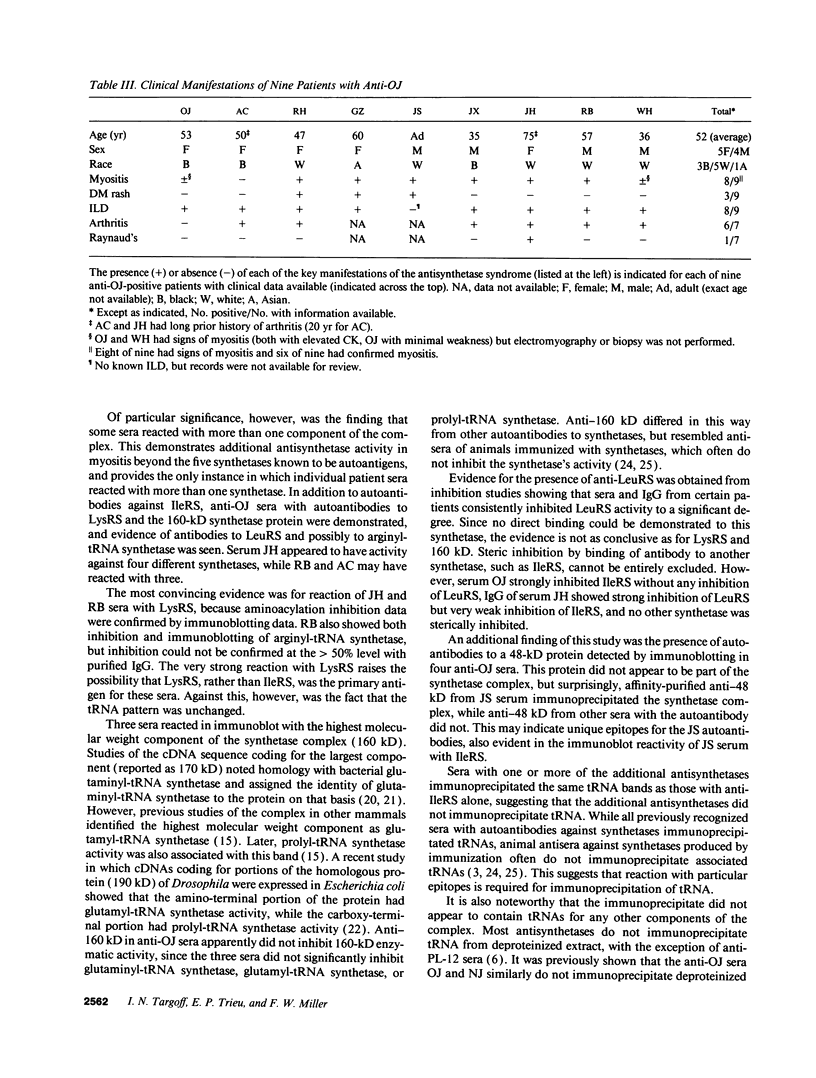
Abstract
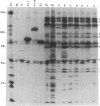
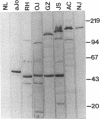
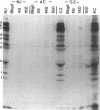
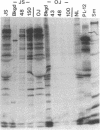
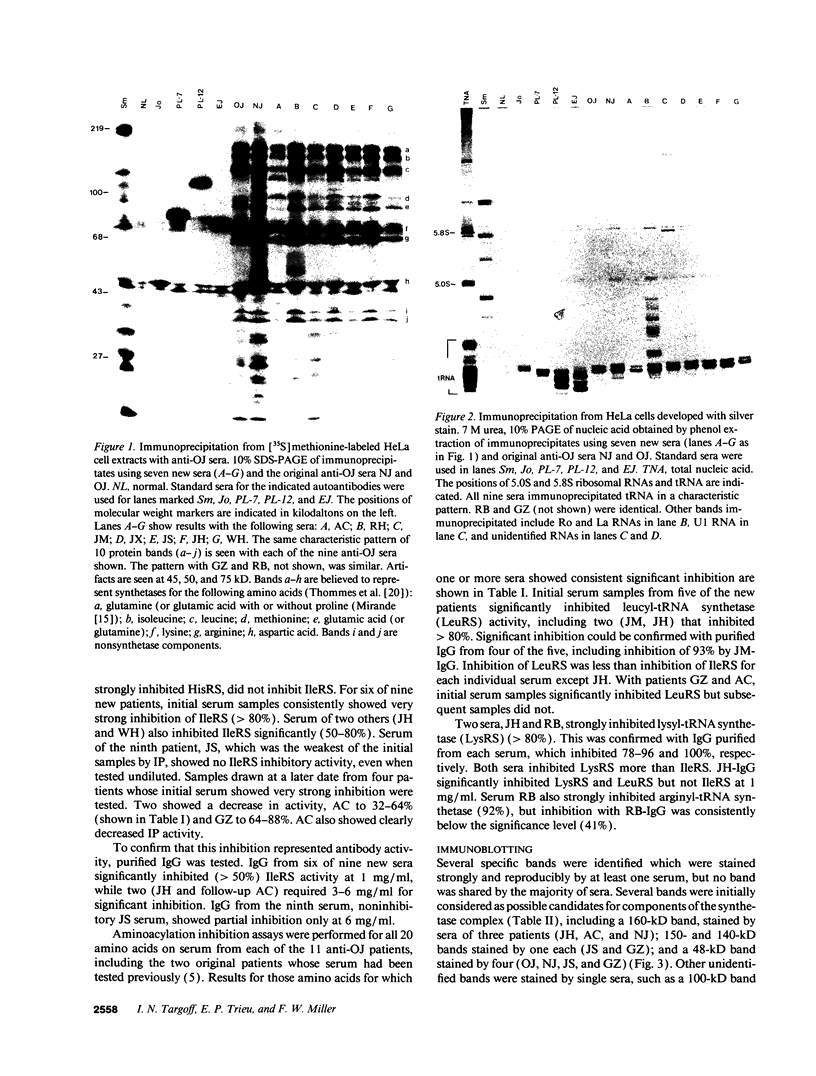
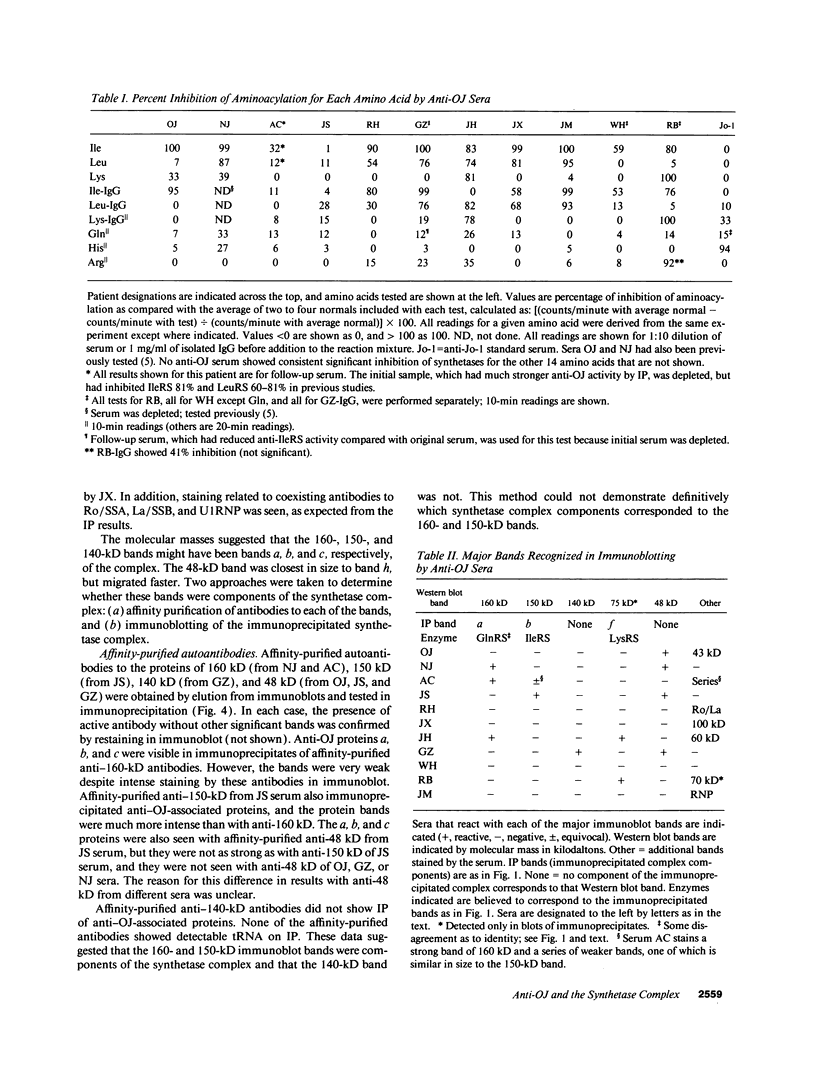
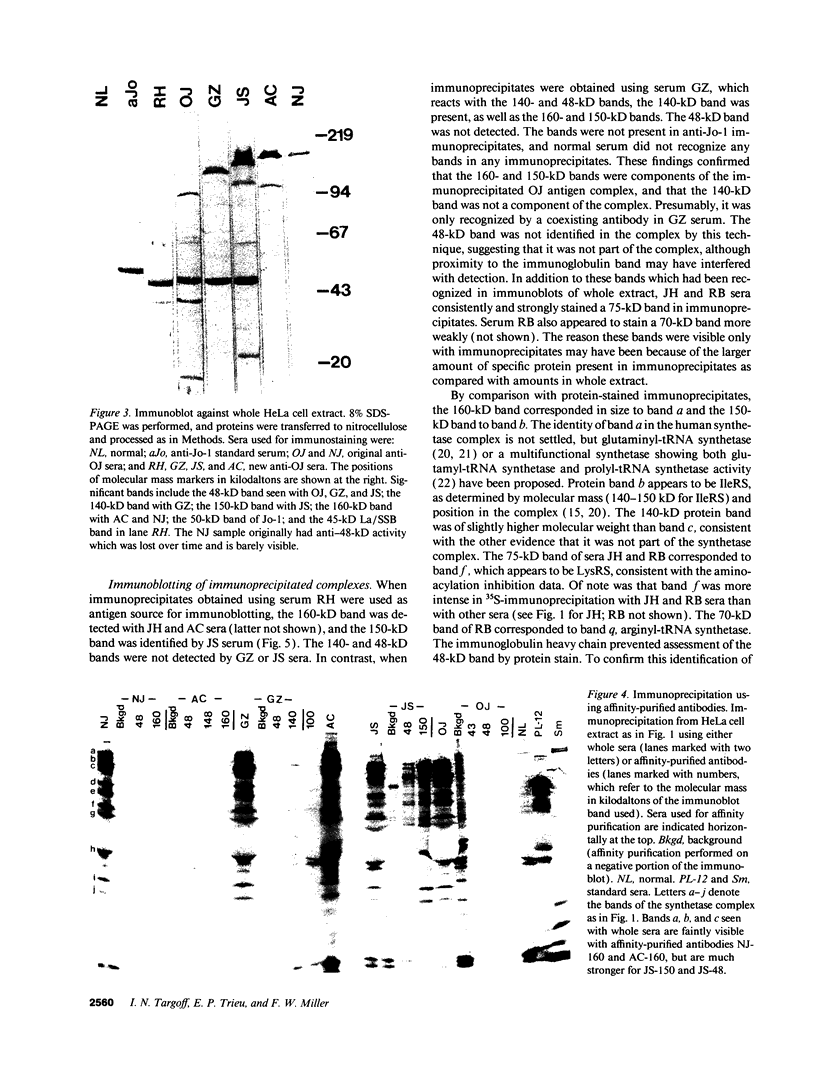
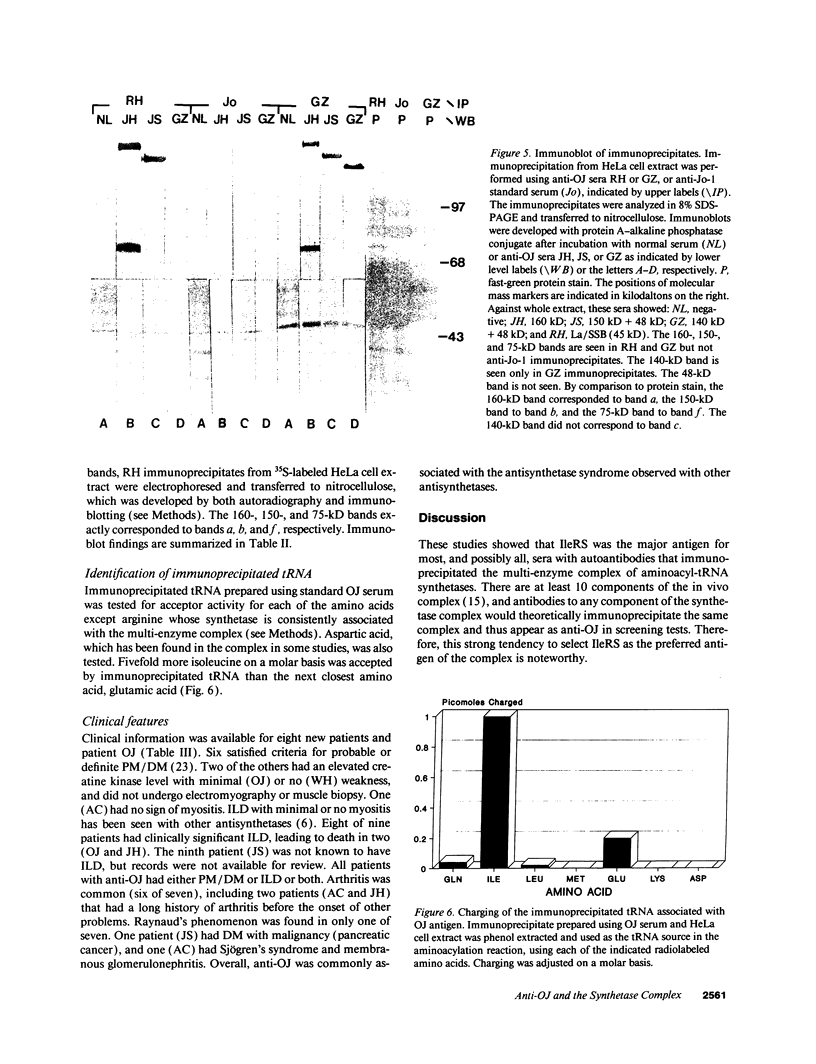
Autoantibodies to five aminoacyl-tRNA synthetases have been reported, and all have been associated with a syndrome of myositis and interstitial lung disease. Four of these synthetases exist free in the cytoplasm, but the fifth, isoleucyl-tRNA synthetase (recognized by anti-OJ autoantibodies), is a component of the multi-enzyme complex containing at least seven synthetases. In an effort to better understand the origins of these antibodies, we examined sera from 11 patients with anti-OJ autoantibodies for evidence of reaction with other components of the complex. All sera showed a characteristic pattern of 10 proteins bands by immunoprecipitation from HeLa cell extract. 10 of 11 sera significantly inhibited isoleucyl-tRNA synthetase enzyme activity. Serum and IgG from four patients also inhibited leucyl-tRNA synthetase activity, and serum and IgG from two inhibited lysyl-tRNA synthetase. Immunoblotting experiments supported reaction of the two sera with lysyl-tRNA synthetase, and revealed additional reactivity of three sera with a 160-kD component believed to be glutaminyl-tRNA synthetase. Despite reaction of some sera with additional synthetases, the immunoprecipitated tRNA appeared the same with all sera, and functioned as tRNA(ile). While reaction with more than one synthetase was seen with some anti-OJ sera, all synthetases targeted by anti-OJ sera were components of the complex, rather than unassociated synthetases. These findings suggest that an initial autoantibody response against isoleucyl-tRNA synthetase was followed by extension to involve other components of the synthetase complex. These observations may have implications for understanding the generation of antisynthetase autoantibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. M., Morgan S. H., Chapman J., Bunn C. C., Mathews M. B., Turner-Warwick M., Hughes G. R. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br Med J (Clin Res Ed) 1984 Jul 21;289(6438):151–152. doi: 10.1136/bmj.289.6438.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975 Feb 20;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerini C., Kerjan P., Astier M., Gratecos D., Mirande M., Sémériva M. A component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J. 1991 Dec;10(13):4267–4277. doi: 10.1002/j.1460-2075.1991.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Dang C. V. Higher eukaryotic aminoacyl-tRNA synthetases in physiologic and pathologic states. Mol Cell Biochem. 1986 Aug;71(2):107–120. doi: 10.1007/BF00214769. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Tan E. M., Traugh J. A. Myositis autoantibody reactivity and catalytic function of threonyl-tRNA synthetase. FASEB J. 1988 May;2(8):2376–2379. doi: 10.1096/fasebj.2.8.2452112. [DOI] [PubMed] [Google Scholar]
- Love L. A., Leff R. L., Fraser D. D., Targoff I. N., Dalakas M., Plotz P. H., Miller F. W. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991 Nov;70(6):360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R., Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984 Aug 1;160(2):420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. W., Waite K. A., Biswas T., Plotz P. H. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9933–9937. doi: 10.1073/pnas.87.24.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: structural domains and their implications. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]
- Nishikai M., Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 1980 Aug;23(8):881–888. doi: 10.1002/art.1780230802. [DOI] [PubMed] [Google Scholar]
- Oddis C. V., Medsger T. A., Jr, Cooperstein L. A. A subluxing arthropathy associated with the anti-Jo-1 antibody in polymyositis/dermatomyositis. Arthritis Rheum. 1990 Nov;33(11):1640–1645. doi: 10.1002/art.1780331106. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Schray B., Thömmes P., Knippers R. Glutaminyl-tRNA synthetase as a component of the high-molecular weight complex of human aminoacyl-tRNA synthetases. An immunological study. Biochim Biophys Acta. 1990 Oct 23;1087(2):226–234. doi: 10.1016/0167-4781(90)90209-k. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Berman L., O'Brien C., Reichlin M. Anti-KJ: a new antibody associated with the syndrome of polymyositis and interstitial lung disease. J Clin Invest. 1989 Jul;84(1):162–172. doi: 10.1172/JCI114136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C. Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase). Am J Med. 1990 Mar;88(3):241–251. doi: 10.1016/0002-9343(90)90149-8. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Reichlin M. Antibody to threonyl-transfer RNA synthetase in myositis sera. Arthritis Rheum. 1988 Apr;31(4):515–524. doi: 10.1002/art.1780310408. [DOI] [PubMed] [Google Scholar]
- Targoff I. N. Autoantibodies in polymyositis. Rheum Dis Clin North Am. 1992 May;18(2):455–482. [PubMed] [Google Scholar]
- Targoff I. N. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J Immunol. 1990 Mar 1;144(5):1737–1743. [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. Measurement of antibody to Jo-1 by ELISA and comparison to enzyme inhibitory activity. J Immunol. 1987 May 1;138(9):2874–2882. [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985 Jul;28(7):796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Trieu E. P., Plotz P. H., Miller F. W. Antibodies to glycyl-transfer RNA synthetase in patients with myositis and interstitial lung disease. Arthritis Rheum. 1992 Jul;35(7):821–830. doi: 10.1002/art.1780350718. [DOI] [PubMed] [Google Scholar]
- Thömmes P., Fett R., Schray B., Kunze N., Knippers R. The core region of human glutaminyl-tRNA synthetase homologies with the Escherichia coli and yeast enzymes. Nucleic Acids Res. 1988 Jun 24;16(12):5391–5406. doi: 10.1093/nar/16.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Akizuki M., Mimori T., Yamagata H., Inada S., Homma M. The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. A marker for a subset of polymyositis with interstitial pulmonary fibrosis. Arthritis Rheum. 1983 May;26(5):604–611. doi: 10.1002/art.1780260505. [DOI] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E., Jaarsma D., Limburg P. C., Kallenberg C. G. Sequential development of antibodies to specific Sm polypeptides in a patient with systemic lupus erythematosus: evidence for independent regulation of anti-double-stranded DNA and anti-Sm antibody production. Arthritis Rheum. 1988 Dec;31(12):1563–1567. doi: 10.1002/art.1780311215. [DOI] [PubMed] [Google Scholar]