Abstract
Objectives
To investigate whether functionally based resistance exercise could improve strength, physical function, and disability among prostate cancer survivors (PCS) on androgen deprivation therapy (ADT); and to explore potential mediators of changes in outcomes from exercise.
Design
Randomized controlled trial.
Setting
Academic medical center.
Participants
PCS (N=51; mean age, 70.2y) on ADT.
Intervention
PCS were randomized to moderate to vigorous intensity resistance training or stretching (placebo control) for 1 year.
Main Outcome Measures
Maximal leg press and bench press strength, objective and self-reported physical function, and self-reported disability. Hierarchical linear modeling was used to test for significant group × time differences adjusting for covariates.
Results
Retention in the study was 84%, and median attendance to supervised classes was 84% in the resistance group. No study-related injuries occurred. Maximal leg strength (P=.032) and bench press strength (P=.027) were improved after 1 year of resistance training, whereas little change occurred from stretching. Self-reported physical function improved with resistance training, whereas decreases occurred from stretching (P=.016). Disability lessened more with resistance training than stretching (P=.018). One-year change in leg press strength mediated the relation between groups (resistance or stretching) and 1-year change in self-reported disability (P<.05).
Conclusions
One year of resistance training improved muscle strength in androgen-deprived PCS. Strengthening muscles using functional movement patterns may be an important feature of exercise programs designed to improve perceptions of physical function and disability. Findings from this study contribute to the mounting evidence that exercise should become a routine part of clinical care in older men with advanced prostate cancer.
Keywords: Activities of daily living, Exercise, Men, Muscle strength, Neoplasm, Rehabilitation, Strength training
Prostate cancer is the most common cancer in older men, with the highest incidence rates in men 70 to 74 years of age.1 The prognosis for most prostate cancer survivors (PCS) is favorable, and >90% of men live at least 15 years past their diagnosis. Up to 70,000 men each year experience prostate specific antigen-only recurrence and often begin treatment with androgen deprivation therapy (ADT) to reduce androgen exposure.2 Median survival in men with prostate specific antigen-only recurrence can be as long as 16 years,3,4 lengthening the time that PCS become susceptible to the combined adverse effects of age, cancer treatment, and inactivity on their health. Prolonged androgen deprivation from ADT has a profound impact on the musculoskeletal system that could place PCS on an accelerated trajectory to disability.5,6
Disability has been conceptualized as resulting from a cascade of declines in which illness and aging lead to physiological impairments (eg, muscle loss, altered gait, fatigue). These impairments lead to declines in physical functioning (eg, reduced mobility, weakness).7–9 Declines in physical function lead to dissability, defined as participation in daily tasks and social activities. PCS on ADT are particularly susceptible to declines along this pathway because androgen deprivation leads to muscle loss of 2% to 4% within 1 to 2 years.5,10 Muscle loss leads to muscle weakening and fatigue, and PCS who are on ADT have lower muscle strength,6,11 have worse performance on objective tests of physical function,6 and report more fatigue and worse physical function11 compared with PCS who are not on ADT or older men without cancer. In older men without cancer, low muscle strength is associated with self-reported functional limitations and both current and future onset disability.12–15 Older adults with disability have increased care needs, are more likely to be admitted to a long-term care facility, and are more likely to die than older adults who remain independent.16,17
In older adults without cancer, resistance training can reverse muscle weakness and improve mobility, thereby reducing the risk of disability.18–20 We have developed a resistance and impact exercise program, Prevent Osteoporosis with Impact + Resistance (POWIR), that has improved risk factors for falls and fractures (eg, increased bone density, muscle strength, balance) in women with21–23 or without cancer.24,25 We have reported preliminary efficacy of the POWIR program to slow bone loss in a 12-month randomized controlled trial in PCS on ADT.26 The purpose of this article is to report on secondary endpoints of that study, including muscle strength, physical function, and disability. We also explored whether changes in strength, objectively measured physical function, or fatigue mediated changes in self-reported function or disability.
Methods
Design and setting
The study was a 12-month single-blind randomized controlled trial comparing 2 parallel groups assigned to a supervised program of POWIR or a placebo control program of seated stretching exercise (FLEX). Outcomes were measured at baseline and at 6 and 12 months. Testing and exercise training took place at Oregon Health & Science University. The Oregon Health & Science University Institutional Review Board approved the study.
Participants
Recruitment procedures are described elsewhere.26 Eligibility criteria were participants with a confirmed diagnosis of prostate cancer, currently receiving ADT but no other cancer treatment, clear of bone metastases in the hip or spine, with bone mineral density T score ≥−2.5, with no antiresorptive medications, with no participation in resistance and/or impact training ≥2 times per week and ≥30 minutes per session, and with physician clearance to exercise. Informed consent was obtained from each participant prior to data collection.
Power analysis
Sample size was estimated from an a priori power analysis to detect differences in our primary aim, bone mineral density, resulting in 25 participants per group.26 Using the same approach based on a 2×3 mixed design analysis of variance and using differences scores for muscle strength from our prior work,25 we determined that 21 participants per group would provide power of .80 to detect a 17% difference in maximal leg strength at the end of the intervention at α<.05.
Interventions
The interventions, including intensity and progression, have been described elsewhere,26 but elements of the program that are salient to this study are described here. Participants in both groups were prescribed an exercise program of 2 supervised classes and 1 home-based session per week for 12 months. The POWIR program was based on our prior interventions in people without cancer27,28 and was aimed to increase musculoskeletal health and function in older adults through resistance plus impact training.29 Free weights were used to apply resistance and included dumbbells, barbells, and weighted vests (loaded as percentage body weight). Resistance exercises were all multijoint and emphasized movements common to activities of daily living, including wall-sits, squats, bent-knee dead lifts, multidirectional lunges, 1-arm row, chest press, lateral raise, and push-ups. Impact exercise, consisting of 50 two-footed jumps, was included to mechanically load the skeleton for bone outcomes.26 For home exercise, resistance bands replaced free weights and body weight replaced weighted vests for upper-body exercises and lower-body exercises, respectively.
Participants in the control group performed a series of seated or lying whole-body stretching and relaxation exercises aimed to minimize weight-bearing forces and muscle activation. For home exercise, control participants followed a written guideline of stretches and relaxation used in class. A gentle exercise placebo group was used as a control rather than a usual care group to equalize attention, maximize retention, and minimize contamination.
Procedures
Tests were administered by trained technicians blinded to group assignment. Randomization was stratified by length of ADT use (>1 or ≤1y) and current aerobic activity (≥90min/wk vs <90min/wk) and occurred after baseline testing.
Measures
Demographics and health status
The prevalence of chronic medical conditions was obtained by self-report using the Charlson Comorbidity Index.30 Other health information and demographics were self-reported using an in-house questionnaire. Energy expended in physical activity (kcal/wk) at baseline and 6 and 12 months was measured by self-report with the Community Health Activities Model Program for Seniors questionnaire for older adults.31 Fatigue was measured using the Schwartz Cancer Fatigue Scale.32 Scores range from 6 to 36, with higher scores indicating worse fatigue.
Muscle strength
Maximal strength of the upper and lower body was evaluated by a 1-repetition maximum leg press and bench press (kg) according to standard protocols33 that we used in prior studies in cancer survivors.22,23,34 Our coefficients of variation for leg and bench press are 6.6% and 7.5%, respectively.
Physical function
Objective physical function was assessed by the Physical Performance Battery (PPB) that consists of 3 timed tests: 5-time chair stand, standing balance, and 4-m usual walk speed.35 Each test is scored 0 (unable) to 4 and then summed. Low scores on the PPB have been shown to predict activities or daily living disability, hospitalization, nursing home admission, and mortality.35–38 We also evaluated changes in chair stand time and walk speed separately. Our coefficients of variation for chair stand and walk speed are 5.9% and 3.1%, respectively.
Self-reported physical function was measured using 3 separate instruments, each with different attributes. For comparison with commonly used questionnaires, we measured physical function with the Medical Outcomes Study 36-Item Short-Form Health Survey physical function subscale, a generic measure for use in general population surveys,39 and the physical function subscale of the European Organization for Research and Treatment of CancerQuality of Life Questionnaire (version 3), a cancer-specific measure.40 To examine conceptually distinct concepts of physical function, we included the Late-Life Function and Disability Instrument (LLFDI), a valid and reliable measure of function and disability in older adults that contains subscales of function, including basic and advanced function of the lower and upper extremities, and a disability scale.41,42 Scales on all 3 instruments range from 0 to 100, with higher scores indicating better function or less disability.
Statistical analysis
We evaluated all hypotheses using an alpha significance level of .05. The intention-to-treat (ITT) analysis was performed using a linear mixed effects modeling approach implemented in the lme package for the R statistical computing environment.a In this model, the trajectory (change) across time in an outcome for each individual is modeled as a random slope coefficient, and group membership (POWIR vs placebo) is modeled as a fixed group effect. A cross-level interaction coefficient is also modeled, which tests whether the groups have significantly different slopes (change) across time. As in repeated-measures analysis of variance, the primary effect of interest is this cross-level interaction. This approach uses the maximum likelihood estimation and allows the estimation of individual change (slopes) across time, even if subjects have missing data at ≥1 time point.43,44 All available information on each subject is used to generate the estimate, but imputed or estimated values for the missing data points are not estimated directly as in multiple imputation approaches. Therefore, it is not necessary to delete subjects who have missing data (eg, listwise deletion) or to use methods (eg, last-observation-carried-forward) that have been shown to produce biased parameter estimates.43 Using maximum likelihood estimation in this way has been shown to perform on par with multiple imputation for generating unbiased parameter estimates when data is missing at random.45 We also performed a sensitivity analysis using a 2 (group) ×3 (time) mixed design analysis of covariance excluding participants who did not complete the study. Additional contrast analyses were performed to determine if group × time interactions were significant at 6 months of exercise. The completer analysis used data from participants with complete baseline and 12-month data and shows whether or not trends observed from ITT analyses that included men who dropped out of the program were similar to trends observed when analyses were limited just to men who finished the trial. Age and time since diagnosis were included as covariates to account for any influence of these variables on exercise tolerance and adaptations. Complete case mediation analyses were performed using Mplus statistical software.b Mplus software allows the direct estimation of indirect (mediating) effects. For ITT mediation analyses, we used a multiple imputation approach to handle missing data because maximum likelihood methods can be more difficult to implement in these models. Multiple imputation routines implemented in Mplus software were used to generate average model results across 5 datasets with imputed values for missing data. The RMediation package implemented in R was then used to estimate 95% confidence intervals for the mediating (indirect) effects. Mediation was tested only for outcomes that showed significant group × time interactions.46
Results
Sample
A total of 467 men showed interest in the study, but most were ineligible because they were not on ADT. A total of 51 men enrolled in the trial and were randomized to the POWIR (n=29) or placebo (n=22) groups (fig 1). Sample sizes were uneven between groups because enrollment ceased prior to completing a randomization block of 20 participants. Regardless, baseline demographic and outcome measures were similar between groups. On average, participants were 70 years old, had multiple comorbidities, were 6 years past diagnosis, and were on ADT for 2.6 years (table 1). A few of the men had metastatic disease or received chemotherapy. The men were equally physically active at baseline.
Fig 1.
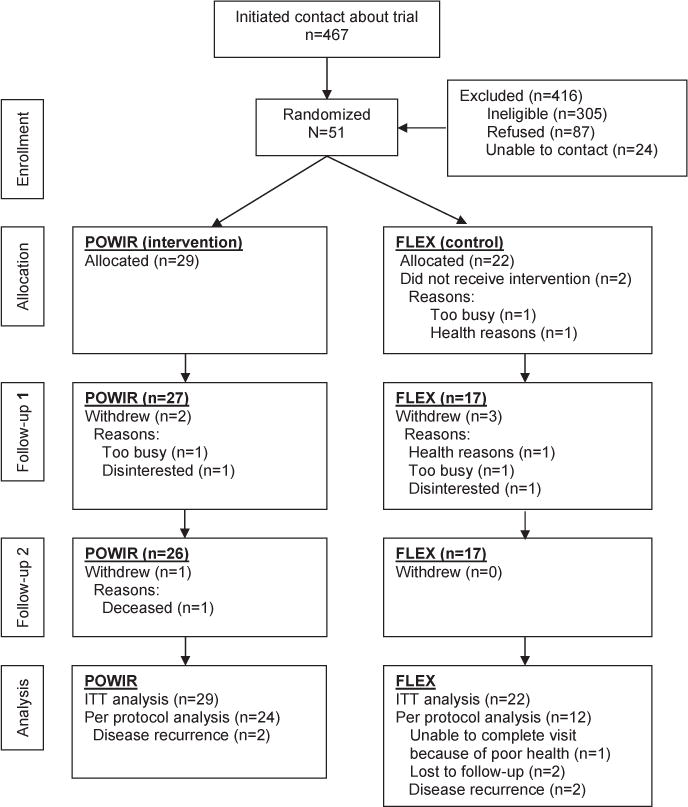
Participant flow throughout the trial.
Table 1.
Baseline clinical characteristics of randomized participants
| Characteristic | POWIR Group (n=29) | FLEX Group (n=22) | P |
|---|---|---|---|
| Age (y) | 69.9±9.3 | 70.5±7.8 | .81 |
| Comorbidity index | 3.9±2.5 | 3.9±3.1 | .99 |
| BMI (kg/m2) | 28.4±4.1 | 29.6±4.8 | .21 |
| Time since diagnosis (mo) | 79.2±58.9 | 75.6±56.0 | .83 |
| Metastatic disease | 27.6 | 13.6 | .23 |
| Received radiation therapy | 44.8 | 50.0 | .71 |
| Received chemotherapy | 6.9 | 13.6 | .42 |
| Length of time on ADT (mo) | 39.0±36.1 | 28.5±29.2 | .27 |
| Physical activity energy expenditure (kcal/d) | 249±241 | 245±300 | .79 |
NOTE. Data are expressed as mean ± SD for continuous data, percentage of sample for categorical data, or as otherwise indicated. Abbreviation: BMI, body mass index.
Retention and adherence
Over 12 months, 6 men withdrew from the study and 2 were lost to follow-up, yielding retention rates of 90% in the POWIR group and 75% in the control group (see fig 1). Final data were not available for 5 men because of disease recurrence (n=4) or poor health (n=1); however, these men still participated in exercise training as much as possible. Men who withdrew from the study were more likely to have received chemotherapy (P=.02) than men who remained in the study, but other characteristics were similar. Missing data may not have been missing at random because disease recurrence and poor health could be related to poorer strength and functional outcomes. However, because most of the attrition occurred in the control condition, any biasing effect would have worked against our hypotheses by boosting the average strength and functional scores of the control condition. Attendance at supervised sessions averaged 83% and 67% and at home sessions averaged 49% and 50% for the POWIR and control groups, respectively. No injuries or adverse events were reported, but 2 men in the POWIR group performed a modified version of the program for most of the intervention because of preexisting limitations.
Outcomes
In ITT analyses, there were significant differences over time between the POWIR and control groups for maximal leg press strength (coefficient for group × time=23.09, SE=8.71, t74=2.65) and maximal bench press strength (coefficient for group time=4.88, SE=2.10, t71=2.14) (table 2 and figs 2A and B). Similar trends were observed in the completer analyses: maximal muscle strength increased over the intervention period in the POWIR group by 7% for bench press and 17% for leg press (see table 2), significantly more than the slight improvements in the control group that were within measurement error. Significant group differences in both strength measures were detected by 6 months.
Table 2.
Initial, midpoint, and final values on outcomes by study group
| POWIR Group
|
FLEX Group
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 6mo | 12mo | Baseline | 6mo | 12mo | P* | P† |
| Objectively measured outcomes | ||||||||
| Bench press 1RM (kg) | 46.8±13.2 | 49.4±15.3‡ | 50.0±16.2 | 44.6±11.3 | 42.2±12.0 | 43.8±13.0 | .01 | .03 |
| Leg press 1RM (kg) | 121.3±33.5 | 137.5±44.3‡ | 142.4±52.2 | 119.9±30.3 | 121.8±33.4 | 120.8±30.6 | .03 | .01 |
| Chair stand (s) | 12.1±2.47 | 10.9±1.9 | 10.3±1.83 | 11.4±1.61 | 11.7±1.8 | 10.6±1.61 | .07 | .09 |
| 4-m usual walk speed (m/s) | 1.31±0.16 | 1.21±0.19 | 1.39±0.18 | 1.20±0.23 | 1.07±0.27 | 1.29±0.29 | .97 | .37 |
| 4-m fast walk (m/s) | 1.98±0.26 | 1.67±0.31 | 2.06±0.31 | 1.88±0.32 | 1.67±0.29 | 2.07±0.31 | .13 | .31 |
| PPB | 10.9±1.1 | 11.4±0.82 | 11.5±0.72 | 10.6±1.7 | 10.9±1.4 | 11.2±1.6 | .88 | .92 |
| Self-report outcomes | ||||||||
| LLFDI | ||||||||
| Overall function | 68.7±9.6 | 69.7±10.7 | 70.0±9.4 | 75.6±19.1 | 73.4±18.7 | 74.4±18.3 | .36 | .21 |
| Basic upper-extremity function | 85.3±11.4 | 85.0±12.3 | 85.1±9.5 | 85.2±17.9 | 81.3±16.0 | 81.1±1.2 | .43 | .15 |
| Basic lower-extremity function | 81.6±13.8 | 82.7±17.2 | 82.8±14.1 | 83.9±20.5 | 83.6±20.7 | 85.4±18.9 | .68 | .40 |
| Advanced lower-extremity function | 63.5±14.5 | 65.3±12.8 | 65.3±13.1 | 69.8±22.4 | 67.6±22.4 | 69.8±21.9 | .32 | .26 |
| Disability limitation | 81.6±13.7 | 85.3±13.5 | 89.8±13.3 | 78.2±17.3 | 79.0±17.4 | 80.9±14.1 | .32 | .02 |
| EORTC QLQC30 | ||||||||
| Physical function | 87.5±14.3 | 92.2±11.7‡ | 93.3±9.0 | 89.7±15.3 | 82.4±20.1 | 86.7±20.7 | <.01 | <.01 |
| Schwartz Cancer Fatigue Scale | ||||||||
| Fatigue | 9.87±4.47 | 9.22±3.46 | 8.83±3.19 | 9.92±3.58 | 9.17±2.98 | 9.83±3.66 | .32 | .06 |
NOTE. Data are presented as unadjusted mean ± SD for participants who completed baseline and 6-month and 12-month follow-up or as otherwise indicated.
Abbreviations: EORTC, European Organization for Research and Treatment on Cancer; 1RM, 1 repetition maximum; QLQC30, Quality of Life Questionnaire.
P value for group × time interaction using all 3 time points from per protocol analysis adjusted for age and time since diagnosis.
P value for group × time interaction using all available time points from ITT analyses adjusted for age and time since diagnosis.
Group × time interaction is significant at the midpoint of the trial tested by planned comparisons between baseline and 6-month time points from per protocol analyses, adjusted for age and time since diagnosis, P<.05.
Fig 2.
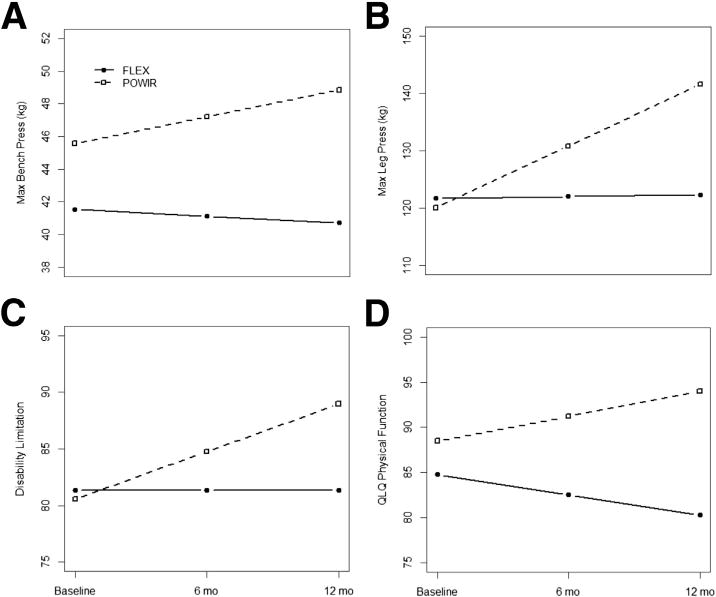
Estimated fixed effects for significant group × time interactions adjusted for age and time since diagnosis: (A) maximum bench press strength, (B) maximum leg press strength, (C) self-report disability limitation, and (D) European Organization for Research and Treatment of Cancer QLQ physical function scale. Estimated values were calculated using the regression equation derived from hierarchical linear modeling analysis using observed data. Abbreviations: max, maximum; QLQ, Quality of Life Questionnaire.
Changes in objective measures of physical function, examined as a summary measure by the PPB or as individual outcomes of the chair stand and walk speed, were not significantly different between groups. However, both ITT and completer analyses showed an observable trend where the POWIR participants decreased the mean time to complete 5 chair stands compared with control participants that approached significance (P=.09 and P=.07 for the ITT and completer analyses, respectively).
In ITT analyses, the POWIR group increased mean self-reported physical function on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire subscale (coefficient for group × time=4.98, SE=1.65, t74=3.02) and decreased disability on the LLFDI subscale (an increase in disability score indicates less dependence with daily activities; coefficient for group × time=4.22, SE=1.71, t74=2.47); these changes significantly differed from decreases in physical function or a slight lessening of disability, respectively, in the control group (figs 2C and D). Significant group × time differences in self-reported physical function using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire remained with the completer analysis, but significance dropped somewhat for disability scores; however, the patterns of change were consistent between the 2 analyses (see table 2). Significant group differences in the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire physical function scale, but not in disability scores, were detectable by 6 months. In both the ITT and completer analyses, there were no significant differences between groups in fatigue or the Medical Outcomes Study 36-Item Short-Form Health Survey or the LLFDI physical function subscales; however, a trend toward greater reductions in fatigue among the POWIR group compared with the control group was observed for the ITT analysis (P=.06) (see table 2), which may be attributable to the larger sample.
Mediation
Mediation analyses were limited to strength, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire physical function, and LLFDI disability outcomes because these showed significant group × time interactions. In ITT analyses, the 12-month change in leg press strength mediated the association between group and self-reported disability (indirect effect, −2.84; 95% confidence interval, 0.08–6.43; P<.05), but not self-reported physical function. Therefore, we would expect a 2.84-unit improvement in self-reported disability in the POWIR group compared with the control group as a result of changes in leg press strength. Mediation completer analyses only resulted in convergent results (indirect effect, −3.52; 95% confidence interval, −0.40 to 7.45; P=.07). These results also held after controlling for age and time since diagnosis. The 12-month change in bench press strength did not mediate associations between the experimental group and self-reported physical function or disability.
Discussion
To our knowledge, this trial is the first to show that exercise training of any type can improve self-reported physical function and lessen disability among older PCS currently on ADT. The observed improvements in muscle strength from POWIR corroborate the findings reported in other trials.34,47,48 Our resistance plus impact training program, POWIR, was originally designed to prevent ADT-related bone loss to reduce fracture risk among PCS.26 After 12 months, men in the POWIR group experienced less bone loss compared with a stretching control group, and now we have shown that POWIR also led to significant gains in strength, better self-reported physical function, and less disability compared with controls. Mediation analysis suggested that improvements in lower-body strength from POWIR could have translated to reductions in self-report disability.
Despite a larger number of studies on exercise benefits for PCS undergoing a variety of treatments (eg, surgery, radiation therapy, ADT), only 3 controlled exercise trials have reported on the efficacy of resistance training, either alone47 or in combination with aerobic exercise,34,48 exclusively in PCS undergoing treatment with ADT. Men in our POWIR program reported significantly better levels of physical function and less disability compared with men in the stretching control group, whereas none of the other trials observed similar changes34,48 or did not measure these outcomes.47 Previous trials evaluated the effect of short-term, 12-week training programs consisting of seated machine-based resistance exercise. In contrast, our training program was 4 times longer so we could evaluate changes in bone health over a 1-year period. Our training program was different from the machine-based training in other trials in that it used free weights to optimally load the skeleton and apply resistance during weight-bearing functional movements. The differences in self-reported outcomes between our trials and other trials suggest that a longer period of training may be necessary before strength gains lead to better functioning and ease of daily tasks recognized by PCS. Also, the functionally based exercises (eg, chair stand, front lunge) characteristic of our training program may better translate to functional movements used in daily activities (ie, weight transfer, climbing stairs) than seated, machine-based resistance exercise.
Consistent with muscle strength, self-report physical function, and disability improvements from the POWIR program, we expected to also find significant improvements in objective measures of physical function. We observed a trend for mean decreased chair stand time among POWIR participants compared with the control group (P=.07–.09), but walk speed increased similarly in both groups. Galvao,34 Cormie,48 and colleagues observed similar trends in chair stand time, with their combined program of resistance and aerobic exercise compared with usual care, but they reported mixed outcomes for usual walk speed that depended on whether men were initial or long-term users of ADT. Because our control group performed seated stretching exercises and experienced some improvement in walk speed, it is possible that lower-intensity stretching was enough to improve this component of function. In fact, the degree of improvements in usual walk speed among our 2 intervention groups was similar to the 6.4% improvement reported from resistance plus aerobic training in long-term ADT users by Galvao,34 suggesting that simply increasing physical activity may be sufficient to improve mobility. We must also consider that men who participated in this trial were well functioning to begin with, as reflected by their relatively high scores on the PPB; therefore, we may have encountered a ceiling effect by using functional tests designed to detect lower limits of physical functioning in older adults.
An important feature of our trial was the measurement of distinct components on the pathway to disability, which we used to determine whether and how exercise training might reduce disability in PCS on ADT. Our mediation analyses identified increases in muscle strength as the only significant mediator of training effects on self-reported disability. In older men without cancer, low muscle strength has been significantly associated with current self-reported functional limitation and disability12,13 and is predictive of future onset disability.14,15 ADT causes accelerated muscle loss and weakening that places PCS on this treatment, most of whom are older and at risk for disability. Our mediation analysis showed that muscle strengthening from a program that includes functionally based lower-body exercise lessened self-reported disability in this vulnerable group of men.
Study limitations
Our study has notable strengths. We are the first to determine the influence of a long-term (eg, 12-mo) exercise program on distinct components along the pathway to disability among PCS on ADT. Prior trials have been much shorter and may not have been long enough to observe trajectories of change, changes in outcomes with a longer time course of adaptation, or long-term tolerance to exercise training among this older group of cancer survivors. Strength and physical function improvements were detectable at 6 months, and these early changes might provide motivation for men to continue exercising to receive the longer-term benefits that preserved their day-to-day abilities. The good adherence of men to moderate- to high-intensity exercise and the maintenance of strength improvements over a 1-year period (see figs 2A and B) is encouraging, given their older age and degree of comorbidities that might limit their tolerance to training over time. Another strength of our study was our resistance training program that was designed around functional movement patterns to strengthen muscles used in everyday activities. Following the principle of specificity,49 performing exercises using functionally based movement patterns are more likely to lead to changes in functional tasks and activities that use these same patterns. Unfortunately, the clinical relevance of our findings remains difficult to predict until minimally important differences using the same measures are established for PCS on ADT. Although the use of an exercise placebo group may have obscured our ability to detect changes in walk speed, it was important to use this type of group instead of usual care given the subjective nature of several outcome measures. Our sample size, although adequate to detect group differences in muscle strength using our full sample and similar to sample sizes in other trials of PCS, may have been insufficient to detect differences in objective physical function measures. Although we used contemporary methods for handling missing data, we did have substantial loss to follow-up that could introduce bias to the extent that the missing data were not missing at random. Adherence to home training was lower than adherence to supervised exercise, suggesting that men may have preferred to exercise in a group setting and that this setting may elicit better improvements than exercising at home. Our sample of PCS on ADT was able and motivated to participate in facility-based training, and this limits the generalizability of our findings. Other PCS may be less motivated and/or able to engage in supervised exercise.
Conclusions
Our findings suggest that POWIR, a 12-month exercise program of functionally based resistance plus impact training, can increase muscle strength, improve self-report function, and reduce self-report disability in PCS on ADT. As evidence of the benefits of exercise mounts, it is increasingly clear that an exercise prescription needs to be a routine component of cancer care and that the design and implementation of exercise programs matter. Our work is designed to develop specific, feasible, and effective exercise programs that can become a routine component in the care of men with advanced prostate cancer.
Acknowledgments
We thank the Oregon State Cancer Registry for their assistance with recruitment.
Supported by the Livestrong Foundation (formerly Lance Armstrong Foundation); and TheraBand, Inc, for providing elastic exercise bands for study participants.
List of abbreviations
- ADT
androgen deprivation therapy
- FLEX
placebo control program of seated stretching exercise
- ITT
intention-to-treat
- LLFDI
Late-Life Function and Disability Instrument
- PCS
prostate cancer survivors
- POWIR
Prevent Osteoporosis with Impact D Resistance
- PPB
Physical Performance Battery
Footnotes
Clinical Trial Registration No.: NCT00660686.
Disclosures: none.
- R software; R Development Core Team c/o Institute for Statistics and Mathematics, Wirtschaftsuniversit ät Wien, Welth-andelsplatz 11020 Vienna, Austria.
- Mplus; Muthén & Muthén, 3463 Stoner Ave, Los Angeles, CA 90066.
References
- 1.American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177:1985–91. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 3.Abern MR, Aronson WJ, Terris MK, et al. Delayed radical prosta-tectomy for intermediate-risk prostate cancer is associated with biochemical recurrence: possible implications for active surveillance from the SEARCH database. Prostate. 2013;73:409–17. doi: 10.1002/pros.22582. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–39. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 6.Galvao DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 7.Bennett JA, Winters-Stone K, Nail L. Conceptualizing and measuring physical functioning in cancer survivorship studies. Oncol Nurs Forum. 2006;33:41–9. doi: 10.1188/06.ONF.41-49. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Young Y, Rubin G, Bandeen-Roche K. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 9.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Saad F, Egerdie B, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30:3271–6. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol. 2002;56:779–86. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 12.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 13.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther. 2007;87:1334–47. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- 14.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–8. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Ann Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- 17.Ostir GV, Carlson JE, Black SA, Rudkin L, Goodwin JS, Markides KS. Disability in older adults 1: Prevalence, causes, and consequences. Behav Med. 1999;24:147–56. doi: 10.1080/08964289.1999.11879271. [DOI] [PubMed] [Google Scholar]
- 18.Hauer K, Rost B, Rutschle K, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J Am Geriatr Soc. 2001;49:10–20. doi: 10.1046/j.1532-5415.2001.49004.x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell AJ, Robertson MC, Gardner M, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing. 1999;28:513–8. doi: 10.1093/ageing/28.6.513. [DOI] [PubMed] [Google Scholar]
- 20.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 21.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27:447–56. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6:189–99. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winters-Stone KM, Dobek J, Nail LM, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24:1637–46. doi: 10.1007/s00198-012-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol A Biol Sci Med Sci. 1998;53:M53–M8. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- 25.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 26.Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc. 2014;46:1482–8. doi: 10.1249/MSS.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winters-Stone K, Snow C. Site-specific response of bone to exercise in premenopausal women. Bone. 2006;39:1203–9. doi: 10.1016/j.bone.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55:M489–M91. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz AL. The Schwartz Cancer Fatigue Scale: testing reliability and validity. Oncol Nurs Forum. 1998;25:711–7. [PubMed] [Google Scholar]
- 33.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 34.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–7. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 36.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 40.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 41.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–M16. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 42.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M22. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 43.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 44.Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry. 2002;47:68–75. [PubMed] [Google Scholar]
- 45.Schlomer GL, Bauman S, Card N. Best practices for missing data management in counseling psychology. J Counsel Psych. 2010;57:1–10. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- 46.Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–9. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 48.Cormie P, Galvão DA, Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2014 Jan 27; doi: 10.1111/bju.12646. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48:987–95. doi: 10.1136/bjsports-2012-091732. [DOI] [PubMed] [Google Scholar]


