Supplemental digital content is available in the text.
Background
CD28 signal blockade after T cell receptor activation is under intense investigation as a tolerance-inducing therapy for transplantation. Our goal is to produce a CD28-specific reagent as a therapy for the prevention of graft rejection and graft-versus-host disease in the canine model of allogeneic hematopoietic cell transplantation.
Methods
We infused a monoclonal mouse anticanine CD28 antibody (1C6 mAb) into 4 dogs and a fragment of antigen-binding (1C6 Fab) into 2 dogs. Pharmacokinetics, pathology, cytokine release, and the crystal structure of 1C6 Fv were evaluated.
Results
Within an hour of an intravenous injection of the 1C6 mAb, the dogs became leukopenic and developed a steroid-refractory cytokine storm. Two of the dogs developed high fevers, one experienced diffuse alveolar hemorrhage and another developed gastrointestinal hemorrhage. The cytokine storm was characterized by elevated plasma levels of monocyte chemotactic protein-1, interferon gamma inducible protein-10, interleukin (IL)-10, IL-6, and tumor necrosis factor-α. In addition, 1 dog showed elevated levels of IL-2, IL-8, and IL-18. In contrast, infusion of 1C6 Fab was well tolerated without any side effects. Dry-coating 1C6 mAb onto tissue culture plates induced CD3-independent proliferation and tumor necrosis factor-α production. Crystal structure analysis revealed that 1C6 binds to canine CD28 in a manner different than previously reported for conventional agonistic or superagonistic antibodies.
Conclusions
These results indicate that dogs and humans develop a similar cytokine storm after infusion of anti-CD28 mAb, providing an appropriate large animal for further study. The 1C6 Fab warrants evaluation as a tolerance-inducing reagent in the canine model of allogeneic hematopoietic cell transplantation.
In the late 1990s, cytotoxic T-lymphocyte antigen 4-Ig was evaluated as an immune-modulating agent in the canine hematopoietic cell transplantation (HCT) model.1,2 CTLA4-Ig binds to B7.1 and B7.2 (CD80, CD86) expressed on antigen-presenting cells and prevents activation of both CD28 (costimulation) and CTLA-4 (coinhibition) expressed on either naive or activated T cells, respectively. When used in conjunction with the immunosuppressive agents, mycophenolate mofetil/cyclosporine or methotrexate/cyclosporine, CTLA4-Ig improved both donor hematopoietic cell engraftment and prevention of graft-versus-host disease (GVHD).1,2 Most recently, after additional data in nonhuman primates,3 the Pediatric Blood and Marrow Transplant Consortium developed a phase 2 clinical trial that uses CTLA4-Ig in combination with methotrexate/cyclosporine for GVHD prevention.
Blockade of the CD28-CD80/CD86 pathway is a promising therapeutic approach to treating a broad spectrum of immune disorders including autoimmunity,4,5 organ transplantation,6,7 hematopoietic graft rejection,1,8 and GVHD.9 However, the immunosuppressive properties of CTLA4-Ig in these applications are limited because the fusion protein not only blocks costimulation through CD28 but also prevents CTLA-4–mediated downregulation of activated T cells. In an effort to surmount this limitation, investigators have examined CD28-specific blockade using monoclonal antibodies (mAb) to CD28. Ideally, an antagonistic anti-CD28 antibody would block CD28 interaction with CD80/CD86 without cross-linking CD28. However, in practice, the majority of anti-CD28 antibodies are agonistic and cross-link CD28.10 Agonistic anti-CD28 antibodies are broadly separated into “superagonist” if they produce extensive cross-linking of CD28 and induce polyclonal T cell activation independent of CD3 ligation, or “conventional” if they produce limited cross-linking of CD28 and require CD3 ligation for T-cell activation.11-15 Some studies have shown that these antibodies can induce tolerance, but the mechanism of tolerance may be due to an agonistic effect on T regulatory cells and not a direct antagonist effect on T cell activation.16-22
Development of CD28-mediated therapies for clinical use has been influenced by the well-publicized phase 1 clinical trial of an anti-CD28 superagonist mAb TGN1412 (CD28-super monoclonal antibody, TeGenero AG).23 All 6 volunteers treated with the antibody became critically ill because of a cytokine storm. This outcome was unexpected based on both in vitro and in vivo studies using rodents and rhesus macaques.24,25 Retrospective modifications to the in vitro assays were able to elicit cytokines using TGN1412.24,26-30 Based on these observations, clinical development of a CD28-targeted therapy has been mostly limited to monovalent forms of anti-CD28 because these forms do not cross-link CD28 and are devoid of cytokine release.31 However, many aspects of our understanding of the TGN1412-mediated cytokine storm remain incomplete including the variability in the severity of the storm witnessed in the test subjects, and whether or not it can be prevented or managed.
To target CD28, we recently produced a number of murine anticanine CD28 antibodies.32 Based on the ability to inhibit mixed leukocyte reactions (MLRs) as effectively as CTLA4-Ig, we chose the clone 1C6 for in vivo studies. We reasoned that 1C6 either as a mAb or a fragment of antigen binding (Fab) could be applied as an agent to induce tolerance for the prevention or treatment of GVHD after HCT for which the canine model has been instrumental.33-35 We present the toxicity results of injecting 1C6 whole mAb and Fab into dogs, follow-up in vitro studies, and the analysis of the biophysical properties of the binding between 1C6 and CD28. The results of these studies correlate well with the mechanisms of action of antihuman CD28 mAbs, particularly those attributed to the superagonist anti-CD28 humanized mAb TGN1412 phase I trial,23 and suggest that the canine is a highly relevant animal for studying CD28-mediated toxicity.
MATERIALS AND METHODS
Experimental Animals
Beagles, Mini Mongrel, Basenji, and Golden Retriever crossbreeds were raised at the Fred Hutchinson Cancer Research Center or purchased from commercial kennels. Animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facilities, and study designs were approved by the Institutional Animal Care and Use Committee. Selection of dogs for MLRs required dog leukocyte antigen (DLA) typing of litters and parents using highly polymorphic microsatellite markers within DLA class I and class II regions36 and DLA-DRB1 gene sequencing.37
MAb and Fab Production
Anti-CD28 clones 5B8 (a mouse anticanine CD28 antibody that does not inhibit MLRs) and 1C6 mAb were generated as described previously.32 A Fab fragment of 1C6 was produced using Ficin (Thermo Fisher Scientific, Waltham, MA). Fc-containing fragments and undigested mAb were removed over mAb Select A (GE Healthcare, Pittsburg, PA). The Fab-containing flow-through was buffer exchanged into phosphate buffered saline using a 10-kDa Amicon stir cell unit (Millipore, Billerica, MA) and assessed by high performance liquid chromatography and sodium dodecyl sulfate polyacrylamide gel electrophoresis. Endotoxin was measured at 3.9 EU/mL using endosafe KTA LAL Assay (Charles River, Wilmington, MA).
Pharmacokinetic Studies
The 1C6 mAb serum clearance was measured in plates coated with goat antimouse IgG1 and detected with horseradish peroxidase-labeled goat antimouse IgG1 (Southern Biotech, Birmingham, AL). 1C6 Fab serum clearance was measured similarly but using goat anti-mouse kappa antibodies (Southern Biotech).
In Vitro Cell Assays
The MLRs were performed as previously described.32,38 Cultures of dog peripheral blood mononuclear cells (PBMCs) were incubated on antibody-coated tissue culture plates based on described methods.24,26,27,29,30 The following antibodies were assessed for stimulation: mouse anti-IgG1 isotype (Southern Biotech), 1C6 mAb, 5B8 mAb, and mouse anticanine CD3 17.6F9, at the concentrations indicated in the subsequent figures in 60 μL of phosphate buffered saline per well. For 3-day proliferation assays, 3H-Thymidine (1 μCi) was added to each well the day before harvest and processed by described Materials and Methods.38
MAP Cytokine Assay
Interleukin (IL)-2, IL-6, IL-8, IL-10 IL-18, TNF-α, and monocyte chemotactic protein (MCP)-1 levels were measured using CCTOMAG-90K Milliplex MAP kit (Millipore). Supernatants were collected after 1 and 24 hours from in vitro cultures of PBMC on antibody-coated plates. Plasma was collected from dogs given 1C6 mAb at various time points.
Flow Cytometry
Canine PBMC were stained with murine anticanine CD3 (an IgG2B clone 17.6F9), rat anticanine CD4 (clone YKiX302.9, Ebioscience, San Diego, CA), either anticanine CD28 (1C6) or IgG1 isotype (Southern Biotech), and then stained with a secondary goat antimouse IgG1 (Southern Biotech). The samples were analyzed using a FACS Canto II (BD Biosciences, San Jose, CA). Data were analyzed using FlowJo Software (Treestar Ashland, OR).
Protein Expression, Purification, Crystallography, and Interaction Analysis
For binding, structural, and interaction analysis see Figure S1 (SDC, http://links.lww.com/TXD/A0). For crystallographic data collection and refinement statistics, see Table S1 (SDC, http://links.lww.com/TXD/A0).
RESULTS
In Vivo Effects of 1C6 mAb
Table 1 summarizes treatment, symptoms, clinical management, laboratory values, and positive macronecropsy and micronecropsy results after administration of either 1C6 mAb (4 dogs) or 1C6 Fab (2 dogs). The initial starting dose of 4 mg/kg for 1C6 mAb was based on previous studies demonstrating positive in vivo effects with CTLA4-Ig.1
TABLE 1.
Toxicity of 1C6 antibodies in dogs
Case 1 (H520)
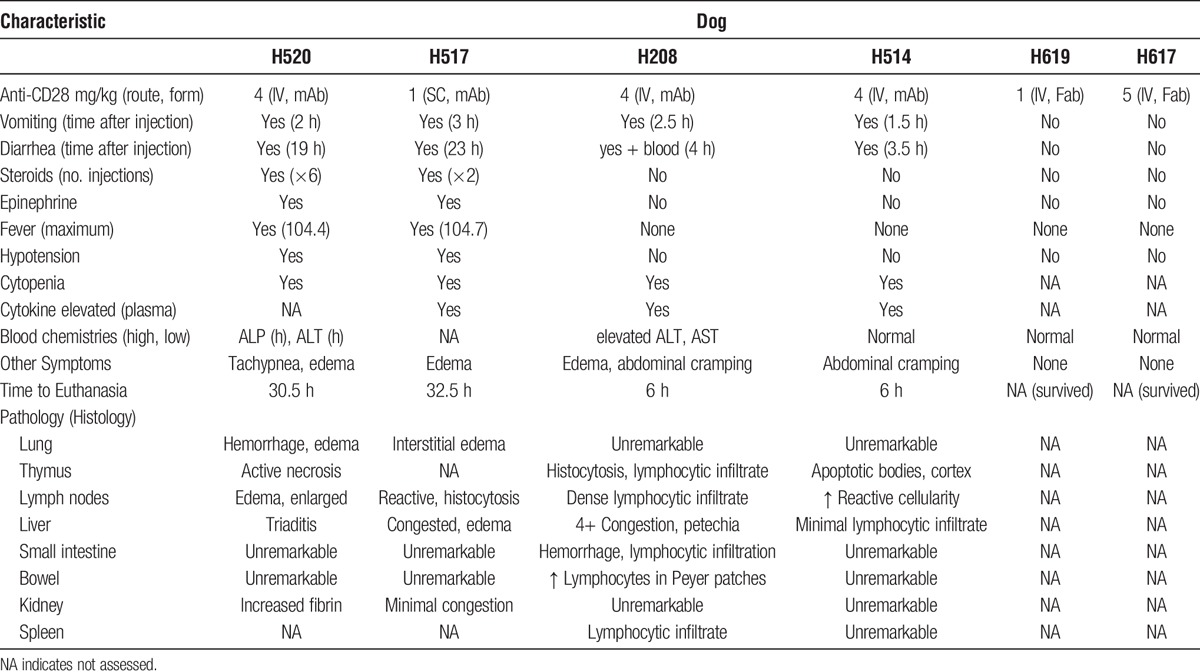
Within 2 hours of the infusion, H520 developed hypotension, recurrent vomiting, diarrhea, and fever. One hour later, H520 was in respiratory distress. Oxygen, intravenous (IV) fluids, and IV dexamethasone (1 mg/kg) were given. There was a transient improvement of symptoms starting 6 hours after administration in which the dog became afebrile, hypotension improved, activity level improved, and the dog took nourishment. However, the symptoms returned overnight. After a total of 30 hours of critical care including another 5 doses of IV dexamethasone, and epinephrine at 0.02 mg/kg IV, the decision to euthanize H520 was made because of unresponsiveness to treatment and overall poor condition. A complete blood count 24 hours after infusion of 1C6 mAb showed a severe lymphopenia. At necropsy, the gross pathology was notable for extreme hemorrhage on the surface of the lungs. The microscopic examination of the lungs was consistent with a diagnosis of diffuse alveolar hemorrhage, and lung macrophages stained positive for 1C6 mAb (Figure 1). Additionally, there was lymphocytic infiltration and edema noted in the microscopic examination of the lung (Figure 1). The thymus showed active necrosis and apoptosis possibly due to the administration of prednisone (not shown).
FIGURE 1.
Histopathology of 4 dogs treated with anti-CD28 (1C6) mAb. The top left panel shows H520 necropsy lung section at 100× magnification demonstrating edema (top arrow), dense lymphocytic infiltrate (middle arrow), and hemorrhage (bottom arrow). The middle left panel shows H520 necropsy lung section at 250× magnification after antimouse peroxidase polymer staining of deparaffinized lung section (arrow points to pulmonary macrophages coated with anti-CD28 mAb). The bottom left panel shows 250× magnification of antirabbit peroxidase polymer control. The top middle panel shows H517 necropsy small intestine section at 100× magnification demonstrating significant hemorrhage on the tips of the villi. The center middle row shows normal small bowel at100× magnification for comparison. The bottom center panel shows H208 necropsy small intestine section at 100× magnification; the arrow points to an enlarged and densely packed Peyer patch. The top right and middle right panel shows H514 necropsy lymph node section at 100× (top) and 250× (middle) magnifications; the arrows point to apoptotic bodies. Slides are stained by hematoxylin and eosin unless otherwise noted.
Case 2 (H517)
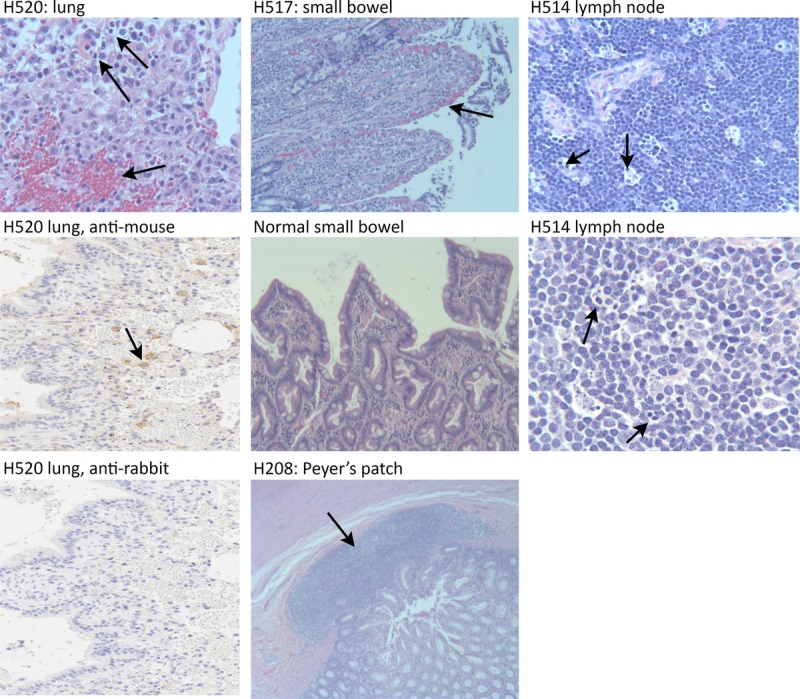
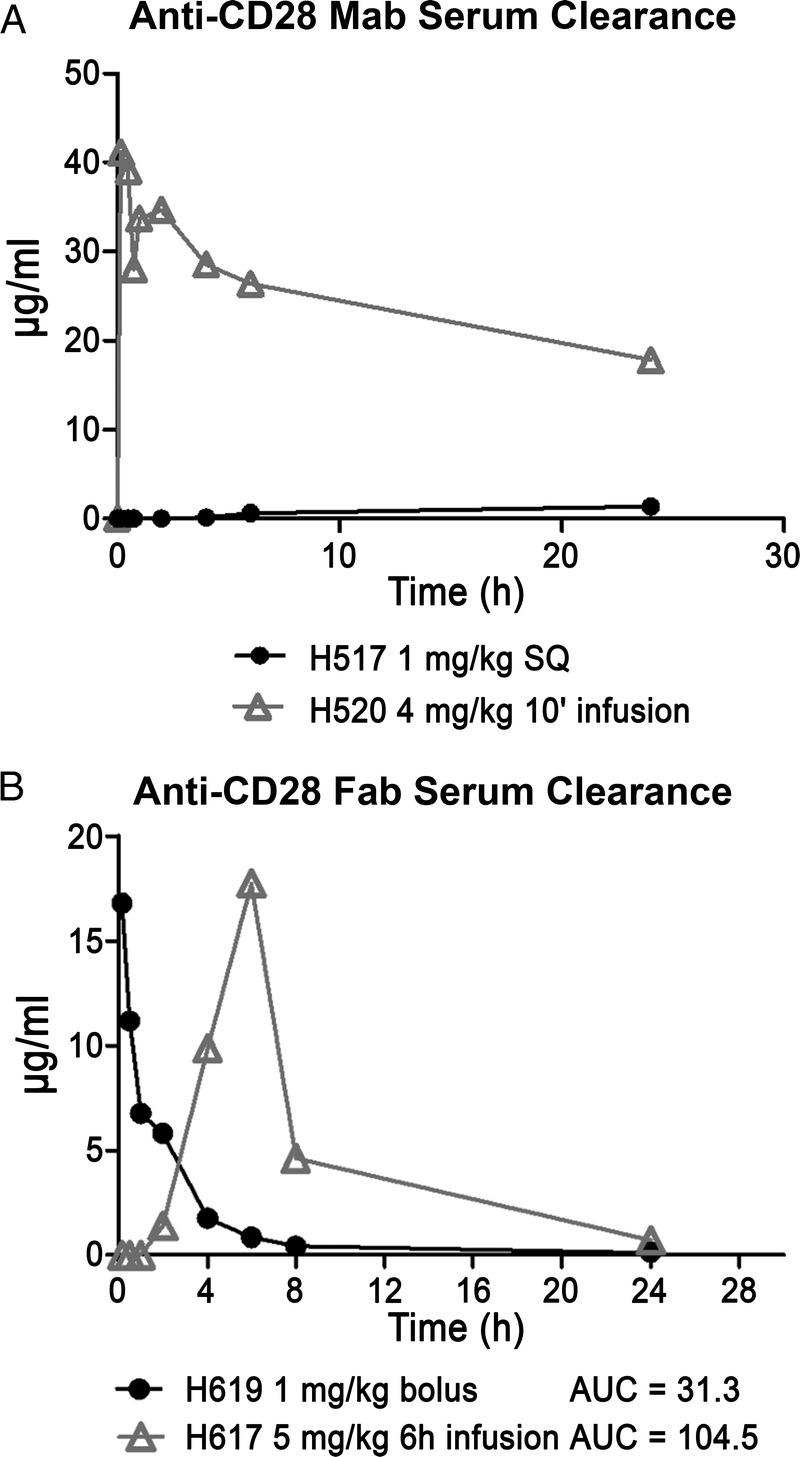
In an effort to reduce/eliminate toxicity, the route of 1C6 mAb infusion was changed from IV to subcutaneous and the dose was decreased to 1 mg/kg. This change in protocol decreased the peak serum concentration of 1C6 mAb to 1.3 μg/mL at 24 hours for H517 (Figure 2). H517 developed a transient fever 5 hours after the subcutaneous injection of 1C6 mAb, but remained active. However, at 22 hours after injection, the dog became febrile, hypotensive, experienced recurrent vomiting, and multiple episodes of diarrhea. Despite 8 hours of critical care, high-dose steroids, empiric antibiotic coverage, and epinephrine infusion, the dog was euthanized due to unresponsiveness to therapy and overall poor condition. A complete blood count of H517 at 24 hours after injection was significant for a severe lymphopenia. At autopsy, the gross pathology was within normal limits. However, the microscopic findings demonstrated hemorrhage on the surface of the intestinal villi (Figure 1). Consistent with the initial physical symptoms, this dog had a relatively stable cytokine profile for the first 4 hours. However, at the 24-hour time point, elevated levels of IL-6, IL-10, interferon gamma inducible protein (IP)-10, and MCP-1, but not IL-2, IL-8, IL-18, and TNF-α were noted (Figure 3).
FIGURE 2.
Serum concentrations of 1C6 mAb and 1C6 Fab. Pharmacokinetic profiles of circulating antibody or antibody fragments from serum collected from 4 dogs injected with 1C6 mAb (A) or 1C6 Fab (B) at the concentrations, routes, and time points indicated.
FIGURE 3.
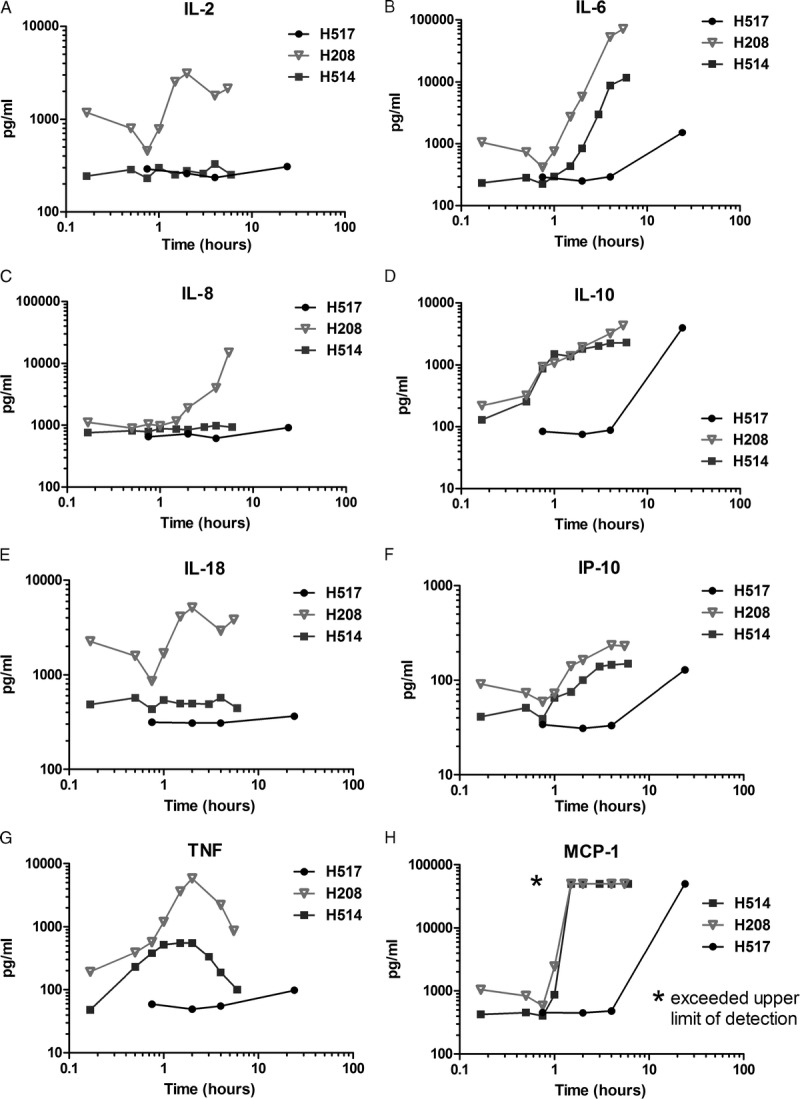
Map cytokine assay. Cytokine levels from plasma samples from 3 dogs treated with 1C6 mAb. Plasma from serial blood samples were collected from dogs injected with 1C6 at 4 mg/kg IV (H208, H514) or 1 mg/kg subcutaneously (H517).
Case 3 (H208) and Case 4 (H514)
To further characterize the cytokine release and peripheral blood lymphopenia after anti-CD28 mAb infusion, 2 dogs were treated with 1C6 mAb at 4 mg/IV in the absence of steroids and only given a single dose of diphenhydramine 30 minutes before the injection of the antibody. Analgesia (buprenorphine IV at 0.005 mg/kg) was administered for signs of significant discomfort. Without supportive care, the study was limited to 6 hours after injection of the antibody to prevent suffering.
H208 did not experience a fever but demonstrated the highest cytokine release. H208 did develop vomiting and diarrhea in the same time course as H520. In addition, edema and cramping were observed (Table 1). Secondary lymphoid tissue involvement was apparent due to the increase in number and size of Peyer patches along the small bowel (Figure 1). Also, severe leukopenia with both lymphocytes and granulocyte depletion occurred within the first hour and remained low for the entire 6-hour experiment (Figure 4). Only TNF-α and IL-10 were elevated within the first hour. By the second hour, elevated levels of IL-2, IL-6, IL-10, IL-18, IP-10, TNF-α, and MCP-1 were noted. Interleukin-8 began to increase at the end of the second hour and continued to rapidly rise for the remainder of the 6-hour experiment. Interleukin-2, IL-18, and TNF-α peaked between 2 and 3 hours and began to decline. IL-6, IL-10, and IP-10 continued to increase during the experiment. MCP-1 exceeded the upper limit of detection by the 2-hour time point, and remained at the upper limit for the remainder of the study (Figure 3).
FIGURE 4.
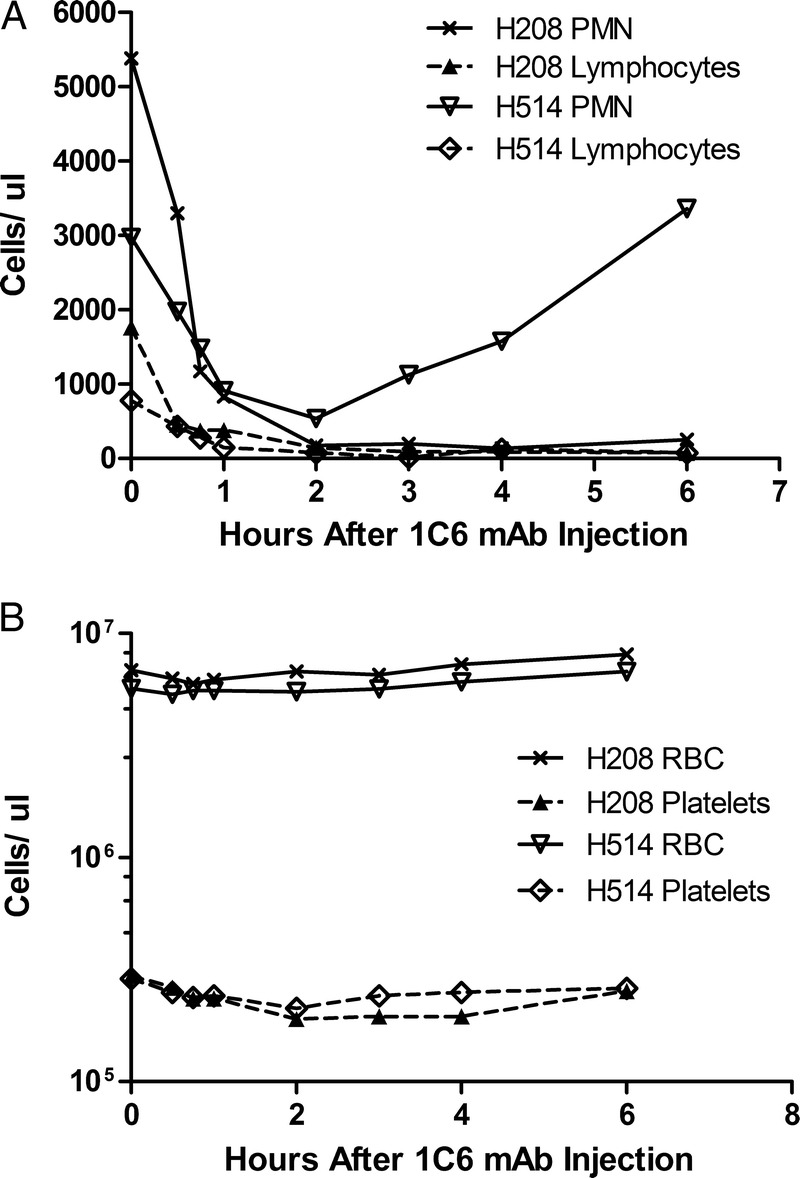
Hematological profile of dogs given 1C6 mAb. After administration of 4 mg/kg 1C6 mAb, complete blood counts were made from serial collections. Counts are shown for polymorphonuclear cells (PMN) and lymphocytes (A), and RBCs and platelets (B) for dogs H208 and H514. Counts from 2 dogs were obtained using an ADVIA 2120i Hematology System (Siemens).
H514 also did not experience a fever, but did demonstrate vomiting, abdominal cramping, and diarrhea 3.5 hours after injection of the antibody. Severe leukopenia with both lymphocytes and granulocyte depletion occurred again within the first hour, but in contrast to H208, the neutrophils recovered to normal levels within 4 hours (Figure 4). On autopsy, there was evidence of a lymphocytic infiltrate in the liver, and the lymph nodes showed apoptotic bodies (Figure 1). Once again, only TNF-α and IL-10 were elevated within the first hour. By the second hour, elevated levels of IL-6, IL-10, IP-10, TNF-α, and MCP-1 were noted. In contrast to H208, H514 did not develop elevated levels of IL-2, IL-8, or IL-18. TNF-α did again peak between 2 and 3 hours and began to decline. IL-6, IL-10, and IP-10 continued to increase during the experiment. Once again, MCP-1 exceeded the upper limit of detection by the 2 hour time point, and remained at the upper limit for the remainder of the study (Figure 3).
In Vivo Effects of 1C6 Fab
Cases 5 (H617) and Case 6 (H619)
To address the toxicity of the 1C6 mAb, we produced a 1C6 Fab that maintained inhibition of MLRs (Figure 5A). 1C6 Fab was injected IV into 2 dogs H619 at 1 mg/kg IV bolus and in a second dog H617 at 5 mg/kg IV infusion over 6 hours. The Fab reached a peak serum concentration of 17 μg/mL when given at 5 mg/kg IV over a 6-hour infusion (Figure 2). As shown in Table 1, 1C6 Fab was well tolerated without signs of toxicity.
FIGURE 5.
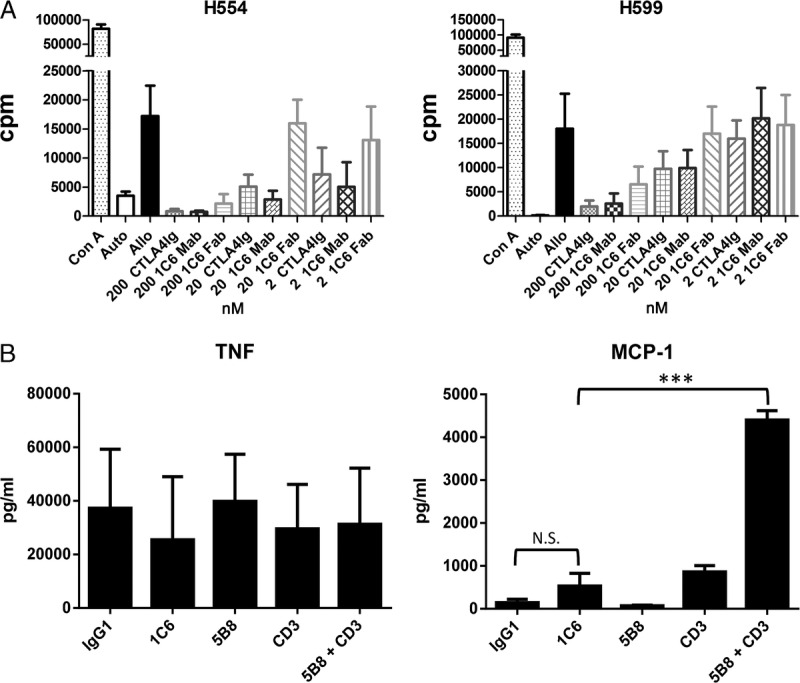
Mixed leukocyte reactions, and MAP cytokine assays from PBMC cultures on antibodies wet-coated to polystyrene tissue culture treated plates. A, One-way mixed leukocyte reaction of PBMC from dogs H554 (left) and H599 (right) were done in the presence and absence of CTLA4-Ig, anti-CD28 mAb, and anti-CD28 Fab at 2, 20, and 200 nM (mean of 9 replicates ± standard deviation). B, PBMC were cultured on polystyrene tissue culture-treated plates previously wet-coated with IgG1 (isotype control), anti-CD28 (1C6), anti-CD28 (5B8), anti-CD3, or anti-CD3 plus anti-CD28 (5B8) for 24 hours. All antibodies were wet-coated at a concentration of 10 μg/mL with the exception of anti-CD3 that was wet-coated at 1 μg/mL. Only the combination of 5B8 and anti-CD3 induced a significant increase in release of MCP-1 from PBMC (***P < 0.0001, Student t test). Data represent the mean and standard deviation of results from 3 dogs.
In Vitro Studies
We selected 1C6 mAb for these initial studies because of the ability of the antibody to inhibit MLRs. 1C6 mAb inhibits MLRs at 20 nanomolar concentrations as effectively as CTLA4-Ig, suggesting that the binding site of the mAb inhibits CD28 interaction with CD80/CD86. Additionally, the 1C6 Fab inhibits MLRs but requires a 10-fold higher concentration to maintain the same level of inhibition as the mAb (Figure 5A).
During the course of these studies, it became clear that the manner in which anti-CD28 antibodies were bound to tissue culture plates has an effect on the cytokine release, reminiscent of the follow-up studies for TGN1412.29,30,39 For example, canine PBMCs cultured on polystyrene tissue culture-treated plates wet-coated with anti-CD28 mAbs 1C6 or 5B8 did not elicit TNF-α or MCP-1 cytokine release. Only the combination of 5B8 with a suboptimal amount of anti-CD3 wet-coated to the plate caused release of MCP-1, but no significant release of TNF-α (Figure 5B). No IL-2 release was detected from any condition (not shown).
In contrast, when PBMCs were cultured on polypropylene plates dry-coated with anti-CD28 mAb cytokine release was detected. After 24 hours of culture, both 1C6 mAb and 5B8 mAb induced significant levels of TNF-α compared to the isotype-matched control IgG1 mAb (P < 0.0001, Student t test) (Figure 6A). However, there was no significant difference in the level of IL-6 release as compared to the isotype control from either 1C6 or 5B8, and significantly less MCP-1 release caused by 1C6 as compared to the isotype control (Figure 6A). No significant amount of IL-2 release was detected from any condition (not shown). After 1 hour of culture, which is the period of time in which TNF-α increases and leukopenia is observed in vivo, no significant amount of IL-2, IL-6, TNF-α, or MCP-1 was detected (not shown).
FIGURE 6.
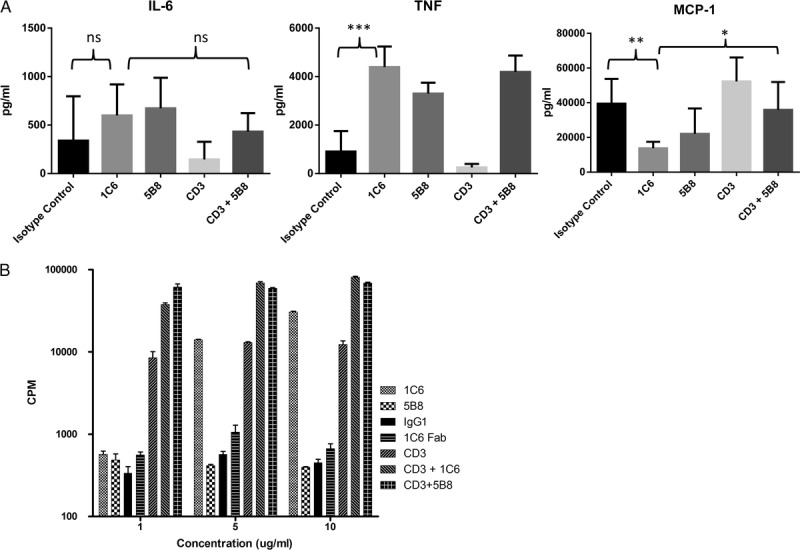
MAP cytokine assays and proliferation assays after PBMC cultures on antibodies dry-coated on polypropylene plates. PBMC were cultured in round bottom polypropylene plates dry-coated with IgG1 (isotype control for 1C6), 1C6, 5B8, or anti-CD3 for 24 hours for the MAP cytokine assay and 3 days for the proliferation assay. A, MAP cytokine assay for IL-6, TNF-α, and MCP-1. All antibodies were dry-coated at a concentration of 10 μg/mL with the exception of anti-CD3 that was dry-coated at 1μg/mL. Data represent mean and standard deviation of results from 3 dogs. 1C6 caused a significant amount of TNF secretion (***P < 0.0001, Student t test). However, 1C6 produced MCP-1 as compared to the isotype control (**P < 0.004, Student t test) or the combination of CD3 and 5B8 (*P < 0.015, Student t test). B, For the 3-day proliferation assay, antibodies were dry-coated at the concentration listed on the horizontal axis, except when combined with anti-CD3, during which times anti-CD3 was added at 1 μg/mL. Data are representative of two independent studies.
Next, proliferation assays were done with PBMCs cultured on polypropylene plates dry-coated with 1C6 mAb, 5B8 mAb, or 1C6 Fab (Figure 6B). Proliferation of PBMCs occurred at 5 and 10 μg/mL concentration of 1C6 mAb, but not at 1 μg/mL. Proliferation was even greater when 1C6 mAb and anti-CD3 were both dry-coated to the wells (Figure 6B). In contrast, 5B8 mAb did not produce proliferation by itself, but proliferation was synergistic when dry-coated with anti-CD3 (Figure 6B). No proliferation occurred in wells dry-coated with 1C6 Fab (Figure 6B).
To assess antibody-mediated proliferation without dry-coating methods, PBMCs were cocultured with plate-bound human umbilical vein endothelial cells (HUVEC) in the presence of soluble 1C6 and 5B8 at 10 μg/mL, and 1C6 and 5B8 at 10 μg/mL with or without anti-CD3 at 1 μg/mL, as described.28 No proliferation was observed after 3 days of culture (not shown), suggesting HUVEC cells were not adaptable for use with canine cells. No canine umbilical vein endothelial cells currently exist to allow for a canine-specific adaptation to this assay.
Cynomologous macaques lost CD28 expression on CD4+ effector memory T cells, explaining why this model failed to predict a cytokine storm in preclinical testing of TGN1412.24 In contrast, all of the canine CD4+ T cells express CD28 as shown in Figure 7.
FIGURE 7.
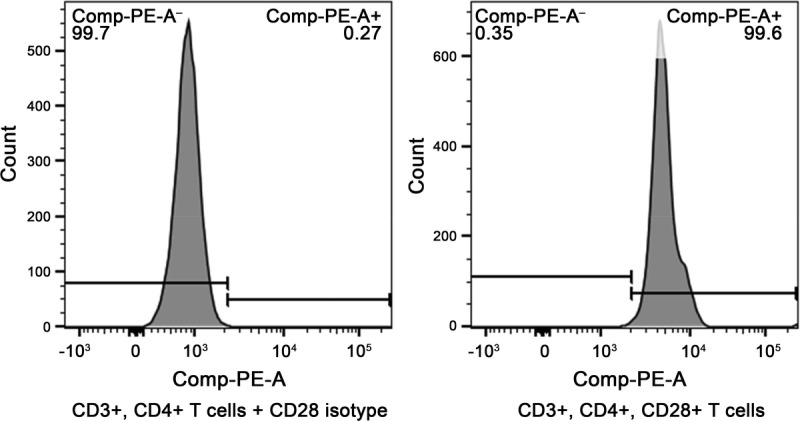
CD28 expression on canine CD4+ T cells. Two representative histogram of CD3+, CD4+ T cells that were either stained with an isotype (left) or 1C6 (right). The data were representative of 5 independent analyses.
Structural Characterization of Canine CD28 and its Interaction With 1C6 Fab
In an effort to compare our results with the TGN1412 trial, we performed biophysical analyses of canine CD28, human CD28, 1C6 Fab, and a precursor human mAb to TGN1412 (5.11A1). Crystal structure of the complex of the canine CD28 ectodomain and 1C6 Fv showed that canine CD28 was structurally homologous to human CD28, as expected from the high sequence identity, recapitulating the homodimerization interface and all salient details of the overall structure (Figure 8A). 1C6 binds to a conformational epitope on canine CD28 that is distinct from that previously observed for antibodies recognizing human CD28 (Figure 8B).11,43 1C6 binds canine CD28 by straddling a pronounced valley in the antibody combining site, the product of relatively short CDR3 loop structures and an extended LCDR2 loop structure (Figure 8C). Computational docking of CD80 onto canine CD28 in the 1C6 complex revealed a small steric clash, suggesting a potential explanation for the ability of 1C6 mAb to inhibit MLRs (Figure 8C). However, it was not possible to definitively conclude that 1C6 binding would competitively inhibit the binding of CD80/CD86 because the small steric clash could be resolved by small rearrangements. Finally, these studies suggest ways in which 1C6 may extensively cross-link CD28, in a similar yet distinct fashion as compared to 5.11A1 (Figure 8F).
FIGURE 8.
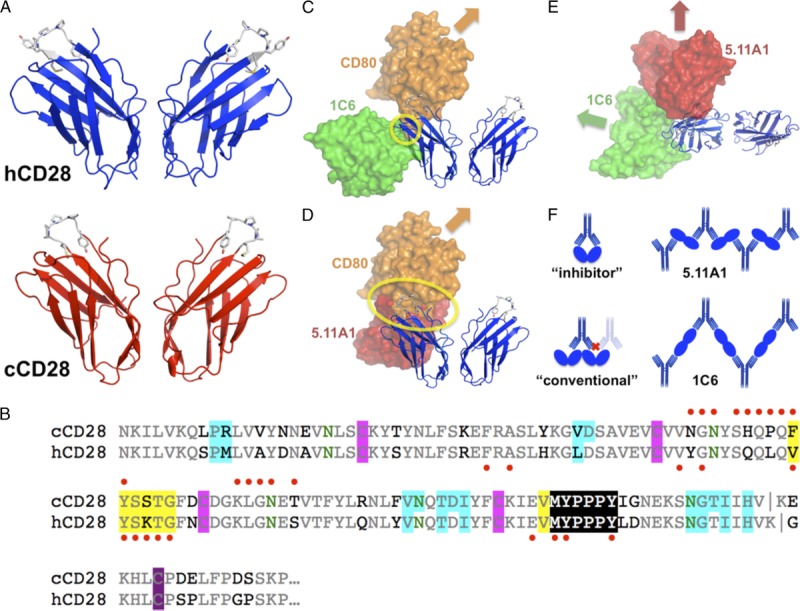
Structural analysis. A, Human CD28 (hCD28) and canine CD28 (cCD28) homodimer structures are conserved, including MYPPPY side-chains. B, CD28 sequences are 83% identical (conserved: gray; nonconserved: black; N-glycan sites: green). Glycosylation is apparent at all 4 sites in the crystals. Disulfide bonds are highlighted (intrachain: magenta; interchain: purple). Homodimer interfaces, determined by PISA,40 are highlighted in blue. Immunodominant epitopes11 are highlighted in yellow. CD28 residues contacting 1C6 are indicated by red dots above the alignment, or contacting 5.11A1 (the TGN1412 parent antibody) by red dots below the alignment. C, The 1C6/cCD28 complex is shown with the 1C6 Fv as a green molecular surface. CD80 (orange) is modeled binding to cCD28, based on the human CD80/CTLA-4 complex (PDB41 accession code 1I8L42). A yellow circle highlights the limited 1C6/CD80 steric clash. D, The position of 5.11A1 (red) on cCD28 is shown as in (C), but the predicted steric clash is more extensive. E, A view orthogonal to (C) and (D) shows the relative positions of 1C6 and 5.11A1. F, Different antibody binding modes are schematized. An idealized inhibitor would engage CD28 homodimers bivalently, without cross-linking, blocking ligand binding. “Conventional” agonist antibodies11 engage CD28 homodimers near the MYPPPY sequence, blocking binding of another antibody to the homodimer, allowing cross-linking of a pair of homodimers, but not formation of extended cross-linked networks. 5.11A1, a “superagonist” antibody,11 enables extensive cross-linking but blocks ligand binding. 1C6 binding permits extensive cross-linking, but generates arrays with distinct geometry and spacing from 5.11A1.
DISCUSSION
Our results demonstrate that 1C6 mAb infusion causes a cytokine storm in dogs similar to the cytokine storm observed in the TGN1412 trial in humans. Further characterization has revealed that 1C6 is capable of producing CD3-independent proliferation of T cells in culture, placing it into a mitogenic or superagonist category of anti-CD28 antibodies.11-15 In contrast, the 1C6 Fab is non-toxic and maintains the ability to inhibit MLRs.
A safe intervention that selectively blocks the CD28/B7 pathway while allowing for CTLA4/B7 inhibitory signal transduction remains an important clinical goal for the induction of tolerance in the treatment or prevention of organ and hematopoietic graft rejection, GVHD, and autoimmune diseases. Since the TGN1412 trial uncovered the cytokine storm, only monovalent forms of anti-CD28 antibodies that do not produce an activated T cell cytokine release have progressed in clinical development.31,44 This assumes that conventional anti-CD28 antibodies or bivalent anti-CD28 antibody fragments without a functional Fc will produce similar uncontrolled cytokine storms. To our knowledge, this premise is not based on trials in a large animal model that has accurately been able to predict cytokine storms in humans.
Monovalent forms have limitations including small size and affinity as compared to the whole antibody. However, the size problem can be overcome with inert linkers, and the affinity of monovalent anti-CD28 forms are considerably stronger than native CD28-CD80/CD86 binding, suggesting that the antibody fragments will efficiently outcompete the native ligand, even though their binding is less avid.31,45 In this respect, our results with the 1C6 Fab warrant combining it with an inert linker, and testing it for the ability to induce tolerance in the canine HCT model.
Multiple therapeutic mAbs in clinical use are associated with cytokine release through distinct mechanisms involving the antibody binding to the target antigen, as well as the Fc portion of the antibody binding to the Fc receptor (FcR) on accessory cells, but the cytokine storm caused by anti-CD28 antibodies differs with respect to cause and the severity. This is because of the CD28 T cell costimulatory receptor target. First, any cross-linking of the CD28 receptor will induce cytokine release from activated T cells. This concept is known from CD28 constructs with mutations in the Fc binding portion preventing FcR binding or from (Fab′)2 constructs where the Fc binding portion was removed, which still induce PI3K activation and resulting cytokine release from activated T cells in culture.31 Second, in contrast to the self-limiting cytokine release of other mAbs, which do not target costimulatory receptors, the initial cytokine release in the setting of ongoing CD28 cross-linking leads to a feedback loop and augmentation of the inflammatory response. This second phase seen in both dogs infused with 1C6 and humans infused with TGN1412 occurs about 12 to 16 hours after infusion after an initial improvement in symptoms.23 Steroids are insufficient treatment for this second phase.
The in vitro results of cytokine release assays after culture of PBMCs with anti-CD28 antibodies are unable to replicate the rapidity or spectrum of the in vivo cytokine storm.24,26-30 The requirement of high concentrations of plate-bound antibody suggests the importance of Fc-FcR cross-linking to elicit cytokines, which is not necessary to produce cytokines from activated T cells as discussed above.31 Interpretations of in vitro cytokine release assays with respect to anti-CD28 mAbs must take into account the microenvironment and T cell subset differences between the peripheral blood cells used in the assay versus the cells in the lymph tissue making up the in vivo epicenter of the cytokine storm. First, there are lower levels of CD4+ effector memory T cells that are contained within the peripheral blood as compared to the high levels located within lymph tissue. This point was made by the lack of response in macaques to TGN1412 because their CD4+ effector memory T cells lose CD28 expression.24 Second, T cells in lymph nodes are more apt to CD28 mediated activation, potentially due to low level priming signals provided in the lymph node microenvironment.39 With respect to the limitations of in vitro assays and previous lack of a large animal model that predicted CD28 mediated cytokine storms, important questions regarding our understanding of anti-CD28 mediated cytokine storms remain.
Canines provide a large animal model for studies needed to address two outstanding questions with respect to anti-CD28–mediated cytokine storms. First is the question regarding whether bivalent anti-CD28 antibodies or fragments induce an uncontrollable cytokine storm. Second is the question regarding whether anti-CD28–mediated cytokine storms can be stopped or prevented. In the TGN1412 trial, the cytokine storm was unexpected, and the treatment of the storm was limited to corticosteroids and daclizumab, an anti-interleukin-2 receptor antagonist mAb.23 Since the TGN1412 study, great progress has been made in a variety of immunotherapies and cytokine release syndromes have become a recognized complication.46 The connection of cytokine storm-mediated mortality in inflammatory conditions, infectious diseases, GVHD, and immunotherapies has stimulated the development of a number of potential and already tested therapies that suggest cytokine storms could be prevented or managed.46,47 If this is the case, then consideration could once again be given to therapies that induce T regulatory cell expansion using anti-CD28 antibodies, which have shown great promise for the treatment of autoimmune diseases,17 solid organ transplant rejection,18-20 and acute GVHD21,22 in rodent models.
In conclusion, canines provide an animal model that accurately predicts the response in humans to anti-CD28 antibody-mediated cytokine storms. We propose canines as a model to test therapies designed to treat or prevent cytokine storms. Additionally, as next generation T cell costimulatory constructs are developed, it will be important to test them for safety in the canine model. Until reagents are developed to block or prevent cytokine storms mediated by whole anti-CD28 mAb, we plan testing an anti-CD28 Fab as a tolerance-inducing therapy in the canine model of allogeneic HCT.
ACKNOWLEDGMENTS
The authors thank Michele Spector, DVM, Alix Joslyn, and the research technicians in the Comparative Medicine facilities for assistance with animal care; Drs. George Georges, Chris Burtner, Bruce Swearingen, Zejing Wang, and Maura Parker for weekend dog care; Helen Crawford and Bonnie Larson for manuscript preparation.
Footnotes
Research reported in this publication was supported by the National Institutes of Health, Bethesda, MD, under award numbers P01CA078902 (RS), P30CA015704, R21 OD010489 (SSG) and R21 A1097786 (RKS), and support from the Gabrielle’s Angel Foundation for Cancer Research (SLR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health; the funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The study was also supported by awards from the Joseph Steiner Krebsstifung, Bern, Switzerland and Lupin Foundation, Metairie, LA, (RS).
The authors declare no conflicts of interest
S.L.R., S.S.G, R.K.S., M.M.G, and D.J.F participated in research design, performance of research, data analysis, and writing of the paper. R.S. participated in research design and writing of the paper. G.E.S., D.M.S., V.K.A, and J.R.H. participated in performance of research.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1. Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999; 94: 2523. [PubMed] [Google Scholar]
- 2. Yu C, Linsley P, Seidel K, et al. Cytotoxic T lymphocyte antigen 4-immunoglobulin fusion protein combined with methotrexate/cyclosporine as graft-versus-host disease prevention in a canine dog leukocyte antigen-nonidentical marrow transplant model. Transplantation. 2000; 69: 450. [DOI] [PubMed] [Google Scholar]
- 3. Miller WP, Srinivasan S, Panoskaltsis-Mortari A, et al. GVHD after haploidentical transplantation: a novel, MHC-defined rhesus macaque model identifies CD28- CD8+ T cells as a reservoir of breakthrough T-cell proliferation during costimulation blockade and sirolimus-based immunosuppression. Blood. 2010; 116: 5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daikh D, Wofsy D, Imboden JB. The CD28-B7 costimulatory pathway and its role in autoimmune disease (Review). J Leukoc Biol. 1997; 62: 156. [DOI] [PubMed] [Google Scholar]
- 5. Buch MH, Vital EM, Emery P. Abatacept in the treatment of rheumatoid arthritis (Review). Arthritis Res Ther. 2008; 10 (Suppl. 1): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992; 89: 11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994; 57: 1701. [PubMed] [Google Scholar]
- 8. Kean LS, Adams AB, Strobert E, et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am J Transplant. 2007; 7: 320. [DOI] [PubMed] [Google Scholar]
- 9. Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000; 164: 4564. [DOI] [PubMed] [Google Scholar]
- 10. Poirier N, Blancho G, Vanhove B. CD28-specific immunomodulating antibodies: what can be learned from experimental models? Am J Transplant. 2012; 12: 1682. [DOI] [PubMed] [Google Scholar]
- 11. Luhder F, Huang Y, Dennehy KM, et al. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med. 2003; 197: 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dennehy KM, Elias F, Zeder-Lutz G, et al. Cutting edge: monovalency of CD28 maintains the antigen dependence of T cell costimulatory responses. J Immunol. 2006; 176: 5725. [DOI] [PubMed] [Google Scholar]
- 13. Dennehy KM, Elias F, Na SY, Fischer KD, Hunig T, Luhder F. Mitogenic CD28 signals require the exchange factor Vav1 to enhance TCR signaling at the SLP-76-Vav-Itk signalosome. J Immunol. 2007; 178: 1363. [DOI] [PubMed] [Google Scholar]
- 14. Singh M, Basu S, Camell C, et al. Selective expansion of memory CD4(+) T cells by mitogenic human CD28 generates inflammatory cytokines and regulatory T cells. Eur J Immunol. 2008; 38: 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanchez-Lockhart M, Kim M, Miller J. Cutting edge: a role for inside-out signaling in TCR regulation of CD28 ligand binding. J Immunol. 2011; 187: 5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beyersdorf N, Ding X, Blank G, Dennehy KM, Kerkau T, Hunig T. Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood. 2008; 112: 4328. [DOI] [PubMed] [Google Scholar]
- 17. Beyersdorf N, Hanke T, Kerkau T, Hunig T. Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases (Review). Ann Rheum Dis. 2005; 64Suppl 4: iv91– iv95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urakami H, Ostanin DV, Hunig T, Grisham MB. Combination of donor-specific blood transfusion with anti-CD28 antibody synergizes to prolong graft survival in rat liver transplantation. Transplant Proc. 2006; 38: 3244. [DOI] [PubMed] [Google Scholar]
- 19. Azuma H, Isaka Y, Li X, et al. Superagonistic CD28 antibody induces donor-specific tolerance in rat renal allografts. Am J Transplant. 2008; 8: 2004. [DOI] [PubMed] [Google Scholar]
- 20. Kitazawa Y, Fujino M, Sakai T, et al. Foxp3-expressing regulatory T cells expanded with CD28 superagonist antibody can prevent rat cardiac allograft rejection. J Heart Lung Transplant. 2008; 27: 362. [DOI] [PubMed] [Google Scholar]
- 21. Beyersdorf N, Ding X, Hunig T, Kerkau T. Superagonistic CD28 stimulation of allogeneic T cells protects from acute graft-versus-host disease. Blood. 2009; 114: 4575. [DOI] [PubMed] [Google Scholar]
- 22. Kitazawa Y, Fujino M, Li XK, et al. Superagonist CD28 antibody preferentially expanded Foxp3-expressing nTreg cells and prevented graft-versus-host diseases. Cell Transplant. 2009; 18: 627. [DOI] [PubMed] [Google Scholar]
- 23. Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006; 355: 1018. [DOI] [PubMed] [Google Scholar]
- 24. Eastwood D, Findlay L, Poole S, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010; 161: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pallardy M, Hunig T. Primate testing of TGN1412: right target, wrong cell. Br J Pharmacol. 2010; 161: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eastwood D, Bird C, Dilger P, et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. Br J Clin Pharmacol. 2013; 76: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Findlay L, Eastwood D, Stebbings R, et al. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J Immunol Methods. 2010; 352: 1. [DOI] [PubMed] [Google Scholar]
- 28. Findlay L, Sharp G, Fox B, et al. Endothelial cells co-stimulate peripheral blood mononuclear cell responses to monoclonal antibody TGN1412 in culture. Cytokine. 2011; 55: 141. [DOI] [PubMed] [Google Scholar]
- 29. Stebbings R, Findlay L, Edwards C, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007; 179: 3325. [DOI] [PubMed] [Google Scholar]
- 30. Stebbings R, Eastwood D, Poole S, Thorpe R. After TGN1412: recent developments in cytokine release assays. J Immunotoxicol. 2013; 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mary C, Coulon F, Poirier N, et al. Antagonist properties of monoclonal antibodies targeting human CD28: role of valency and the heavy-chain constant domain. mAbs. 2013; 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graves SS, Stone DM, Loretz C, et al. Antagonistic and agonistic anti-canine CD28 monoclonal antibodies: tools for allogeneic transplantation. Transplantation. 2011; 91: 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Storb R, Raff RF, Appelbaum FR, et al. FK506 and methotrexate prevent graft-versus-host disease in dogs given 9.2 Gy total body irradiation and marrow grafts from unrelated DLA-nonidentical donors. Transplantation. 1993; 56: 800. [DOI] [PubMed] [Google Scholar]
- 34. Thomas ED. Transplantation of hematopoietic progenitor cells with emphasis on the results in children. Turkish J Pediatr. 1995; 37: 31. [PubMed] [Google Scholar]
- 35. Yu C, Storb R, Deeg HJ, et al. Tacrolimus (FK506) and methotrexate regimens to prevent graft-versus-host disease after unrelated dog leukocyte antigen (DLA) nonidentical marrow transplantation. Bone Marrow Transplant. 1996; 17: 649. [PubMed] [Google Scholar]
- 36. Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996; 62: 876. [DOI] [PubMed] [Google Scholar]
- 37. Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication). Tissue Antigens. 1998; 52: 397. [DOI] [PubMed] [Google Scholar]
- 38. Lee WS, Suzuki Y, Graves SS, et al. Canine bone marrow-derived mesenchymal stromal cells suppress alloreactive lymphocyte proliferation in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant. 2011; 17: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romer PS, Berr S, Avota E, et al. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011; 118: 6772. [DOI] [PubMed] [Google Scholar]
- 40. Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007; 372: 774. [DOI] [PubMed] [Google Scholar]
- 41. Berman HM, Westbrook J, Feng Z, et al. The protein data bank. Nucleic Acids Res. 2000; 28: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stamper CC, Zhang Y, Tobin JF, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses [erratum appears in Nature 2001 May 31;411(6837):617]. Nature. 2001; 410: 608. [DOI] [PubMed] [Google Scholar]
- 43. Evans EJ, Esnouf RM, Manso-Sancho R, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005; 6: 271. [DOI] [PubMed] [Google Scholar]
- 44. Poirier N, Mary C, Dilek N, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab’ antibody. Am J Transplant. 2012; 12: 2630. [DOI] [PubMed] [Google Scholar]
- 45. Poirier N, Blancho G, Vanhove B. Alternatives to calcineurin inhibition in renal transplantation: belatacept, the first co-stimulation blocker (Review). Immunotherapy. 2010; 2: 625. [DOI] [PubMed] [Google Scholar]
- 46. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment (Review). Nat Rev Drug Discov. 2010; 9: 703. [DOI] [PubMed] [Google Scholar]


