cell proliferation, cytokine/chemokine production, and chemotaxis (4). Four human BDs (i.e. HBD-1, -2, -3, and -4) have been identified in human skin to date (5, 6). HBD3 is reported to activate monocytes through TLR 1 and 2, but the activating effects of other HBDs remains unknown (7).
Previous reports have highlighted the role of human cathelicidin (also known as LL37), an alarmin, in promoting microbial or self-nucleic acid uptake and IFN-α production leading to exacerbated skin inflammation in autoimmune diseases (8). Normally, self-nucleic acids released by damaged host tissue have little or no impact on innate immune response since they are unable to gain access to the endosomal compartments and TLR9. However, LL37 was shown to break innate tolerance to extracellular nucleic acids released by dying cells by forming complexes with the released self-nucleic acids and by promoting their transport into intracellular compartments containing TLR7 and TLR9, leading to chronic pDC activation and type 1 IFN production (2). Surprisingly, cathelicidin peptides released or induced during tissue damage were sufficient, but not required for pDC activation, suggesting redundancy of this pathway and the presence of additional factors that control the immunogenicity of extracellular host derived nucleic acids (2, 8). Hence, we hypothesized that in addition to LL37, BDs like HBD2 and 3 may be important contributors to ongoing inflammation and may promote uptake of nucleic acids and IFN-α production by human pDCs and promote activation of bystander myeloid DCs in the course of host defense and in chronic inflammatory conditions including autoimmune pathologies.
Materials and methods
Mice and Reagents
Female wild-type (wt) C57BL/6 mice, 6 to 8 weeks old, were provided by the Animal Production Facility of the NCI. NCI is approved by the American Association for the Accreditation of Laboratory Animal Care International and follows the Public Health Service policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council, Washington, DC).
Recombinant HBD2 and 3 were purchased from Peprotech and used at 10μg/ml unless otherwise indicated. Human genomic DNA isolated from placenta, skin or human leukocytes was purchased from BioChain (Thurmont, MD) and used at 20μg/mL. Human CpG ODN D 35 sequence 5’GGTGCATCGATGCAGGGGGG3’ and K3 sequence ATCGACTCTCGAGCGTTCTC (phosphorothioate nucleotides are underlined) were used at 1μM. For in vivo experiments mouse CpG ODN 1555 (GCTAGACGTTAGCGT) was used. ODNs were synthesized at the Center for Biologics Evaluation and Research core facility (Bethesda, MD). For some experiments FITC-conjugated-CpG ODNs were used. All antibodies used for flow cytometry analysis were purchased from BD/Pharmingen (San Diego, CA), including FITC-conjugated mouse antihuman CD83, FITC-conjugated mouse antihuman CD80, PE-conjugated mouse antihuman CD86, as well as FITC- and/or PE-conjugated, isotype-matched mouse IgG1, κ, IgG2a, κ and IgG2b.
Cell culture and treatments
Human peripheral blood enriched in mononuclear cells was obtained from healthy donors by leukapheresis (Transfusion Medicine Department, Clinical Center, National Institutes of Health, and Bethesda, MD, with an approved human-subject agreement). The blood was centrifuged through Ficoll-Hypaque; Sigma (St Louis, MO), and peripheral blood mononuclear cells (PBMCs) collected at the interface were washed with PBS and centrifuged through isoosmotic Percoll (Pharmacia, Uppsala, Sweden) gradient. The plasmacytoid dendritic cell subpopulation was purified by negative selection using The Plasmacytoid Dendritic Cell Isolation Kit II, Miltenyi Biotech, (Auburn, CA) and then further purified as BDCA4+ve lineage-vecells using fluorescence-activated cell sorting on a FACSAria (BD Bioscience) to reach 99% purity.
For IFN-α measurements, purified pDCs were plated at 0.7-1×106 cells/mL in RPMI+10% FCS and treated with either human genomic DNA (Biochain) or CpG ODN/s alone or in complex with various alarmins as indicated. Supernatants were collected after 18-24 hours for cytokine measurements and analyzed using a multiplex immunoassay system, (Aushon Biosciences). For flow-cytometry experiments CpG D35 or K3 FITC or human DNA labeled with Alexa 488 (Molecular Probes) was incubated with or without HBD2 or 3 for 30’ at RT. Preformed complexes were added to pDCs re-suspended in Binding medium (BM) (RPMI 1640 medium with 1% BSA). After 30 min, cells were washed with BM and analyzed by flow cytometry using a FACS Scan cytometer (BD Biosciences). For some experiments pDCs were plated in the presence or absence of defensins alone or in complex with CpG ODNs and analyzed by FACS for costimulatory molecules after 24 hrs. For some experiments enriched monocytes were obtained from PBMCs using a percoll gradient. Monocyte derived immature DCs (Mo-iDC) were generated as described previously (9). Subsequently, DC were incubated in fresh G4 (RPMI 1640 + 10% FBS + 50 ng/ml of GMCSF and IL-4) medium in the absence or presence of pDC supernatants for 24–48 h in a CO2 (5%) incubator.
Luciferase assays
HEK293 T cells were seeded on 6-well plates (2×105 cells per well) and transiently transfected with Fugene 6 (Roche) with either 250ng of TLR9 or TLR4 expression plasmids, 10ng of IRF7, and 250ng of IFNA4 luciferase reporter plasmid. Cells were harvested after 24 hours and replated in a 96-well plate and stimulated as indicated for 24h. CpG-D-ODN complexed with N-[1-(2,3-Dioleoyloxy)]-N,N,N-trimethylammonium propane methylsulfate (DOTAP) was used as a positive control for its ability to induce IFN-α in cell lines expressing TLR9/IRF7 (2). Renilla luciferase reporter gene (pRL-TK) was simultaneously transfected as an internal control for transfection efficiency. Luciferase activity was measured with a dual-luciferase reporter assay system (Promega). IRF7 and IFNA4 plasmids were a kind gift from Dr Michel Gilliet (University of Texas M. D. Anderson Cancer Center).
Confocal microscopy
Purified human pDCs were resuspended in RPMI medium supplemented with 2% FCS; GIBCO-Invitrogen (Carlsbad, CA). An 8 well LabTek chamber slide (NalgeNunc) was precoated using Cell-Tak (BD Biosciences) in alkaline PBS for 1h at 37°C. Slides were washed with water and left to dry for 30 minutes at room temperature. pDCs treated with Alexa 488 labeled DNA or with DNA-HBD3 complexes were fixed with 1% paraformaldehyde and added to coated well at 105 cells/well. Cells were left to adhere for 30 minutes at 37°C and stained with wheat germ agglutinin tetramethylrhodamine (Texas Red) to determine the cell contour. Cells were imaged using a 60X oil objective on an Olympus FV1000 confocal microscope at a digital zoom of 1X or 5X.
Isotheral Titration Calorimetry
The DNA-protein interactions were studied using an isothermal titration microcalorimeter iTC200 (GE Healthcare/Microcal, Northampton, MA) at 25°C or 37°C. The experiments were done either with DNA in the syringe (concentration 0.16 mM) and the protein in the calorimeter cell (concentration 0.022mM) or with protein in the syringe (concentration 0. 44 mM) and DNA in the calorimeter cell (concentration 0.044 mM). All experiments were done at least in duplicate.
In vivo treatments
Eight-week-old C57BL/6 (3 mice/group) were injected intravenously with 0.1 mL PBS alone, HBD3 (10 μg/mouse), mouse CpG K 1555 (5 μg/mouse) or preformed HBD3-CpG (5μg of CpG + 10μg of HBD3) complexes. Mice were euthanized after 24 hrs and serum and spleen cells were analyzed for cytokine profile by multiplex ELISA (Meso Scale Discovery) and cellular analysis respectively. For adjuvant experiments six-eight-week-old C57BL/6 mice (4 mice/group) were immunized intraperitoneally with either 50μg of OVA alone or in the presence of HBD3 (20μg/mouse) or CpG 1555 (10μg/mouse) or preformed CpG-HBD3 (10μg of CpG + 20μg of HBD3) complexes. Mice were boosted twice at day 10 and day 21 and euthanized one week after final boost. Serum was collected at day 14 and day 28 for determining OVA specific titers and spleens were collected for cellular analysis and cytokine measurements. For measuring OVA specific response after immunization (1×106/0.2 ml/well) cells from individual spleen from each immunized group were stimulated with 50μg/ml of OVA and supernatants were collected after 72 h and measured by multiplex ELISA (meso scale discovery)
For skin inflammation studies six-eight-week-old C57BL/6 mice (4 mice/group) were injected subcutaneously on right flank with 200 μl of sterile PBS alone, HBD3 (10μg/mouse), CpG (20μg/mouse) or CpG + HBD3 complexes. 24 hours later mice were euthanized using CO2 inhalation. Site of injection was examined and any abnormality was recorded. A 15 × 10 mm longitudinal section of skin surrounding the injection site was removed and fixed flat in 10% neutral buffered formalin. After fixation, two longitudinal sections were taken, through the midline and parasagittal, for each skin sample. Tissues were then routinely processed, paraffin-embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (HE). Stained sections were evaluated by a boarded veterinary pathologist.
Statistical analysis
Data were analyzed using paired two-tailed Student’s t-test comparing untreated or DNA or CpG treated samples with HBD2 or 3-treated or complex treated samples, using GraphPad Prism software (GraphPad Software, Inc). For some experiments one way ANOVA was used.
RESULTS
Recombinant human beta defensin 2 and 3 form complexes with host DNA and promote IFN-α production by pDCs
It has been reported previously that pDCs infiltrate injured tissue in both transient and chronic inflammation. Furthermore, HBD2 and 3 are highly expressed in ex vivo cultured and injured human skin as well as in chronic inflammatory diseases likes ulcerative colitis, rheumatoid arthritis and psoriasis. Since injured epithelium is an excellent source of host nucleic acids as well as HBD2 and 3, we hypothesized that both HBD2 and 3 may complex to DNA released by damaged tissues or cells and may promote IFN-α production by self DNA similar to LL37. As shown in Figure 1A recombinant HBD3 when mixed with human genomic DNA forms large complex DNA nets. To further assess whether these nets were able to bind to pDCs, Alexa 488 labeled DNA was mixed with HBD3 and the resulting complex was then added to pDC cultures. As seen in Figure 1B human DNA was readily detected bound to and internalized by pDCs by FACS analysis, when given in complex with HBD3, but not when delivered alone. Consequently HBD 3 was able to physically bind to DNA and form stable nets further enabling self-nucleic acid to bind to pDCs. Confocal analysis further confirmed that HBD3 promoted uptake of labeled DNA (green) by the pDCs (Fig.1C). Interestingly when the complexes were added to purified pDCs, substantial amounts of IFN-α were produced after 24 hours of treatment (Fig. 1D) and was dependent on the dose of DNA (Fig 1.E). Similarly, human beta defensin 2 (HBD2) another defensin with alarmin activity, also promoted rapid uptake of DNA (Fig. 2A) and subsequent IFN-α production by pDCs indicating a complex interplay between these endogenous mediators and nucleic acids (Fig. 2B).
Figure 1. HBD 3 interacts with self DNA and activates pDCs.
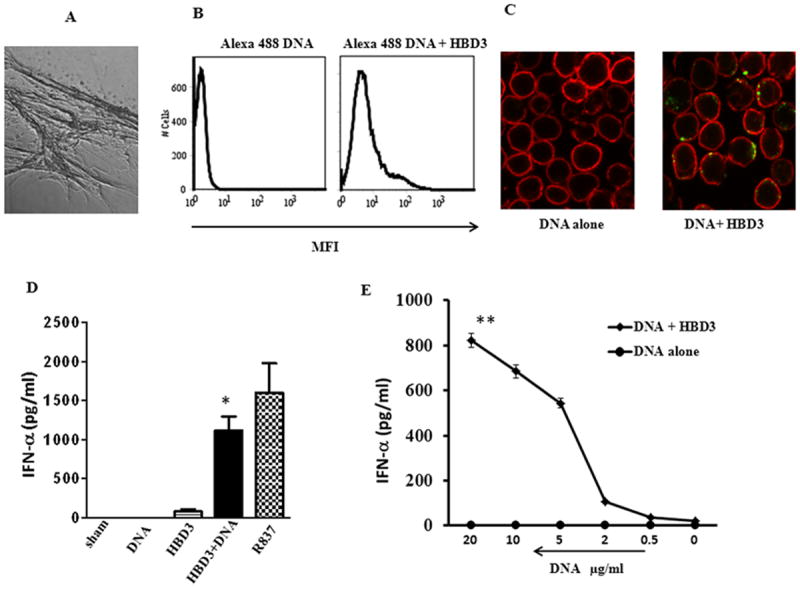
HBD3 (2μM) was mixed with genomic DNA from fetal skin (10μg/ml) for 30 minutes. Resulting complexes were analyzed light microscopy 40X (A). Results shown are one of 10 independent experiments. Human genomic DNA labeled with Alexa 488 (5μg) was mixed with HBD3 (1μM) for 30 minutes at RT and added to pDC cultures. Cells were analyzed after 30 minutes by (B) FACS. One representative experiment out of five is shown or (C) Confocal. Alexa 488 labeled DNA is in green. Cell contour is in red. One representative experiment out of four is shown. (D) HBD 3 (2μM) or genomic DNA (10μg/ml) was added to pDC cultures either alone or after complex formation. IFN-α levels were measured after 24 hrs. One representative experiment out of six is shown. (E) Different doses of human genomic DNA were allowed to form complexes with HBD 3 (2μM) and resulting complexes were added to pDC cultures. IFN-α levels were measured after 24 hrs. One representative experiment out of three is shown. Statistical significance was determined using Student’s t-test. Error bars indicate SEM. ** P <0.01
Figure 2. HBD2 binds self DNA and induces IFN production by pDCs.
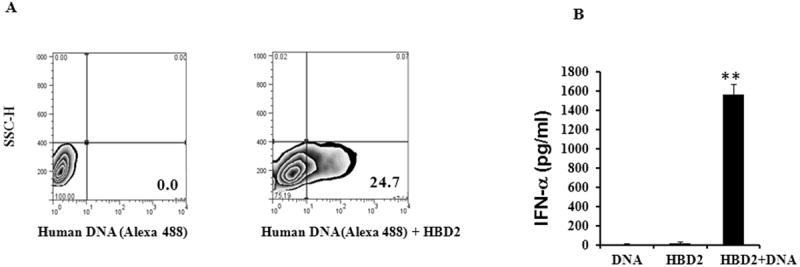
(A) Human genomic DNA labeled with Alexa 488 (5μg) was mixed with HBD2 (1μM) for 30 minutes at RT and added to pDC cultures. Cells were analyzed by FACS after 30 minutes. One representative experiment out of five is shown. (B) For IFN-α measurements HBD2 (2μM) was mixed with genomic DNA from fetal skin (10μg/ml) for 30 minutes. Resulting complexes were added to pDC cultures and supernatants were collected after 18-24 hrs and IFN-α was measured. Statistical significance was determined using Student’s t-test. Error bars indicate SEM. One representative experiment out of five is shown. ** P <0.001
On the contrary DNA, HBD2 or HBD3 by themselves did not induce IFN-α production (Fig. 1D and 2B). This suggested that complexes of HBD2 or HBD3 with self-DNA became potent activators of pDC activation when transported into TLR-containing endosomes.
Interferon alpha production by HBD3-DNA complexes is TLR9 dependent
Since pDCs sense DNA through TLR9 in endosomes, we examined whether TLR9 was involved in recognition of self DNA-HBD3 complexes. As seen in Fig. 3A, HBD3–DNA complexes activated the IFN-α A4 promoter in IRF-7/TLR-9-transfected HEK293 cells, but not in TLR4 expressing cells. Additionally, pretreatment of pDCs with suppressive ODN A151 (TTAGGG)4 (which inhibits multiple forms of immune activation including CpG-induced stimulation via TLR9) specifically inhibited IFN-α induction by HBD3–DNA complexes (Fig. 3B). Thus the defensin DNA complexes clearly activate human pDCs via TLR9.
Figure 3. HBD3-DNA mediated IFN-α production is dependent on TLR9.
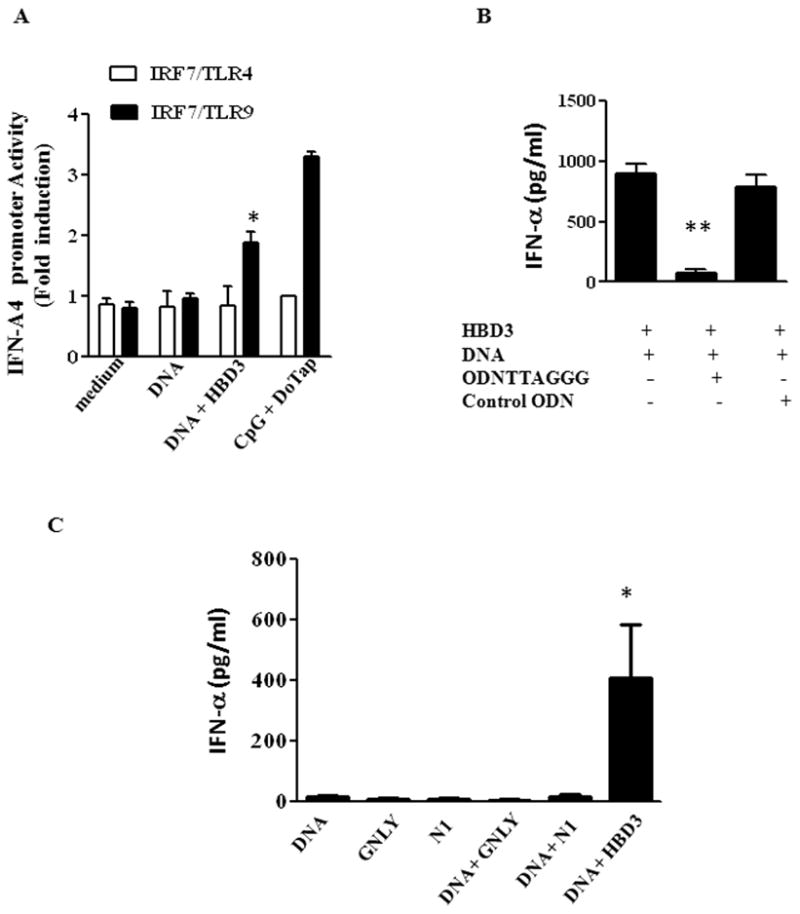
(A) IFN A4 promoter activity in HEK cells transfected with IRF7/TLR4 or IRF/TLR9 after treatment with HBD3/DNA complexes. CpG/DoTap was used as positive control. One representative experiment out of two is shown. Error bars indicate SEM. * P<0.05, ** P <0.01. (B) pDCs were pretreated with either ODN TTAGGG or control ODN (10:1 ODN/DNA ratio) before adding HBD3-DNA complexes. One representative experiment out of two is shown. (C) pDCs were treated with indicated alarmins in the presence or absence of DNA. IFN-α levels were measured after 24 hrs. One representative experiment out of two is shown. Error bars indicate SEM. (ANOVA one-way analysis of variance)* P<0.05** P <0.001
To validate whether this enhancement of IFN-α production was due to their high cationic charge or was an intrinsic property of HBD2 and 3 we treated pDC with DNA complexed with another antimicrobial peptide granulysin, a highly positively charged molecule and another nuclear binding protein HMGN1 which activate APCs through TLR4 (9, 10) Surprisingly, we did not observe any enhancement of DNA induced IFN-α by either granulysin or HMGN1 (Fig. 3C) suggesting that the bimolecular interaction between HBD2 or 3 and DNA was based on more than their positive charge.
Recombinant HBD2 and 3 both enhance CpG binding/uptake and IFN-α production by pDCs
To further elucidate the effect of HBD2 and 3 on pDC activation we used a human D mix CpG oligonucleotide, a much more potent stimulus than self DNA, to activate human pDCs. Plated cells were incubated with CpG-D mix either alone or complexed with HBD2 or 3 as described in Methods. Culture supernatants were analyzed for IFN-α production after 24 hrs.
As reported previously, CpG-D stimulated the production of substantial amounts of IFN-α (Fig. 4A). However when CpG-D ODN was complexed with HBD3, production of IFN-α was increased several fold (Fig. 4A). This effect was also observed with HBD2 (Fig. 4B). On the contrary, both HBD2 and HBD3 were unable to induce IFN-α production by pDCs on their own, indicating their capacity to activate required complex formation with nucleic acids (Fig. 1D and 2B).
Figure 4. HBD3 forms complexes with CpG and enhances CpG induced IFN-α.
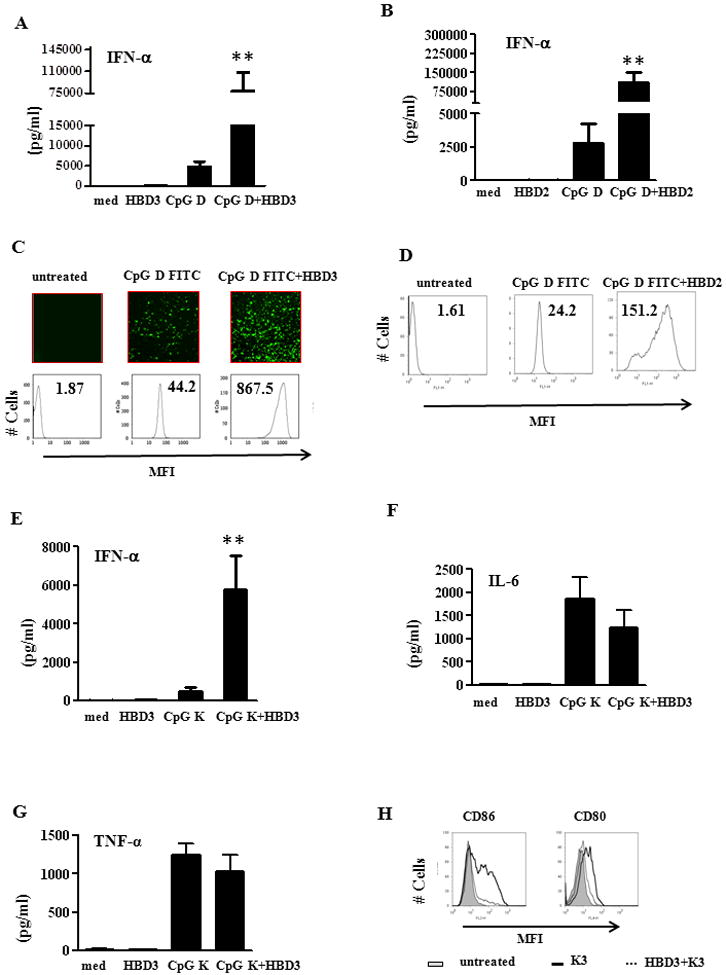
(A) HBD3 (2μM) or CpG ODN D mix was added to pDC cultures either alone or after complex formation. IFN alpha levels were measured after 24 hrs. One representative experiment out of six is shown. Error bars indicate SEM. (statistical significance was measured using student t test). (B) IFN-α levels from pDCs treated with either HBD2 or CpG D alone or after complex formation. One representative experiment out of five is shown (C) CpG ODN D35 labeled with FITC (1μM) was mixed with HBD3 (2μM) for 30 minutes at RT and added to pDC cultures. Cells were analyzed by light microscopy and FACS for internalization. One representative experiment out of five is shown (D) FITC labeled CpG (1μM) was mixed with HBD2 (2 μM) for 30 min at RT and added to pDCs. Cells were analyzed by FACS after 30 minutes. One representative experiment out of five is shown. CpG K3 (1μM) was complexed with HBD3 (2μM) for 30 min and added to pDCs cultures. After 24 hrs supernatants were measured for (E) IFN-α (F) IL-6 and (G) TNF-α. (H) CpG K3 (1μM) was complexed with HBD3 (2μM) and added to pDCs cultures. Cells were analyzed by FACS for CD86 and CD80 respectively after 24 hrs. One representative experiment out of three is shown Error bars indicate SEM. (statistical significance was measured using student t test). * P<0.05, ** P <0.001
We observed that both HBD2 and 3 formed large microparticles when complexed with CpG D-35 labeled with FITC (Fig. 4C (top panel) and data not shown). These complexes were highly stable and promoted rapid internalization of CpG when associated with either HBD3 (Fig. 4C or HBD2 (Fig. 4D). Since type D ODNs (also known as A type) are potential IFN-α producers due to their retention in early endosomes, we investigated whether HBD 3 could associate with CpG K (a type B ODN), which generally activate pDCs to induce phenotypic maturation and TNF-α and IL-6 secretion. We observed that HBD3 forms similar aggregates when incubated with CpG K3 and further promoted IFN-α secretion by pDCs (Fig. 4E) rather than IL-6 or TNF-α (Fig. 4F and G). Consequently these complexes also down regulated CpG K induced phenotypic maturation of pDCs as seen in Figure 4H, indicating that the K type ODNs were converted to IFN-α producers by HBD3.
HBD3 induced IFN-α production by pDC activates myeloid DCs
Type 1 interferons produced by pDCs are not only instrumental in inhibiting virally infected cells but also in activating other cells types including dendritic cells. Tissue damage coupled with a dysregulated epithelial expression of HBD3 may elicit the sustained accumulation and activation of pDCs in inflammatory conditions like psoriasis. Consequently, aberrant production of IFNs may lead activation of bystander myeloid dendritic cells that initiate local T-cell-mediated autoimmune inflammation. Therefore, we treated human monocyte derived DCs (MoDCs) with supernatants of pDCs treated with either HBD3 alone or in combination with DNA or CpG ODNs. As shown in Figure 5, supernatants of pDCs from cells treated with complexes of HBD3 with self DNA or CpG K induced maturation of MoDCs as evident by enhanced surface expression of costimulatory molecules when compared to supernatants from cells treated with HBD3 or DNA or CpG K alone. This further confirmed that HBD3 expression and release during tissue damage or infection may regulate or contribute towards an ongoing inflammation by binding to self DNA or viral nucleic acids.
Figure 5. Supernatants of pDC treated with HBD3-DNA complexes activate bystander myeloid cells.
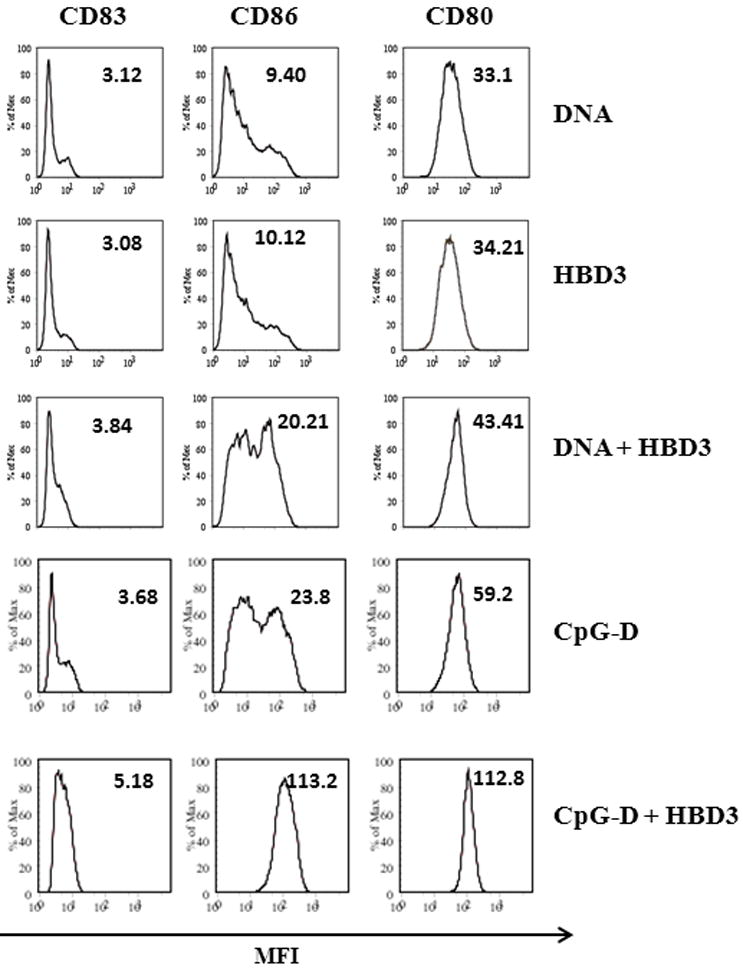
MoDCs were cultured for 48 hrs with supernatants of pDCs treated with either DNA (10μg/ml) or CpG ODN D type (1μM) or HBD3 (2μM) alone or in combination as shown. Cell surface expression of co- stimulatory molecules was measured by FACS. One representative experiment out of two is shown
HBD3 complexes to CpG D in a 2:1 molar ratio
Since thermodynamic studies provide relevant energetic information to understand the stability and specificity of protein-nucleic acid interaction (11, 12) we performed ITC experiments to elucidate the nature of the complexes formed between CpG D35 and HBD3. ITC directly measures the heat released or absorbed during a biomolecular binding event. Measurement of this heat allows accurate determination of binding affinity (KA), reaction stoichiometry (n), free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), thereby providing a complete thermodynamic profile of the molecular interaction in a single experiment. Binding isotherms were generated using CpG D ODN as a ligand and HBD3 as an acceptor (“direct” titration) and vice versa (“reverse” titration). Each isotherm peak represents a heat change associated with the injection of a small volume of ligand into the ITC reaction cell. As successive amounts of the ligand are titrated into the ITC cell, the quantity of heat absorbed or released is in direct proportion to the amount of binding ligand. As the system reaches saturation, the heat signal diminishes until only heats of ligand dilution are observed. A binding curve was then obtained from a plot of the heats from each injection against the ratio of ligand and binding partner in the cell (Fig. 6). The binding curves were analyzed with the appropriate binding model.
Figure 6. Isothermal titration calorimetry (ITC) experiment with DNA CpG ODN35 binding to protein HBD3.
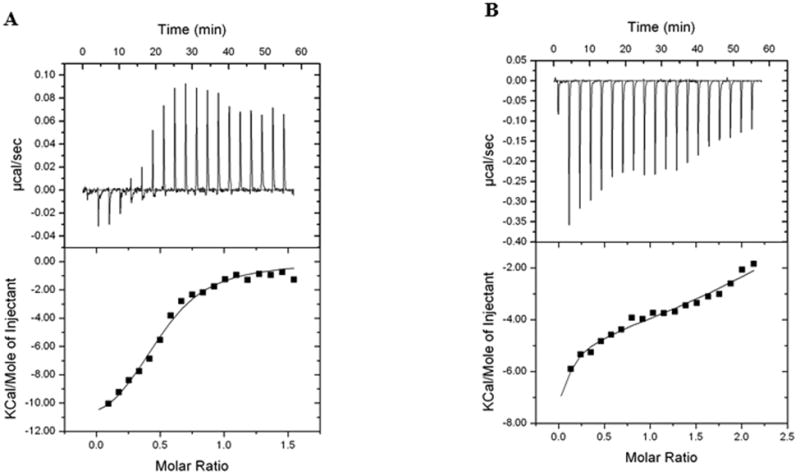
The exothermic binding isotherm (above) was integrated to give the enthalpy change plotted as a function of the molar ratio of DNA/protein (panel A) or protein/DNA (Panel B). The integrated enthalpy change data were used to calculate the binding affinity. One representative experiment out of two is shown.
The direct titration (Fig. 6A) was satisfactorily fitted with a basic “One Set of Sites” model. The binding affinity KA had a value of 4.5E5 ± 0.8E5 M-1, ΔH was -1.270 E4 ± 816.9 cal/mol, ΔS was -16.7 cal/mol/deg (all data for 25 degrees). The stoichiometry (a number of binding sites available on each molecule in the cell) was close to 0.5, i.e. two molecules of protein associated with one DNA molecule.
The relatively high enthalpy value (compared with TΔS input) indicates the enthalpy-driven nature (resulting generally from H-bonding, van der Waals forces, etc.) of binding mechanism, while the entropy contribution, generally an input from hydrophobic interactions, still prevails.
Interestingly, the reaction temperature increase from 25°C to 37 °C did not change the binding affinity, keeping the enthalpy-entropy compensation at the same level, (data not shown), though ΔH underwent typical growth, indicating increased bond formation.
One could expect for the reverse titration (protein solution titrated into DNA) the stoichiometry to be around 2, as a reciprocal to 0.5. But the observed thermogram’s (Fig. 6B) appearance represents more complicated mechanism than just simple “One set of sites” model which worked quite well for direct titration. The search for better fit, in assumption of presence of at least of two sequential binding processes, produced a set of data helping better understand how the binding happens. The first binding step had KA around 1.0E4 M-1, the second one – around 4.9E5 ± 0.5E5 M-1. The major finding here is that the second value statistically matches to the KA value for direct titration (4.5E5 M-1). It is important that the stoichiometry value for this step, though cannot be determined precisely due to software limitations, can be graphically placed in the range 1.4 – 1.8, i.e. tends to stoichiometry 2 as a reciprocal to value 0.5 for direct injection. In addition to that, the sum of enthalpies for two steps of reverse titration gives ΔH = -0.97E4 cal/mol, which statistically is similar to the enthalpy for direct titration.
HBD3/CpG complexes act as proinflammatory mediator and enhance antigen specific immune responses in vivo
It is well documented that in systemic autoimmune diseases like SLE, self-DNA-alarmin complexes are found to be a constituent of the circulating immune complexes isolated from patient sera (13, 14). These complexes further exacerbate the severity of the diseases and contribute to excessive inflammation by enhancing the stability and uptake of self DNA and subsequent immune activation. Therefore to further elucidate the role of defensin/DNA complexes in promoting inflammation in vivo we administered HBD3 complexed with suboptimal doses of CpG ODN1555 systemically in C57/B6 mice and analyzed the cytokine profile in serum and changes in spleen cell populations after 24 hrs. Similar to the human CpG ODN, HBD3 formed stable complexes with mouse CpG ODN1555 (data not shown) and served as a model to the floating immune complexes found in autoimmune inflammatory conditions. As expected mice immunized with HBD3-CpG complexes dramatically increased the immunostimulatory potential of CpG ODN1555 and significantly enhanced the production of inflammatory cytokines including IL-6, IFN-γ, IL-12p70, IL-10 and IFN-α in the serum (Fig.7A-E). Subsequently, we observed a two to three fold increase in F480 +ve macrophages, CD11c+ve DCs and Ly6C+ve PDCA1+ve pDCs in the spleen. No significant changes were observed in mice immunized with CpG alone (Fig. 7F).
Figure 7. HBD3-CpG complexes act as proinflammatory mediator in vivo.
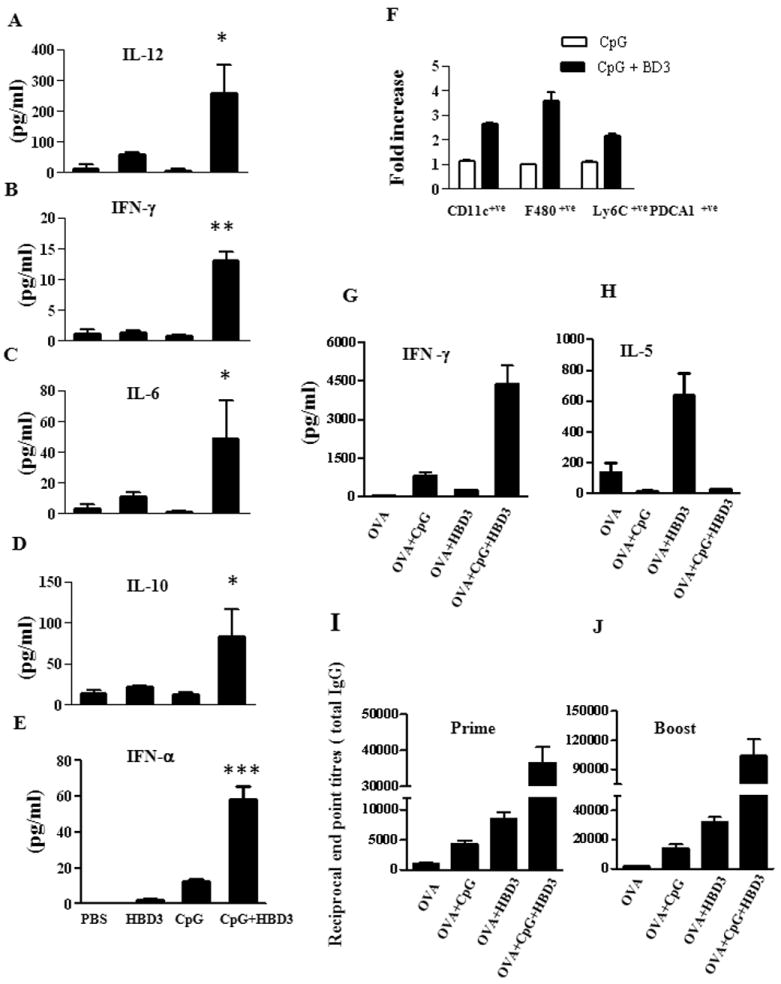
C57/BL6 mice (3 mice per group) were treated intravenously with PBS alone or HBD3 (10μg/mouse) or CpG (5 μg/mouse) or HBD-CpG complexes (HBD3/CpG molar ratio 2:1). (A-E) Serum cytokines profile after 24 hrs of immunization. (F) The indicated subpopulations of APCs were determined by flow cytometry and calculated as fold increase induced by HBD3-CpG over the CpG alone group. One representative experiment out of two is shown. C57/BL6 mice (4 mice per group) were immunized with either OVA alone or in the presence of HBD3 or CpG ODN1555 or preformed CpG-HBD3 complexes. Mice were boosted twice at day 10 and day 21 and euthanized one week after final boost. Splenocytes from individual mice were taken after the final boost and treated ex vivo with 50μg/ml of OVA and supernatants were collected after 72h and IL-5 (G) and IFN-γ (H) were measured by multiplex ELISA. Total IgG levels in serum from each group of mice were measured after 1st (I) and booster immunization (J). Error bars indicate SEM. (ANOVA one-way analysis of variance). * P<0.05, ** P <0.01, *** P <0.001
To further analyze whether these complexes were able to enhance induce antigen specific immune responses we immunized mice intraperitoneally with OVA alone or in the presence of HBD3 or CpG ODN1555 or preformed CpG-HBD3 complexes. Mice were boosted as indicated in methods section. Serum was collected for determining OVA specific titers and individual spleens were analyzed for cytokine measurements. Splenocytes from mice immunized with OVA in the presence of CpG-HBD3 complexes induced at least 3-5 fold higher IFN-γ (Fig.7G) suggestive of a Th1 response. In contrast mice immunized with OVA+HBD3 in absence of CpG induced high levels of IL-5 (Fig. 7H) as observed before; HBD3 promotes Th2 responses (Tewary et al unpublished observations), no significant changes were observed in mice immunized with OVA+ CpG or with OVA+CPG+HBD3 complexes. Nevertheless, we observed a 5-10 fold increase in serum anti-OVA antibody titers in group immunized with OVA in combination with preformed complexes in comparison to mice immunized with OVA+CpG or OVA+HBD3 (data not shown). These data indicated that HBD-CpG complexes enhanced OVA-specific cellular and humoral immune responses and had adjuvant activity.
HBD3-CpG complexes induce skin inflammation
Eight week old mice (4 mice per group) were injected subcutaneously on right flank with either PBS alone or HBD3 (10μg/mouse) or CpG (20μg/mouse) or CpG+HBD3 complexes. 24 hours later mice were euthanized and skin sections were analyzed for recruitment of inflammatory cells. As seen in Figure 8 we observed an increased epidermal hyperplasia and inflammation in mice injected with CpG-HBD3 complexes as compared to either CpG or HBD3 alone. Skin sections of mice injected with HBD3 alone showed increased infiltration of inflammatory cells at the site of injection which correlated with our previous observations. However, HBD3-CpG complexes induced more severe inflammation with enhanced infiltration of neutrophils and lymphocytic cells as observed by H&E staining, thus indicating that persistence of nucleic acid - HBD3 complexes may initiate and augment inflammatory conditions.
Figure 8. HBD3-CpG complexes induce skin inflammation.
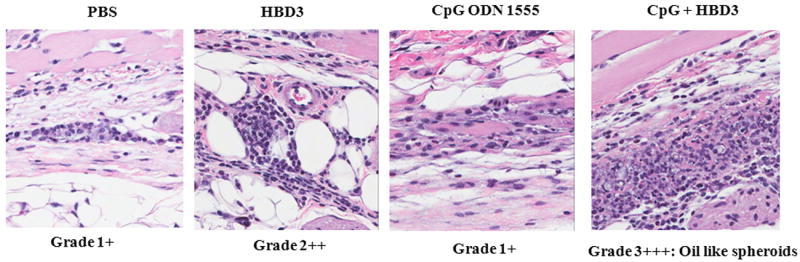
Microscopic analysis of skin sections for recruitment of inflammatory cells after injection with either PBS or HBD3 or CpG or HBD3 + CpG. Shown are representative H&E stained sections from one mouse from each treated group. Positive signs indicate degree of severity of inflammation. All mice showed similar degree/scores of inflammation.
Discussion
Our results provide further support for the hypothesis that alarmins interact with multiple host cell receptors to promote the recruitment and activation of inflammatory cells. LL37 has been shown to have chemotactic effects by interacting with FPR2 (15) and has been proposed to activate inflammatory cells through P2X7 (16) and by complexing with DNA through TLR9. Human beta defensins have been shown to be chemotactic by interacting with CCR2 (17) and CCR6 (18) Although murine BD2 has been reported to activate inflammatory cells through TLR4 (19) and HBD3 has been reported to use TLR1 and 2 (7), their capacity to bind DNA and activate TLR9 adds a potent activating pathway that can account for their alarmin activity.
Therefore, our findings are indicative of a novel interaction between beta defensins and IFN-α-producing cells like pDCs. Our results demonstrate for the first time that cationic antimicrobial peptides like HBD2 and 3 which are epithelial cell products convert self-nucleic acid into an activator of TLR9 in human pDCs.
Binding studies revealed an intricate nature of complexes which included both energy and enthalpy driven hydrogen bonding, van der waals and ionic interactions as well as entropy driven hydrophobic interactions. The interactions basically revealed a stoichiometry of one CpG molecule interacting with 2 molecules of HBD3 and were a one to one or of a sequential nature (reverse titration), depending on the nature and concentration of ligand. It is important to discuss the seemingly important fitting model difference between direct and reverse titrations. For the direct injection, the protein molecules are in excess, while for the reverse one the DNA molecules are. In the first case, the DNA site has a capacity to accommodate two protein moieties, and, since the latter are in excess, they immediately use available opportunity. For the reverse titration, at the first step only one protein molecule joins the DNA binding site until all of the will be half-occupied; then, the second protein molecules start to join the complex up to complete the filling capacity. ITC performed at 37°C generated similar binding isotherms, indicating that the interactions were highly stable at physiological temperatures.
The mixture of antimicrobial proteins found at the site of inflammation due to neutrophil or epithelial cell activation can determine the levels of proinflammatory cytokines including type 1 interferons which may exacerbate the development of autoimmune diseases like lupus and psoriasis or even result in the development of cancer through STAT2 activation (15, 20) HBD2 and 3 have been reported to be elevated in patients with chronic inflammatory conditions including psoriasis, SLE, rheumatoid arthritis and in IBD (15, 20-23) correlating with an increase in keratinocyte and neutrophil presence and activity. Thus, beta defensins 2 and 3 in this context appear to function as an enhancer of inflammation mediated by IFN-α and subsequent activation of myeloid DCs at the site of infection or tissue damage.
These findings raise the possibility that HBD2 and HBD3 released at sites of tissue damage/infection may complex with self or foreign DNA present in the immediate environment with a number of diverse effects on the host as follows : (i) These complexes could play a physiological role in supporting host homeostasis by facilitating elimination of soluble viral/bacterial nucleic acid complexes (ii) Conversely, alarmin/DNA complexes might have pathological effect on the host by supporting the induction of autoimmune disease. Such a scenario would mimic the reported effect of combining LL37 with host DNA in increasing susceptibility to lupus and arthritis (24). These interactions are important in the physiologic response to tissue injury, infection and cancer and can be used to make more potent vaccine adjuvants. As evident from Figure 7 the ability of HBD2 and 3 to enhance cellular uptake of nucleic acids can lead to improved DNA vaccine formulations by promoting their uptake by various cells resulting in an enhanced immune response. (iii) Moreover; synthetic complexes of HBD2/3 with immunomodulatory ODN may have therapeutic value. Defensins also synergistically enhance the activity of immunomodulatory ODNs and this activity can be harnessed to therapeutic advantage. It is likely that CpG–HBD complexes will accelerate wound healing (25) (and aid in tumor elimination), while suppressive (SUP) ODN–HBD complexes will inhibit excessive inflammatory/autoimmune (26).
Literature suggests that chromatin binding proteins like HMGB1 associate with DNA released from necrotic cells, thereby inducing TLR9 mediated responses to self-DNA, rather than acting as a proinflammatory mediator (27). Some reports suggest that HMGB1 associates with receptor for advanced glycosylation end products (RAGE) at the cell surface which further mediates internalization/uptake of self-nucleic /HMGB1 leading to induction of TLR9 mediated responses (27). Similarly, numerous other alarmins including LL37 (2) and several members of the defensin family including several alpha defensins (Tewary et al, unpublished observations) bind to DNA indicating a potential pathological involvement in inflammatory conditions. One major question arises whether this phenomenon of facilitating entry of self-nucleic acids into immune cells is an intrinsic feature of the defensin family ? Since all defensins are also antimicrobial in nature, our results raise the possibility that the presence of these peptides during infection may increase the probability of detecting rare or low levels of microbial ligands including bacterial and viral nucleic acids; however, this high affinity of recognition may apparently lead to potentially recognizing self-nucleic acids that may be present in the local milieu as well. Although defensins and LL37 when complexed to nucleic acids are equipotent in stimulating immune cells, we do not know whether they are redundant or play unique roles. Defensins and LL37 both are also products of epithelial cells and it is likely that they have similar effects during autoimmune pathologies. On the other hand LL-37 unlike beta defensins is also present in neutrophils and may therefore be more evident at sites of acute inflammation. Our studies comparing LL-37 and HBD 3 complexed to DNA show them to be equipotent. (Tewary et al unpublished observations). It is also crucial to determine whether all the alarmin family members are capable of nucleic acid sensing and/or whether their effect is compartmentalized. It is likely that the role these proteins play in self/nonself discrimination between self and microbial nucleic acids may depend upon their expression levels in a particular pathological condition. However, future studies are warranted to confirm these hypotheses.
Acknowledgments
We would like to thank Ms Anna Trivett, Ms Kelli Czarra and Ms Megan Karwan for their technical help and assistance. We would also like to acknowledge Dr Diana Haines (PHL, FNL) for her expert analysis of histopathology skin sections and careful reviewing of the manuscript.
This work was supported by National Cancer Institute, National Institutes of Health Contract HHSN261200800001E. This work was also supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Footnotes
Conflict of interest: The authors have no competing financial interests. Dr Klinman and members of his laboratory hold or have applied for patents concerning the activity of CpG ODN. The rights to all such patents have been transferred to the U.S. government.
References
- 1.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 2.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 3.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niyonsaba F, Ogawa H. Protective roles of the skin against infection: implication of naturally occurring human antimicrobial agents beta-defensins, cathelicidin LL-37 and lysozyme. J Dermatol Sci. 2005;40:157–168. doi: 10.1016/j.jdermsci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, Steinau HU, Yao F, Onderdonk AB, Steinstraesser L, Eriksson E. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11:220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 7.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci U S A. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–3474. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, Klinman D, Gioannini T, Weiss JP, Furusawa T, Bustin M, Oppenheim JJ. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012 doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 12.Jelesarov I, Bosshard HR. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. Journal of Molecular Recognition. 1999;12:3–18. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<3::AID-JMR441>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Tian, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 14.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 17.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-Defensin 2 and 3 and Their Mouse Orthologs Induce Chemotaxis through Interaction with CCR2. Journal of Immunology. 2010;184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Chertov O, Bykovskaia N, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OMZ, Oppenheim JJ. beta-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 19.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 20.Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics. 2010;125:1237–1247. doi: 10.1542/peds.2009-3289. [DOI] [PubMed] [Google Scholar]
- 21.Vordenbaumen S, Fischer-Betz R, Timm D, Sander O, Chehab G, Richter J, Bleck E, Schneider M. Elevated levels of human beta-defensin 2 and human neutrophil peptides in systemic lupus erythematosus. Lupus. 2010;19:1648–1653. doi: 10.1177/0961203310377089. [DOI] [PubMed] [Google Scholar]
- 22.Zilbauer M, Jenke A, Wenzel G, Postberg J, Heusch A, Phillips AD, Noble-Jamieson G, Torrente F, Salvestrini C, Heuschkel R, Wirth S. Expression of human beta-defensins in children with chronic inflammatory bowel disease. PLoS One. 2010;5:e15389. doi: 10.1371/journal.pone.0015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, van de Kerkhof PC, Traupe H, de Jongh G, den Heijer M, Reis A, Armour JA, Schalkwijk J. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frese S, Diamond B. Structural modification of DNA--a therapeutic option in SLE? Nat Rev Rheumatol. 2011;7:733–738. doi: 10.1038/nrrheum.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Yamamoto M, Shimosato T, Klinman DM. Accelerated wound healing mediated by activation of Toll-like receptor 9. Wound Repair Regen. 2010;18:586–593. doi: 10.1111/j.1524-475X.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L, Ito S, Ishii KJ, Klinman DM. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheum. 2005;52:651–658. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 27.Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic Acid Recognition by the Innate Immune System. Annual Review of Immunology. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]


