Abstract
Transport of vitamin D3 from its sites of cutaneous synthesis into the circulation has been assumed to be via the plasma vitamin D binding protein (DBP). We studied vitamin D transport from the skin in seven healthy volunteers who received whole body irradiation with 27 mJ/cm2 dosage of ultraviolet B light (290-320 nm). Samples of venous blood were collected serially in EDTA and immediately chilled. In KBr, plasma samples were ultracentrifuged to provide a rapid separation of proteins of density < and > 1.3 g/ml. Upper and lower phases and serial fractions were analyzed for vitamin D3 (extraction, HPLC), cholesterol (enzyme assay), and human DBP (hDBP) (radial immunodiffusion). Total plasma vitamin D (basal level < 1 ng/ml) increased by 10 h and peaked at 24 h (9 +/- 1 ng/ml). 98% of the D3 remained at the density > 1.3 layers for up to 7 d, whereas cholesterol (> 85%) was detected at density < 1.3 and all of the hDBP was at density > 1.3. In three volunteers who each ingested 1.25 mg of vitamin D2, the total plasma D2 increased to 90 +/- 32 ng/ml by 4 h, and the D2 was evenly distributed between the upper and lower layers at 4, 8, and 24 h after the dose, indicating a continuing association of the vitamin with chylomicrons and lipoproteins, as well as with hDBP. Actin affinity chromatography removed D3 from plasma of irradiated subjects, indicating the association of the D3 with DBP. These findings indicate that endogenously synthesized vitamin D3 travels in plasma almost exclusively on DBP, providing for a slower hepatic delivery of the vitamin D and the more sustained increase in plasma 25-hydroxycholecalciferol observed after depot, parenteral administration of vitamin D. In contrast, the association of orally administered vitamin D with chylomicrons and lipoproteins allows for receptor-mediated, rapid hepatic delivery of vitamin D, and the reported rapid but less-sustained increases in plasma 25-hydroxycholecalciferol.
Full text
PDF
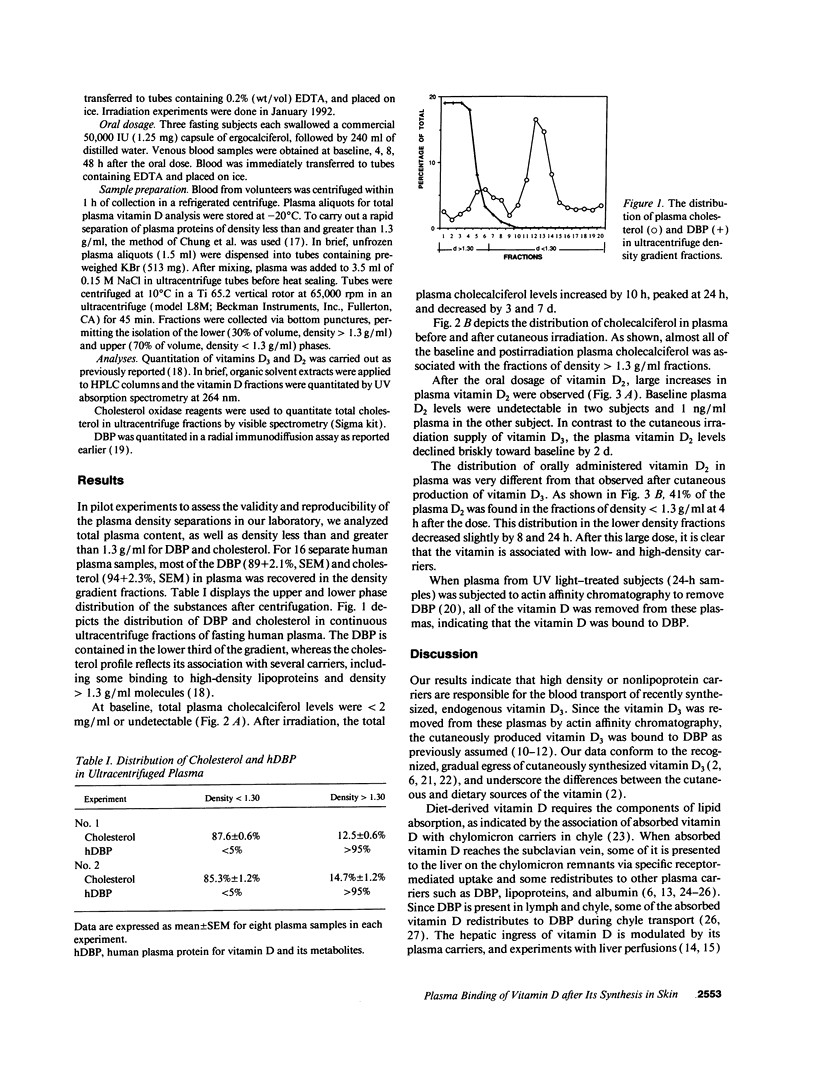

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avioli L. V. Absorption and metabolism of vitamin D3 in man. Am J Clin Nutr. 1969 Apr;22(4):437–446. doi: 10.1093/ajcn/22.4.437. [DOI] [PubMed] [Google Scholar]
- Avioli L. V., Lee S. W., McDonald J. E., Lund J., DeLuca H. F. Metabolism of vitamin D3-3H in human subjects: distribution in blood, bile, feces, and urine. J Clin Invest. 1967 Jun;46(6):983–992. doi: 10.1172/JCI105605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragry J. M., France M. W., Boucher B. J., Cohen R. D. Metabolism of intravenously administered cholecalciferol in man. Clin Endocrinol (Oxf) 1979 Nov;11(5):491–495. doi: 10.1111/j.1365-2265.1979.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- Dueland S., Helgerud P., Pedersen J. I., Berg T., Drevon C. A. Plasma clearance, transfer, and distribution of vitamin D3 from intestinal lymph. Am J Physiol. 1983 Oct;245(4):E326–E331. doi: 10.1152/ajpendo.1983.245.4.E326. [DOI] [PubMed] [Google Scholar]
- Dueland S., Pedersen J. I., Helgerud P., Drevon C. A. Absorption, distribution, and transport of vitamin D3 and 25-hydroxyvitamin D3 in the rat. Am J Physiol. 1983 Nov;245(5 Pt 1):E463–E467. doi: 10.1152/ajpendo.1983.245.5.E463. [DOI] [PubMed] [Google Scholar]
- Dueland S., Pedersen J. I., Helgerud P., Drevon C. A. Transport of vitamin D3 from rat intestine. Evidence for transfer of vitamin D3 from chylomicrons to alpha-globulins. J Biol Chem. 1982 Jan 10;257(1):146–150. [PubMed] [Google Scholar]
- Fraser D. R. The physiological economy of vitamin D. Lancet. 1983 Apr 30;1(8331):969–972. doi: 10.1016/s0140-6736(83)92090-1. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Abrams J., Walgate J. Affinity chromatography with 25-hydroxycholecalciferol ester in the isolation of the binding protein for vitamin D and its metabolites from human serum. Metab Bone Dis Relat Res. 1981;3(1):43–46. doi: 10.1016/s0221-8747(81)80022-7. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Aden D. P., Aw T. C. Plasma carriers influence the uptake of cholecalciferol by human hepatoma-derived cells. J Bone Miner Res. 1989 Apr;4(2):243–247. doi: 10.1002/jbmr.5650040217. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Jennings A. S., Aw T. C. Vitamin D uptake and metabolism by perfused rat liver: influences of carrier proteins. Endocrinology. 1988 Jul;123(1):498–504. doi: 10.1210/endo-123-1-498. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Jr, Hahn T. J. Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature. 1973 Aug 24;244(5417):515–517. doi: 10.1038/244515a0. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Kowalski M. A., Lange E. E. Selective, rapid removal of the vitamin D-binding protein and its sterol ligands from human and bovine plasma. Anal Biochem. 1985 Apr;146(1):96–102. doi: 10.1016/0003-2697(85)90401-4. [DOI] [PubMed] [Google Scholar]
- Holick M. F., MacLaughlin J. A., Clark M. B., Holick S. A., Potts J. T., Jr, Anderson R. R., Blank I. H., Parrish J. A., Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980 Oct 10;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
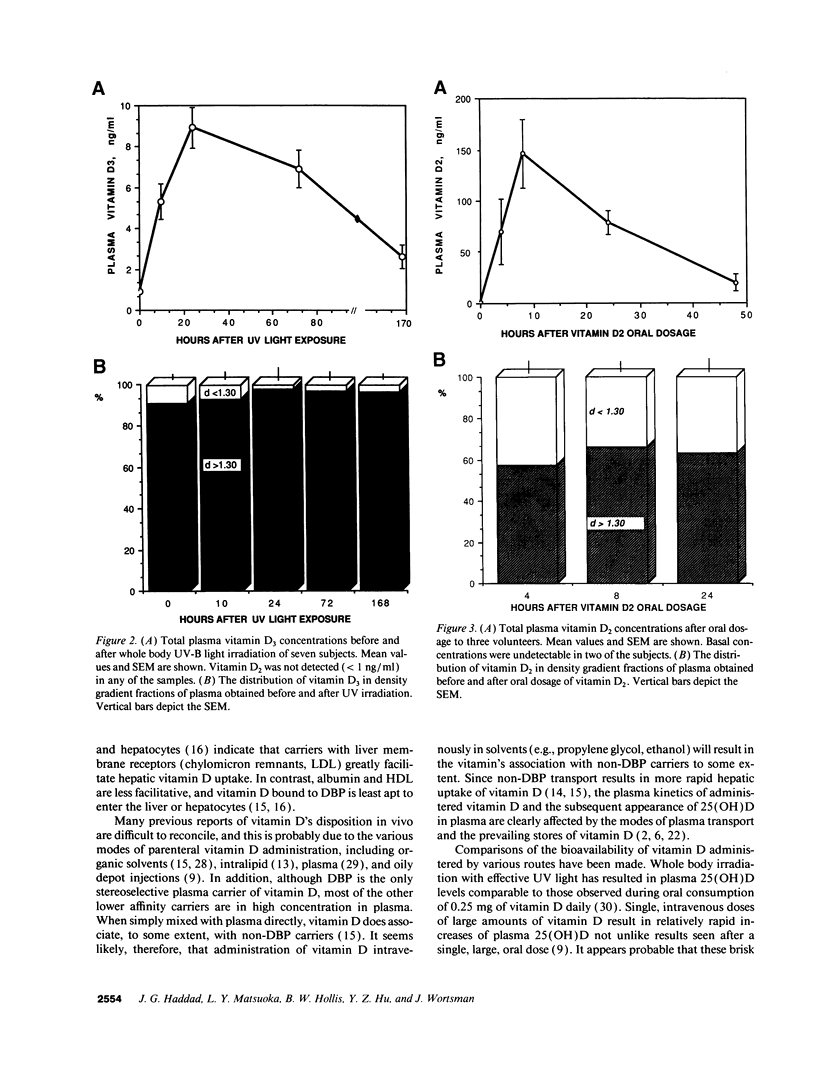
- Holick M. F., MacLaughlin J. A., Doppelt S. H. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981 Feb 6;211(4482):590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- Holick M. F. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed Proc. 1987 Apr;46(5):1876–1882. [PubMed] [Google Scholar]
- Hollis B. W., Frank N. E. Solid phase extraction system for vitamin D and its major metabolites in human plasma. J Chromatogr. 1985 Sep 13;343(1):43–49. doi: 10.1016/s0378-4347(00)84566-1. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Paul A. A., Black A. E., Cole T. J., Mandal A. R., Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J. 1979 Aug 4;2(6185):303–305. doi: 10.1136/bmj.2.6185.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Sedrani S. H., Douglas J. Interrelationships in rats of tissue pools of cholecalciferol and 25-hydroxycholecalciferol formed in u.v. light. Biochem J. 1986 Jan 15;233(2):535–540. doi: 10.1042/bj2330535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. W., Henry H. 1,25-Dihydroxycholecalciferol--a hormonally active form of vitamin D. Recent Prog Horm Res. 1974;30(0):431–480. [PubMed] [Google Scholar]
- Okano T., Masuda S., Ishimine M., Murai J., Yamamoto Y., Kobayashi T. Existence of vitamin D3 and 25-hydroxyvitamin D3 in rat lymph. Chem Pharm Bull (Tokyo) 1983 Sep;31(9):3233–3241. doi: 10.1248/cpb.31.3233. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., Garry P. J., Hunsaker L. A., Hunt W. C., Goodwin J. S. Nutritional status in a healthy elderly population: vitamin D. Am J Clin Nutr. 1982 Dec;36(6):1225–1233. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- Poskitt E. M., Cole T. J., Lawson D. E. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br Med J. 1979 Jan 27;1(6158):221–223. doi: 10.1136/bmj.1.6158.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojanasathit S., Haddad J. G. Hepatic accumulation of vitamin D3 and 25-hydroxyvitamin D3. Biochim Biophys Acta. 1976 Jan 14;421(1):12–21. doi: 10.1016/0304-4165(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Silver J., Berry E. Vitamin D uptake by the perfused rat liver is determined by its transport protein. Miner Electrolyte Metab. 1982 Jun;7(6):298–304. [PubMed] [Google Scholar]
- Stamp T. C., Haddad J. G., Twigg C. A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet. 1977 Jun 25;1(8026):1341–1343. doi: 10.1016/s0140-6736(77)92553-3. [DOI] [PubMed] [Google Scholar]
- Stamp T. C., Round J. M. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature. 1974 Feb 22;247(5442):563–565. doi: 10.1038/247563a0. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Haddad J. G., Jr, Walters D. D., Stamp T. C. Vitamin D bioavailability: serum 25-hydroxyvitamin D levels in man after oral, subcutaneous, intramuscular, and intravenous vitamin D administration. J Clin Endocrinol Metab. 1979 Jun;48(6):906–911. doi: 10.1210/jcem-48-6-906. [DOI] [PubMed] [Google Scholar]