Abstract
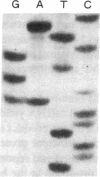
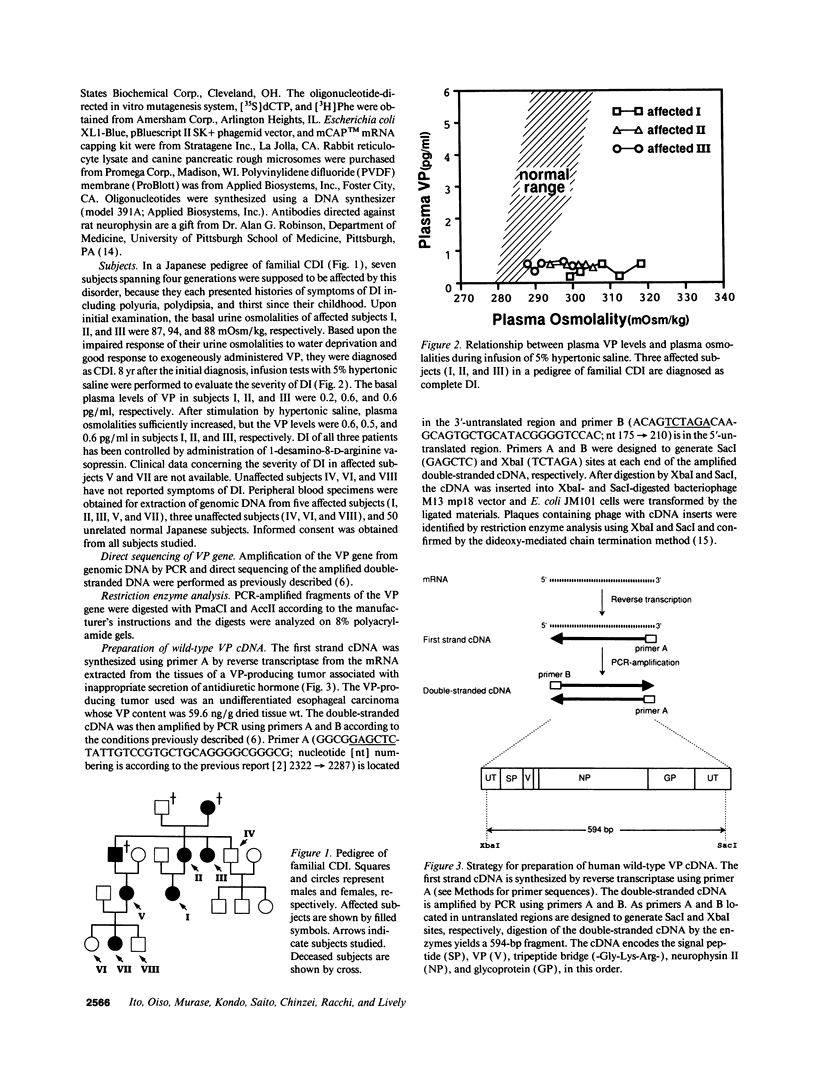
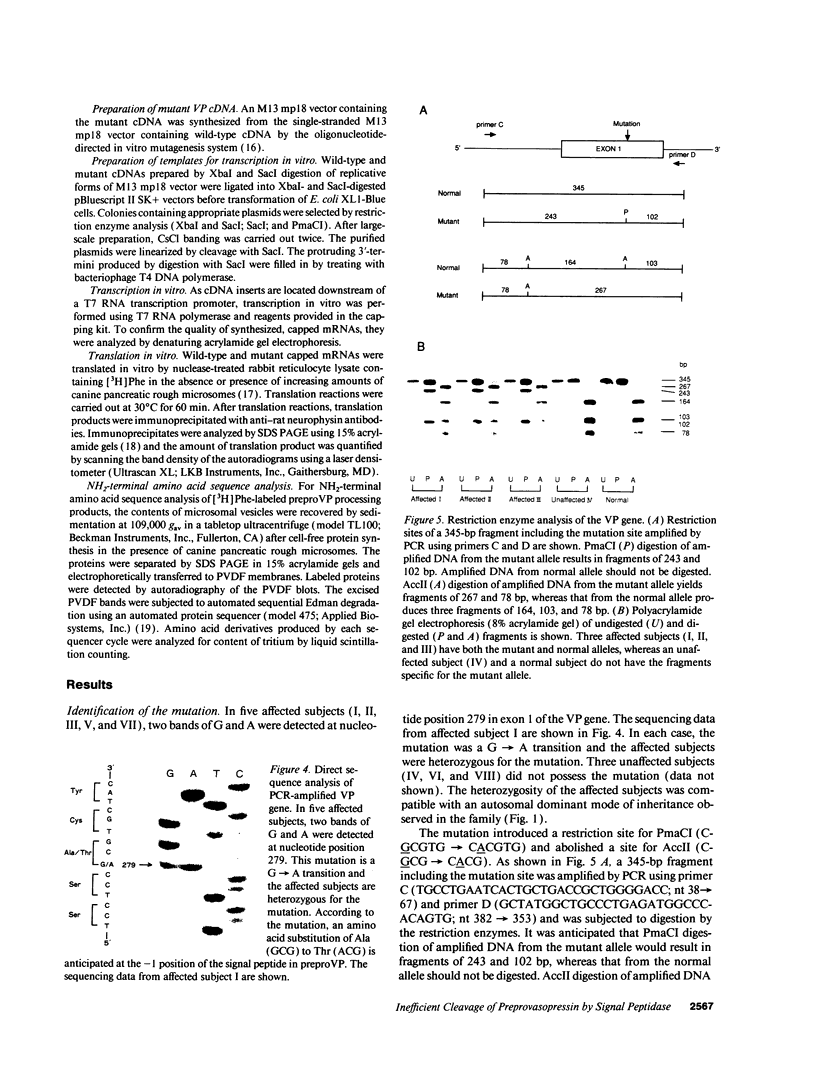
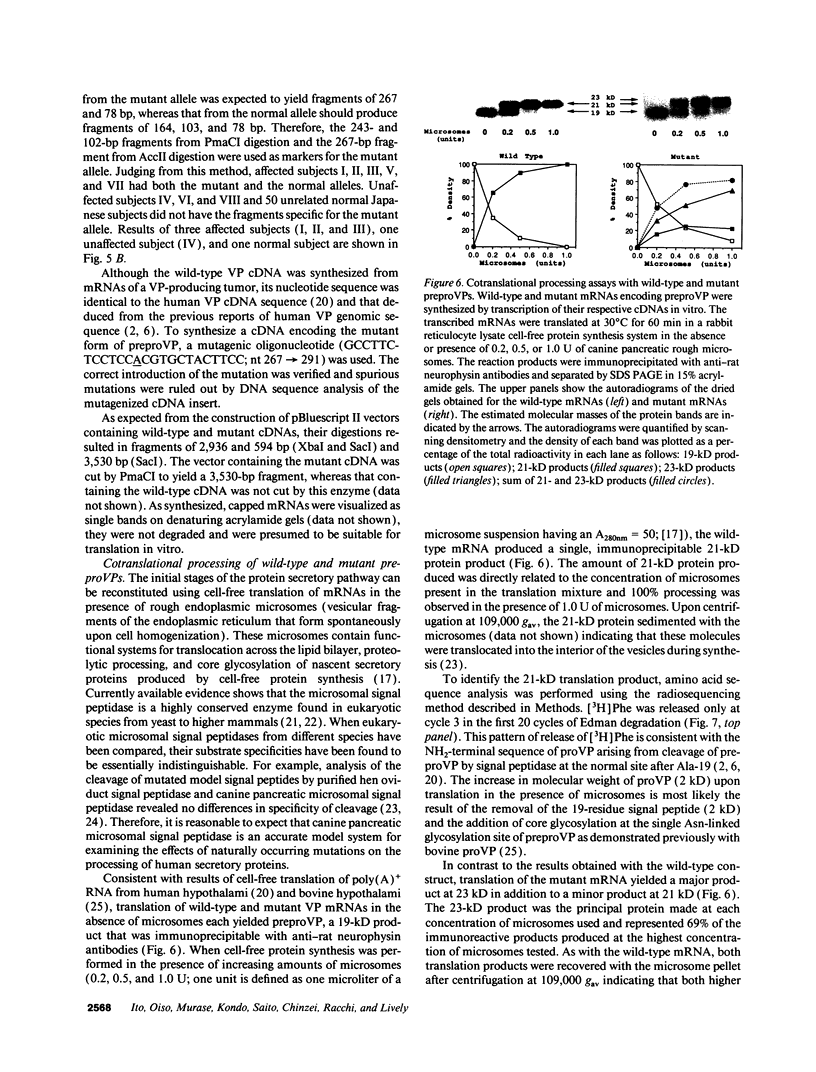
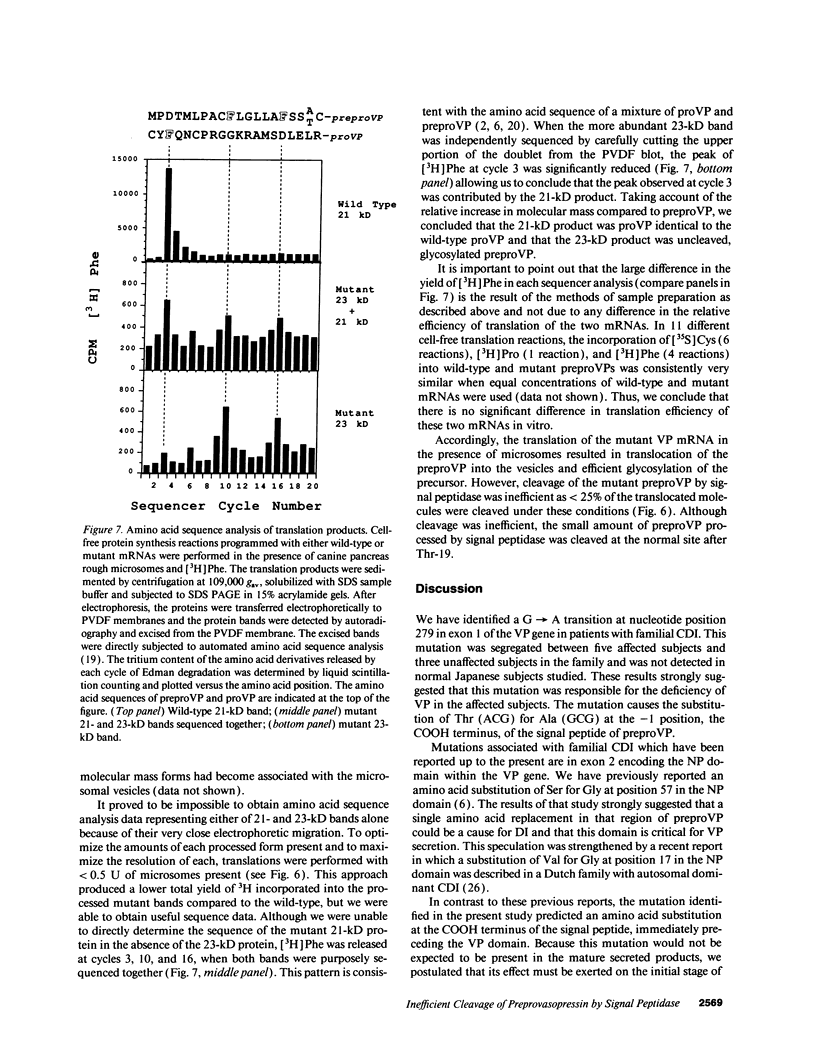
A transition of G to A at nucleotide position 279 in exon 1 of the vasopressin gene has been identified in patients with familial central diabetes insipidus. The mutation predicts an amino acid substitution of Thr (ACG) for Ala (GCG) at the COOH terminus of the signal peptide in preprovasopression (preproVP). Translation in vitro of wild-type and mutant mRNAs produced 19-kD preproVPs. When translated in the presence of canine pancreatic rough microsomes, wild-type preproVP was converted to a 21-kD protein, whereas the mutant mRNA produced proteins of 21 kD and 23 kD. NH2-terminal amino acid sequence analysis revealed that the 21-kD proteins from the wild-type and the mutants were proVPs generated by the proteolytic cleavage of the 19-residue signal peptide and the addition of carbohydrate. Accordingly, mutant preproVP was cleaved at the correct site after Thr-19, but the efficiency of cleavage by signal peptidase was < 25% that observed for the wild-type preproVP, resulting in the formation of a predominant glycosylated but uncleaved 23-kD product. These data suggest that inefficient processing of preproVP produced by the mutant allele is possibly involved in the pathogenesis of diabetes insipidus in the affected individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Horst S. A., Gardella T. J., Baba H., Levine M. A., Kronenberg H. M. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990 Oct;86(4):1084–1087. doi: 10.1172/JCI114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Bahnsen U., Oosting P., Swaab D. F., Nahke P., Richter D., Schmale H. A missense mutation in the vasopressin-neurophysin precursor gene cosegregates with human autosomal dominant neurohypophyseal diabetes insipidus. EMBO J. 1992 Jan;11(1):19–23. doi: 10.1002/j.1460-2075.1992.tb05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis P. H., Robertson G. L. Vasopressin function in familial cranial diabetes insipidus. Postgrad Med J. 1981 Jan;57(663):36–40. doi: 10.1136/pgmj.57.663.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Myles T., Peach R. J., Donaldson D., George P. M. Albumin Redhill (-1 Arg, 320 Ala----Thr): a glycoprotein variant of human serum albumin whose precursor has an aberrant signal peptidase cleavage site. Proc Natl Acad Sci U S A. 1990 Jan;87(1):26–30. doi: 10.1073/pnas.87.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Cioffi J. A., Allen K. L., Lively M. O., Kemper B. Parallel effects of signal peptide hydrophobic core modifications on co-translational translocation and post-translational cleavage by purified signal peptidase. J Biol Chem. 1989 Sep 5;264(25):15052–15058. [PubMed] [Google Scholar]
- Daly M., Bruce D., Perry D. J., Price J., Harper P. L., O'Meara A., Carrell R. W. Antithrombin Dublin (-3 Val----Glu): an N-terminal variant which has an aberrant signal peptidase cleavage site. FEBS Lett. 1990 Oct 29;273(1-2):87–90. doi: 10.1016/0014-5793(90)81057-u. [DOI] [PubMed] [Google Scholar]
- Folz R. J., Nothwehr S. F., Gordon J. I. Substrate specificity of eukaryotic signal peptidase. Site-saturation mutagenesis at position -1 regulates cleavage between multiple sites in human pre (delta pro) apolipoprotein A-II. J Biol Chem. 1988 Feb 5;263(4):2070–2078. [PubMed] [Google Scholar]
- Ito M., Mori Y., Oiso Y., Saito H. A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Invest. 1991 Feb;87(2):725–728. doi: 10.1172/JCI115052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Schmale H., Richter D. Glycosylation of the arginine vasopressin/neurophysin II common precursor. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1230–1236. doi: 10.1016/s0006-291x(81)80143-x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. B., D'Ercole A. J., Robertson G. L. Radioimmunoassay of vasopressin in familial cental diabetes insipidus. J Pediatr. 1982 Jan;100(1):76–81. doi: 10.1016/s0022-3476(82)80238-2. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mohr E., Hillers M., Ivell R., Haulica I. D., Richter D. Expression of the vasopressin and oxytocin genes in human hypothalami. FEBS Lett. 1985 Nov 25;193(1):12–16. doi: 10.1016/0014-5793(85)80069-7. [DOI] [PubMed] [Google Scholar]
- Müller M. Proteolysis in protein import and export: signal peptide processing in eu- and prokaryotes. Experientia. 1992 Feb 15;48(2):118–129. doi: 10.1007/BF01923506. [DOI] [PubMed] [Google Scholar]
- Newsome A. L., McLean J. W., Lively M. O. Molecular cloning of a cDNA encoding the glycoprotein of hen oviduct microsomal signal peptidase. Biochem J. 1992 Mar 1;282(Pt 2):447–452. doi: 10.1042/bj2820447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Hoeltzli S. D., Allen K. L., Lively M. O., Gordon J. I. Residues flanking the COOH-terminal C-region of a model eukaryotic signal peptide influence the site of its cleavage by signal peptidase and the extent of coupling of its co-translational translocation and proteolytic processing in vitro. J Biol Chem. 1990 Dec 15;265(35):21797–21803. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Racchi M., Watzke H. H., High K. A., Lively M. O. Human coagulation factor X deficiency caused by a mutant signal peptide that blocks cleavage by signal peptidase but not targeting and translocation to the endoplasmic reticulum. J Biol Chem. 1993 Mar 15;268(8):5735–5740. [PubMed] [Google Scholar]
- Riddell D. C., Mallonee R., Phillips J. A., Parks J. S., Sexton L. A., Hamerton J. L. Chromosomal assignment of human sequences encoding arginine vasopressin-neurophysin II and growth hormone releasing factor. Somat Cell Mol Genet. 1985 Mar;11(2):189–195. doi: 10.1007/BF01534707. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Seif S. M., Huellmantel A. B., Platia M. P., Haluszczak C., Robinson A. G. Isolation, radioimmunoassay and physiologic secretion of rat neurophysins. Endocrinology. 1977 May;100(5):1317–1326. doi: 10.1210/endo-100-5-1317. [DOI] [PubMed] [Google Scholar]
- Sheer D. G., Yuen S., Wong J., Wasson J., Yuan P. M. A modified reaction cartridge for direct protein sequencing on polymeric membranes. Biotechniques. 1991 Oct;11(4):526–533. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Watzke H. H., Wallmark A., Hamaguchi N., Giardina P., Stafford D. W., High K. A. Factor XSanto Domingo. Evidence that the severe clinical phenotype arises from a mutation blocking secretion. J Clin Invest. 1991 Nov;88(5):1685–1689. doi: 10.1172/JCI115484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]